Evaluation of the Surface Performance of Mortar Matrix Subjected to Sodium Chloride Solution Modified with Hybrid Nanosilica Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
2.3.1. Rebound Surface Hardness
2.3.2. Rapid Chloride Migration (RCM) Test
2.3.3. X-ray Diffraction (XRD)
2.3.4. Thermogravimetric and Differential Thermogravimetric (TG-DTG) Analyses
2.3.5. Thermodynamic Modeling
3. Results and Discussion
3.1. Surface Hardness Change
3.2. RCM
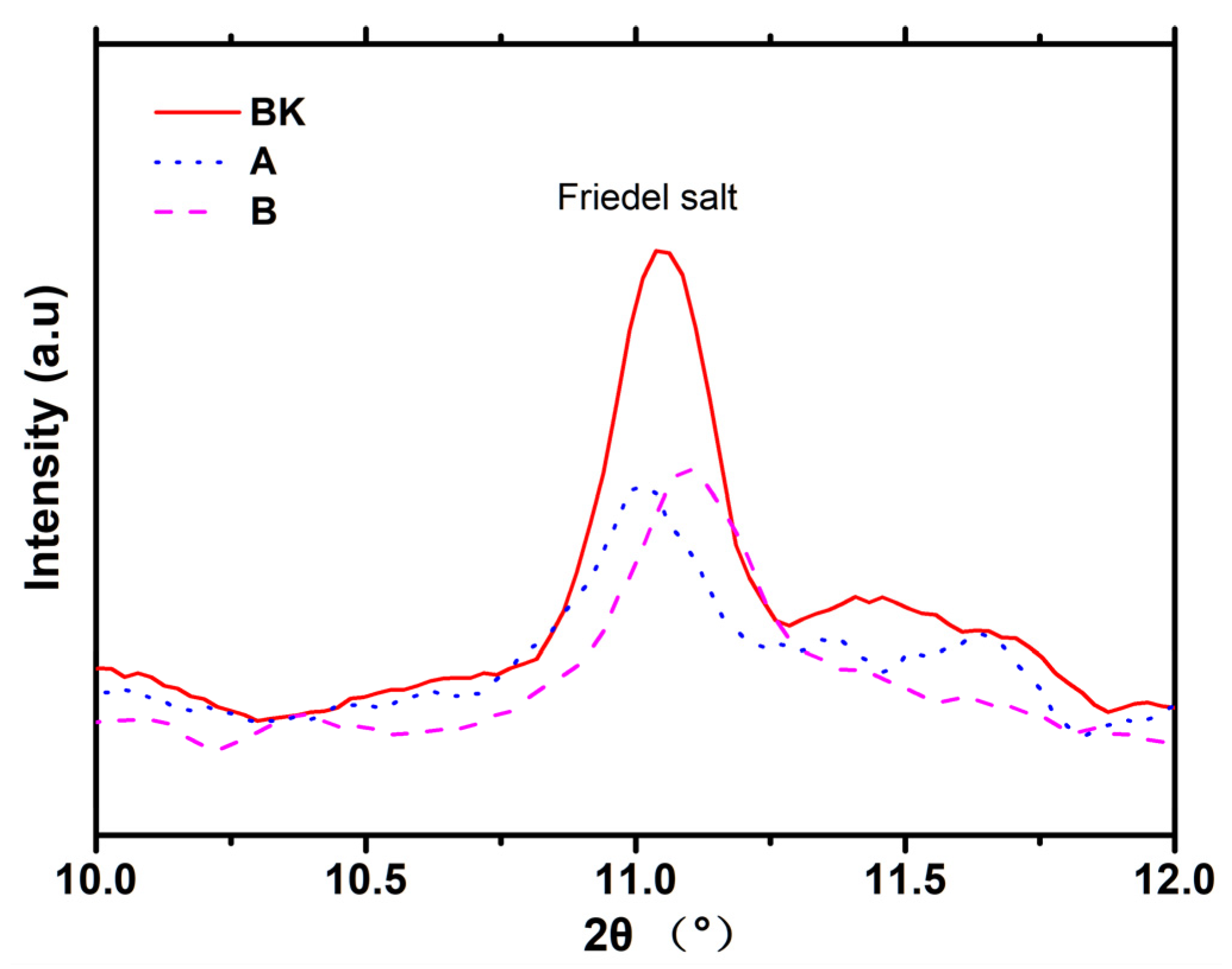
3.3. XRD
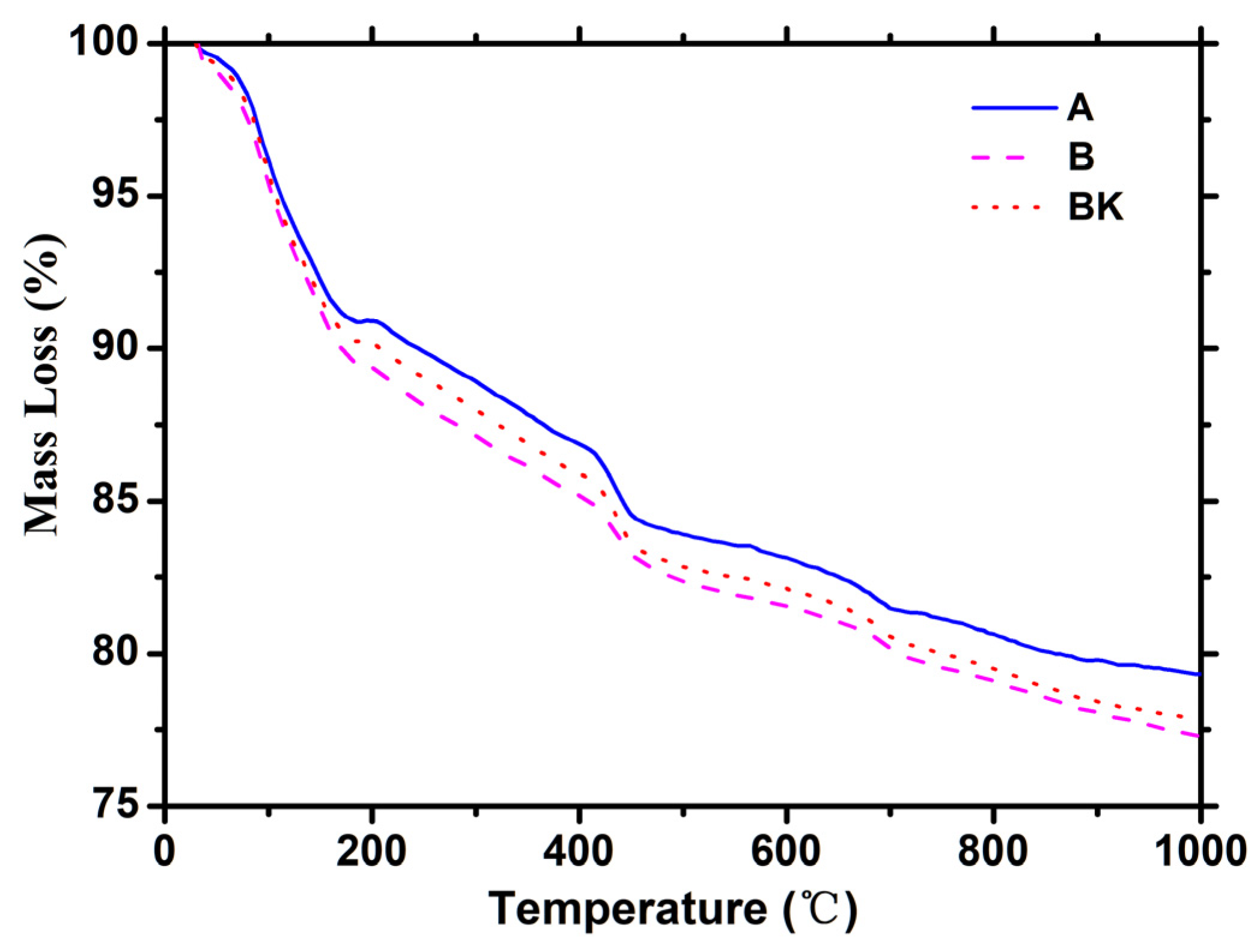
3.4. TG-DTG
3.5. Thermodynamic Modeling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, P.J.M.; Miller, S.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, K.; Krakowiak, K.J.; Bauchy, M.; Hoover, C.G.; Masoero, E.; Yip, S.; Ulm, F.J.; Levitz, P.; Pellenq, R.J.M.; Del Gado, E. Mesoscale texture of cement hydrates. Proc. Natl. Acad. Sci. USA 2016, 113, 2029–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trung, N.T.; Alemi, N.; Haido, J.H.; Shariati, M.; Baradaran, S.; Yousif, S.T. Reduction of cement consumption by producing smart green concretes with natural zeolites. Smart Struct. Syst. 2019, 24, 415–425. [Google Scholar]
- Shariati, M.; Rafie, S.; Zandi, Y.; Fooladvand, R.; Gharehaghaj, B.; Mehrabi, P.; Shariat, A.; Trung, N.T.; Salih, M.N.; Poi-Ngian, S. Experimental investigation on the effect of cementitious materials on fresh and mechanical properties of self-consolidating concrete. Adv. Concr. Constr. 2019, 8, 225–237. [Google Scholar]
- Nosrati, A.; Zandi, Y.; Shariati, M.; Khademi, K.; Aliabad, M.D.; Marto, A.; Mu’azu, M.A.; Ghanbari, E.; Mahdizadeh, M.B.; Shariati, A.; et al. Portland cement structure and its major oxides and fineness. Smart Struct. Syst. 2018, 22, 425–432. [Google Scholar]
