Methane Emissions from Livestock Slurry: Effects of Storage Temperature and Changes in Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dairy Manure, Fattening Pig Manure
2.2. Physical–Chemical Analysis
2.3. Experimental Procedures
2.3.1. Storage Experiments
Comparison with IPCC Methodology Tier 2
2.3.2. Biochemical Methane Potential Tests
2.4. Data Analysis
2.4.1. Statistical Analysis
2.4.2. Kinetics Analysis
3. Results and Discussion
3.1. Manure Characteristics
3.2. Methane Emissions during Storage
3.2.1. Dairy Manure
3.2.2. Fattening Pig Manure
3.3. Methane Yield during the Biochemical Methane Potential Test
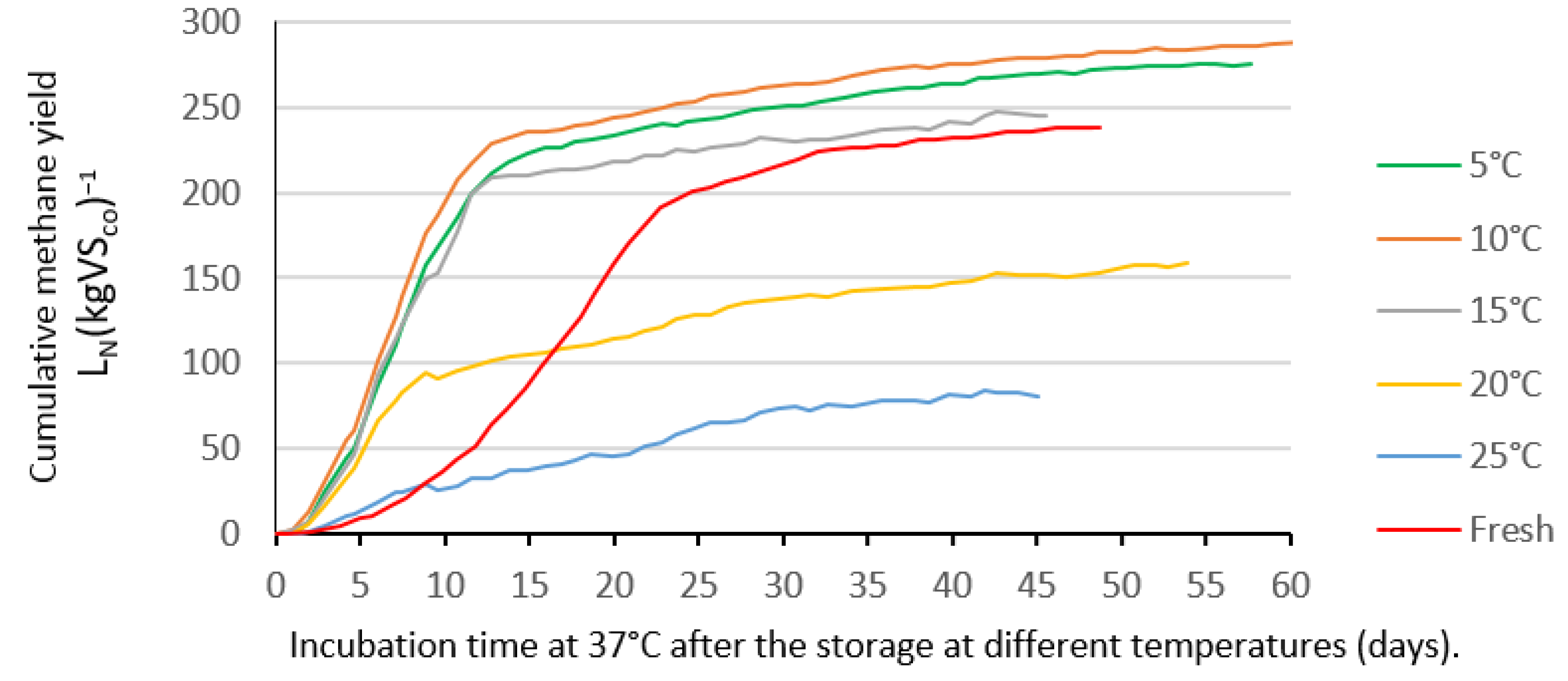
3.3.1. Dairy Manure
3.3.2. Fattening Pig Manure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. Sustain. Dev. Efforts Eradicate Poverty. 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 19 January 2022).