- Zhang, P.; Sha, D.; Li, Q.; Zhao, S.; Ling, Y. Effect of Nano Silica Particles on Impact Resistance and Durability of Concrete Containing Coal Fly Ash. Nanomaterials 2021, 11, 1296. [Google Scholar] [CrossRef]
- Rupasinghe, M.; Nicolas, R.S.; Mendis, P.; Sofi, M.; Ngo, T. Investigation of strength and hydration characteristics in nano-silica incorporated cement paste. Cem. Concr. Compos. 2017, 80, 17–30. [Google Scholar] [CrossRef]
- Lyu, K.; Garboczi, E.J.; Gao, Y.; Miao, C.; Liu, X. Relationship between fine aggregate size and the air void system of six mortars: I. Air void content and diameter distribution. Cem. Concr. Compos. 2022, 131, 104599. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Zhang, T.; Mei, J.; Qi, H.; Chen, P.; Wang, J. Chloride binding in sound and carbonated cementitious materials with various types of binder. Constr. Build. Mater. 2014, 68, 82–91. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Lura, P.; Müller, H.S.; Han, S.; Zhao, T. Application of neutron imaging to investigate fundamental aspects of durability of cement-based materials: A review. Cem. Concr. Res. 2018, 108, 152–166. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; de Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement–metakaolin–limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Martın-Pérez, B.; Zibara, H.; Hooton, R.D.; Thomas, M.D.A. A study of the effect of chloride binding on service life predic-tions. Cem. Concr. Res. 2000, 30, 1215–1223. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. Corrosion protection of the reinforcing steels in chloride-laden con-crete environment through epoxy/polyaniline–camphorsulfonate nanocomposite coating. Corros. Sci. 2015, 90, 239–247. [Google Scholar] [CrossRef]
- Franzoni, E.; Varum, H.; Natali, M.E.; Bignozzi, M.C.; Melo, J.; Rocha, L.; Pereira, E. Improvement of historic reinforced concrete/mortars by impregna-tion and electrochemical methods. Cem. Concr. Compos. 2014, 49, 50–58. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Chang, H.; Wang, X.; Wang, Y.; Li, S.; Wang, J.; Liu, J.; Feng, P. Influence of Low Vacuum Condition on Mechanical Performance and Microstructure of Hardened Cement Paste at Early Age. Constr. Build. Mater. 2022, 346, 128358. [Google Scholar] [CrossRef]
- Franzoni, E.; Pigino, B.; Pistolesi, C. Ethyl silicate for surface protection of concrete: Performance in com-parison with other inorganic surface treatments. Cem. Concr. Compos. 2013, 44, 69–76. [Google Scholar] [CrossRef]
- Medeiros, M.H.; Helene, P.R.D.L. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.N.D.R.E.A.; Bolzoni, F.A.B.I.O.; Berra, M.A.R.I.O.; Pastore, T.; Ormellese, M.A.R.C.O. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Zhu, H. Potential application of geopolymers as protection coatings for marine concrete: II. Microstructure and anticorrosion mechanism. Appl. Clay Sci. 2010, 49, 7–12. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wei, Z.; Jiang, L.; She, W.; Lyu, K. Synthesis of nanoSiO2@graphene-oxide core-shell nanoparticles and its influence on mechanical properties of cementitious materials. Constr. Build. Mater. 2020, 236, 117619. [Google Scholar] [CrossRef]
- Gu, Y.; Ran, Q.; She, W.; Shu, X.; Liu, J. Effects and mechanisms of surface-treatment of cementitious materials with nanoSiO2@PCE core-shell nanoparticles. Constr. Build. Mater. 2018, 166, 12–22. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, X.; Huang, Y.; Zhang, P.; Tian, Q.; Liu, J. Investigation of moisture transport in cement-based materials using low-field nuclear magnetic resonance imaging. Mag. Concr. Res. 2019, 73, 252–270. [Google Scholar] [CrossRef]