- Duwe, M.; Hope, E.; Morales, D.L. Climate Laws in Europe: Good Practices in Net-Zero Management. Eur. Clim. Found. 2020. Available online: https://www.ecologic.eu/sites/files/publication/2020/climatelawsineurope_fullreport_0.pdf (accessed on 19 January 2022).
- FAO. Emissions from Agriculture and Forest Land. Global, Regional and Country Trends 1990–2019. FAOSTAT Anal. Br. Ser. No 25. 2021. Available online: https://www.fao.org/publications/card/en/c/CB5293EN/ (accessed on 19 January 2022).
- FAO. FAOSTAT Statistical Database. Food and Agriculture Organization of the United Nations. 2019. Available online: https://www.fao.org/faostat/en/#home (accessed on 2 July 2021).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2021. Available online: https://www.ipcc.ch/report/sixth-assessment-report-working-group-i/ (accessed on 7 September 2021).
- BMU. 2050—Principles and Goals of the German Government’s Climate Policy; Federal Ministry for the Environment: Berlin, Germany, 2016. [Google Scholar]
- Sajeev, E.P.M.; Winiwarter, W.; Amon, B. Greenhouse Gas and Ammonia Emissions from Different Stages of Liquid Manure Management Chains: Abatement Options and Emission Interactions. J. Environ. Qual. 2018, 47, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Petersen, S.O.; Møller, H.B. Algorithms for calculating methane and nitrous oxide emissions from manure management. Nutr. Cycl. AgroecoSyst. 2004, 69, 143–154. [Google Scholar] [CrossRef]
- Ouatahar, L.; Bannink, A.; Lanigan, G.; Amon, B. Modelling the effect of feeding management on greenhouse gas and nitrogen emissions in cattle farming systems. Sci. Total Environ. 2021, 776, 145932. [Google Scholar] [CrossRef]
- Andersen, D.S.; van Weelden, M.B.; Trabue, S.L.; Pepple, L.M. Lab-assay for estimating methane emissions from deep-pit swine manure storages. J. Environ. Manag. 2015, 159, 18–26. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X. Chapter 26—Manure Treatment and Utilization in Production Systems. In Animal Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 455–467. [Google Scholar] [CrossRef]
- FAO. Greenhouse Gas Emissions from the Dairy Sector: A Life Cycle Assessment. 2010. Available online: https://www.uncclearn.org/resources/library/greenhouse-gas-emissions-from-the-dairy-sector-a-life-cycle-assessment/ (accessed on 11 November 2021).
- Petersen, S.O. Greenhouse gas emissions from liquid dairy manure: Prediction and mitigation. J. Dairy Sci. 2018, 101, 6642–6654. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, C.; Lawlor, P.G.; Jiang, Y.; Gardiner, G.E.; Xie, S.; Nghiem, L.D.; Zhan, X. Greenhouse gas emissions from different pig manure management techniques: A critical analysis. Front. Environ. Sci. Eng. 2017, 11, 11. [Google Scholar] [CrossRef]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. 2019. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/index.html (accessed on 22 April 2021).