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials–A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Norhasri, M.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Parghi, A.; Shahria Alam, M. Effects of curing regimes on the mechanical properties and durability of poly-mer-modified mortars–an experimental investigation. J. Sustain. Cem.-Based Mater. 2016, 5, 324–347. [Google Scholar]
- Saloma; Nasution, A.; Imran, I.; Abdullah, M. Improvement of Concrete Durability by Nanomaterials. Procedia Eng. 2015, 125, 608–612. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.; Cheng, X.; Qian, J.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of surface-treatment of nano-SiO2 on the transport properties of hardened cement pastes with different water-to-cement ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Shirzadi Javid, A.A.; Ghoddousi, P.; Zareechian, M.; Habibnejad Korayem, A. Effects of Spraying Various Nanoparticles at Early Ages on Improving Surface Charac-teristics of Concrete Pavements. Int. J. Civ. Eng. 2019, 17, 1455–1468. [Google Scholar] [CrossRef]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of modified nanoparticles on hydrophobicity of concrete with organic film coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Li, R.; Hou, P.; Xie, N.; Ye, Z.; Cheng, X.; Shah, S.P. Design of SiO2/PMHS hybrid nanocomposite for surface treatment of cement-based materials. Cem. Concr. Compos. 2018, 87, 89–97. [Google Scholar] [CrossRef]
- Gu, Y.; Ran, Q.; She, W.; Liu, J. Modifying Cement Hydration with NS@PCE Core-Shell Nanoparticles. Adv. Mater. Sci. Eng. 2017, 2017, 3823621. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.Y.; Yu, Q.L.; Brouwers, H.J.H. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem. Concr. Res. 2018, 105, 81–90. [Google Scholar] [CrossRef]
- GBT50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Chinese National Standards: Beijing, China, 2009.