- Dalby, F.R.; Hafner, S.D.; Petersen, S.O.; Vanderzaag, A.; Habtewold, J.; Dunfield, K.; Chantigny, M.H.; Sommer, S.G. A mechanistic model of methane emission from animal slurry with a focus on microbial groups. PLoS ONE 2021, 16, e0252881. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. Microbiology and biochemistry of anaerobic digesters: An overview. In Bioreactors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–26. [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZa, A. Ammonia and greenhouse gas emissions from slurry storage—A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Baral, K.R.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.H.; Olesen, J.E.; Petersen, S.O. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Cárdenas, A.; Ammon, C.; Schumacher, B.; Stinner, W.; Herrmann, C.; Schneider, M.; Weinrich, S.; Fischer, P.; Amon, T.; Amon, B. Methane emissions from the storage of liquid dairy manure: Influences of season, temperature and storage duration. Waste Manag. 2021, 121, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Moset, V.; Wahid, R.; Ward, A.; Møller, H.B. Modelling methane emission mitigation by anaerobic digestion: Effect of storage conditions and co-digestion. Environ. Technol. 2019, 40, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Mostafa, A.; Lim, K.-H.; Kim, I.; Kim, D.-H. Automatic temperature rise in the manure storage tank increases methane emissions: Worth to cool down! Sci. Total Environ. 2022, 823, 153533. [Google Scholar] [CrossRef]
- Sokolov, V.; VanderZaag, A.; Habtewold, J.; Dunfield, K.; Wagner-Riddle, C.; Venkiteswaran, J.; Gordon, R. Greenhouse gas emissions from gradually-filled liquid dairy manure storages with different levels of inoculant. Nutr. Cycl. Agroecosyst. 2019, 115, 455–467. [Google Scholar] [CrossRef]
- Sommer, S.G.; Petersen, S.O.; Sørensen, P.; Poulsen, H.D.; Møller, H.B. Methane and carbon dioxide emissions and nitrogen turnover during liquid manure storage. Nutr. Cycl. AgroecoSyst. 2007, 78, 27–36. [Google Scholar] [CrossRef]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Clemens, J.; Trimborn, M.; Weiland, P.; Amon, B. Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Philippe, F.X.; Nicks, B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric. Ecosyst. Environ. 2015, 199, 10–25. [Google Scholar] [CrossRef]
- VDI4630; Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Beuth Verlag: Berlin, Germany, 2016. Available online: https://www.beuth.de/de/technische-regel/vdi-4630/244849582 (accessed on 24 January 2022).
- Sommer, S.G.; Olesen, J.E.; Petersen, S.O.; Weisbjerg, M.R.; Valli, L.; Rodhe, L.; Beline, F. Region-specific assessment of greenhouse gas mitigation with different manure management strategies in four agroecological zones. Glob. Chang. Biol. 2009, 15, 2825–2837. [Google Scholar] [CrossRef]
- Dalby, F.R.; Hafner, S.D.; Petersen, S.O.; VanderZaag, A.C.; Habtewold, J.; Dunfield, K.; Chantigny, M.H.; Sommer, S.G. Understanding methane emission from stored animal manure: A review to guide model development. J. Environ. Qual. 2021, 50, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Elsgaard, L.; Olsen, A.B.; Petersen, S.O. Temperature response of methane production in liquid manures and co-digestates. Sci. Total Environ. 2016, 539, 78–84. [Google Scholar] [CrossRef]
- Massé, D.I.; Masse, L.; Claveau, S.; Benchaar, C.; Thomas, O. Methane emissions from manure storages. Trans. ASABE 2008, 51, 1775–1781. [Google Scholar] [CrossRef]
- Petersen, S.O.; Olsen, A.B.; Elsgaard, L.; Triolo, J.M.; Sommer, S.G. Estimation of methane emissions from slurry pits below pig and cattle confinements. PLoS ONE 2016, 11, e0160968. [Google Scholar] [CrossRef] [PubMed]
- Vanderzaag, A.C.; MacDonald, J.D.; Evans, L.; Vergé, X.P.C.; Desjardins, R.L. Towards an inventory of methane emissions from manure management that is responsive to changes on Canadian farms. Environ. Res. Lett. 2013, 8, 035008. [Google Scholar] [CrossRef]
- Im, S.; Petersen, S.O.; Lee, D.; Kim, D.H. Effects of storage temperature on CH4 emissions from cattle manure and subsequent biogas production potential. Waste Manag. 2020, 101, 35–43. [Google Scholar] [CrossRef]
- Feng, L.; Ward, A.J.; Moset, V.; Møller, H.B. Methane emission during on-site pre-storage of animal manure prior to anaerobic digestion at biogas plant: Effect of storage temperature and addition of food waste. J. Environ. Manag. 2018, 225, 272–279. [Google Scholar] [CrossRef]
- DIN38404; German Standard Methods for the Examination of Water, Waste Water and Sludge—Physical and Physico-Chemical Characteristics (Group C)—Part 5: Determination of pH Value (C 5). Beuth Verlag: Berlin, Germany, 2009. Available online: https://www.din.de/de (accessed on 24 January 2022).