- Luping, T.; Nilsson, L. Rapid determination of the chloride diffusivity in concrete by applying an electric field. Mater. J. 1993, 89, 49–53. [Google Scholar]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2012, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Shi, C.; Zhang, J.; Jia, L.; Chong, L. Effect of inorganic surface treatment on surface hardness and carbonation of cement-based materials. Cem. Concr. Compos. 2018, 90, 218–224. [Google Scholar] [CrossRef]
- Kong, D.; Pan, H.; Wang, L.; Corr, D.J.; Yang, Y.; Shah, S.P.; Sheng, J. Effect and mechanism of colloidal silica sol on properties and microstructure of the hardened cement-based materials as compared to nano-silica powder with agglomerates in micron-scale. Cem. Concr. Compos. 2019, 98, 137–149. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P. Chloride in cement. Adv. Cem. Res. 2015, 27, 63–97. [Google Scholar] [CrossRef]
- Wang, D.; Yang, P.; Hou, P.; Zhang, L.; Zhou, Z.; Cheng, X. Effect of SiO2 oligomers on water absorption of cementitious materials. Cem. Concr. Res. 2016, 87, 22–30. [Google Scholar] [CrossRef]
- Chu, H.; Zhang, B.; Zhao, S.; Guo, M.; Liang, Y.; Jiang, L.; Song, Z. Effect of electric current on the stability of bound chloride. Cem. Concr. Compos. 2019, 103, 71–79. [Google Scholar] [CrossRef]
- Singh, L.P.; Goel, A.; Bhattachharyya, S.K.; Ahalawat, S.; Sharma, U.; Mishra, G. Effect of Morphology and Dispersibility of Silica Nanoparticles on the Mechanical Behav-iour of Cement Mortar. Int. J. Concr. Struct. Mater. 2015, 9, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.P.; Ali, D.; Sharma, U. Studies on optimization of silica nanoparticles dosage in cementitious system. Cem. Concr. Compos. 