- VDLUFA. Bestimmung von flüchtigen stickstoffhaltigen Basen B. Durch Destillation. In Die Chemische Untersuchung Von Futtermitteln, Methodenbuch Band III, Kap. 4.8.2, 3rd ed.; Supplementary Delivery 2006; VDLUFA-Verlag: Darmstadt, Germany, 2006. [Google Scholar]
- Weissbach, F.; Kuhla, S. Substance Losses in Determining the Dry Matter Content of Silage and Green Fodder: Arising Errors and Possibilities of Correction. Uebersichten zur Tierernaehrung. 1995. Available online: https://agris.fao.org/agris-search/search.do?recordID=DE97A8972 (accessed on 24 January 2022).
- VDLUFA. 6.5.1 Bestimmung der Neutral-Detergenzien-Faser nach Amylasebehandlung (aNDF) Sowie nach Amylasebehandlung und Veraschung (aNDFom)—Band III die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- VDLUFA. 6.5.2 Bestimmung der Säure-Detergenzien-Faser (ADF) und der Säure-Detergenzien-Faser nach Veraschung (ADFom)—Band III die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- VDLUFA. 6.5.3 Bestimmung des Säure-Detergenzien-Lignins (ADL)—Band III die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Helffrich, D.; Oechsner, H. The Hohenheim biogas yield test: Comparison of different laboratory techniques for the digestion of biomass. Agrartech. Forsch. 2003, 9, 27–30. Available online: https://www.cabdirect.org/cabdirect/abstract/20033112266 (accessed on 24 January 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2022; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 19 January 2022).
- Pererva, Y.; Miller, C.D.; Sims, R.C. Existing empirical kinetic models in biochemical methane potential (BMP) testing, their selection and numerical solution. Water 2020, 12, 1831. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Riet, K.V. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef]
- Lo, H.M.; Kurniawan, T.A.; Sillanpää, M.E.T.; Paia, T.Y.; Chiang, C.F.; Chao, K.P.; Liu, M.H.; Chuang, S.H.; Banks, C.J.; Wang, S.C.; et al. Modeling biogas production from organic fraction of MSW co-digested with MSWI ashes in anaerobic bioreactors. Bioresour. Technol. 2010, 101, 6329–6335. [Google Scholar] [CrossRef]
- Morais, N.W.S.; Coelho, M.M.H.; de Oliveira, M.G.; Mourão, J.M.M.; Pereira, E.L.; dos Santos, A.B. Kinetic Study of Methanization Process Through Mathematical Modeling in Biochemical Methane Potential Assays from Four Different Inoculants. Water. Air. Soil Pollut. 2021, 232, 423. [Google Scholar] [CrossRef]
- Dalgaard, P.; Koutsoumanis, K. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J. Microbiol. Methods 2001, 43, 183–196. [Google Scholar] [CrossRef]
- Petersen, S.O.; Andersen, A.J.; Eriksen, J. Effects of Cattle Slurry Acidification on Ammonia and Methane Evolution during Storage. J. Environ. Qual. 2012, 41, 88–94. [Google Scholar] [CrossRef]
- Tavares, J.M.R.; Filho, P.B.; Coldebella, A.; Oliveira, P.A.V. The water disappearance and manure production at commercial growing-finishing pig farms. Livest. Sci. 2014, 169, 146–154. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Qiao, Y.; Li, S.; Chen, Y.; Hu, C. Mitigation of carbon and nitrogen losses during pig manure composting: A meta-analysis. Sci. Total Environ. 2021, 783, 147103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Drosg, B.; Braun, R.; Bochmann, G.; Al Saedi, T. Analysis and Characterisation of Biogas Feedstocks; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar] [CrossRef]