2016, 70, 60–68. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, T.; Tan, H.; Liu, X.; Mei, J.; Jiang, W.; Qi, H.; Gu, B. Effect of TIPA on Chloride Immobilization in Cement-Fly Ash Paste. Adv. Mater. Sci. Eng. 2018, 2018, 1326053. [Google Scholar] [CrossRef] [Green Version]
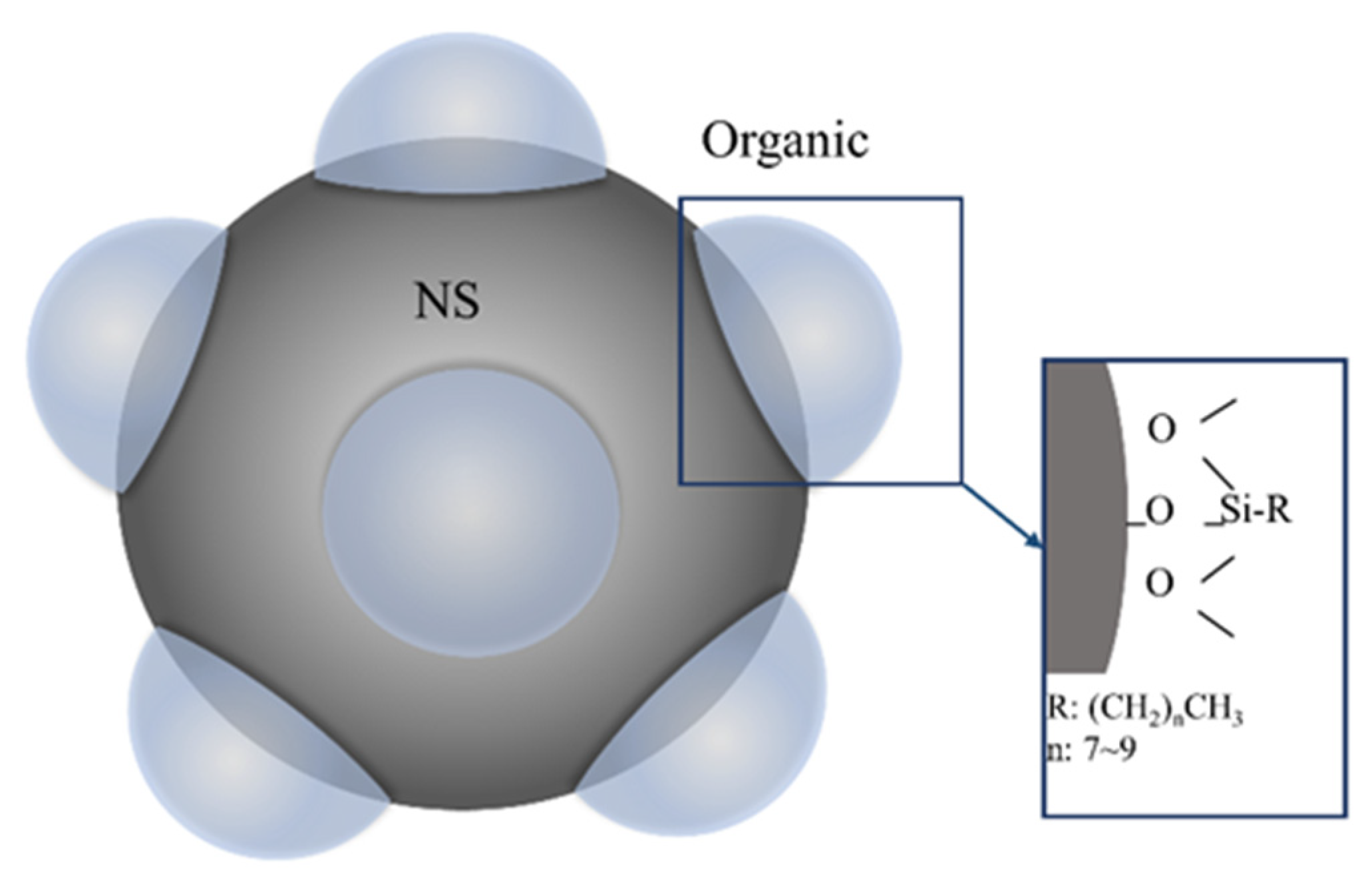







| CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | K2O | Na2O |
|---|---|---|---|---|---|---|---|
| 62.83 | 20.5 | 5.61 | 1.7 | 3.84 | 3.07 | 1.31 | 0.21 |
| Sample | Water to Cement Ratio | HN Content (%) |
|---|---|---|
| BK | 0.35 | 0 |
| A | 0.35 | 1 |
| B | 0.35 | 2 |
| Sample | A | B | BK |
|---|---|---|---|
| CH content (%) | 12.17 | 11.49 | 12.56 |
| Friedel salt content (%) | 6.43 | 6.12 | 6.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, K.; Xu, J.; Gu, Y.; Xia, K.; Wang, L.; Liu, W.; Xie, X. Evaluation of the Surface Performance of Mortar Matrix Subjected to Sodium Chloride Solution Modified with Hybrid Nanosilica Cement Paste. Sustainability 2022, 14, 9876. https://doi.org/10.3390/su14169876
Lyu K, Xu J, Gu Y, Xia K, Wang L, Liu W, Xie X. Evaluation of the Surface Performance of Mortar Matrix Subjected to Sodium Chloride Solution Modified with Hybrid Nanosilica Cement Paste. Sustainability. 2022; 14(16):9876. https://doi.org/10.3390/su14169876
Chicago/Turabian StyleLyu, Kai, Junjie Xu, Yue Gu, Kailun Xia, Lei Wang, Weiwei Liu, and Xian Xie. 2022. "Evaluation of the Surface Performance of Mortar Matrix Subjected to Sodium Chloride Solution Modified with Hybrid Nanosilica Cement Paste" Sustainability 14, no. 16: 9876. https://doi.org/10.3390/su14169876