- ASAE. Manure Production and Characteristics; ASAE: Washington, DC, USA, 2000. [Google Scholar]
- Sommer, S.G.; Clough, T.J.; Balaine, N.; Hafner, S.D.; Cameron, K.C. Transformation of Organic Matter and the Emissions of Methane and Ammonia during Storage of Liquid Manure as Affected by Acidification. J. Environ. Qual. 2017, 46, 514–521. [Google Scholar] [CrossRef]
- Adghim, M.; Abdallah, M.; Saad, S.; Shanableh, A.; Sartaj, M. Assessment of the biochemical methane potential of mono- and co-digested dairy farm wastes. Waste Manag. Res. 2020, 38, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Massé, D.I.; Croteau, F.; Patni, N.K.; Masse, L. Methane emissions from dairy cow and swine manure slurries stored at 10 °C and 15 °C. Can. Biosyst. Eng./Genie Biosyst. Canada 2003, 45, 1–6. [Google Scholar]
- Zhang, W.; Zhang, F.; Li, Y.X.; Zeng, R.J. Free acetic acid as the key factor for the inhibition of hydrogenotrophic methanogenesis in mesophilic mixed culture fermentation. Bioresour. Technol. 2018, 264, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, F.; Li, Y.X.; Zeng, R.J. Inhibitory effects of free propionic and butyric acids on the activities of hydrogenotrophic methanogens in mesophilic mixed culture fermentation. Bioresour. Technol. 2019, 272, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lendormi, T.; Jaziri, K.; Béline, F.; Le Roux, S.; Bureau, C.; Midoux, C.; Barrington, S.; Dabert, P. Methane production and microbial community acclimation of five manure inocula during psychrophilic anaerobic digestion of swine manure. J. Clean. Prod. 2022, 340, 130772. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R.; Arteaga, V.; Rumsey, T.; Mitloehner, F.M. Volatile fatty acids and alcohols production during anaerobic storage of dairy manure. Trans. ASABE 2011, 54, 599–607. [Google Scholar] [CrossRef]
- Habtewold, J.; Gordon, R.; Sokolov, V.; VanderZaag, A.; Wagner-Riddle, C.; Dunfield, K. Reduction in methane emissions from acidified dairy slurry is related to inhibition of methanosarcina species. Front. Microbiol. 2018, 9, 2806. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, J.; Gordon, R.; Sokolov, V.; VanderZaag, A.; Wagner-Riddle, C.; Dunfield, K. Targeting bacteria and methanogens to understand the role of residual slurry as an inoculant in stored liquid dairy manure. Appl. Environ. Microbiol. 2018, 84, e02830-17. [Google Scholar] [CrossRef] [PubMed]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure-Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Sommer, S.G.; Husted, S. The chemical buffer system in raw and digested animal slurry. J. Agric. Sci. 1995, 124, 45–53. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between biogas yield and chemical composition of energy crops. Bioresour. Technol. 2014, 174, 316–320. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Popovic, O.; Jensen, L.S. Storage temperature affects distribution of carbon, VFA, ammonia, phosphorus, copper and zinc in raw pig slurry and its separated liquid fraction. Water Res. 2012, 46, 3849–3858. [Google Scholar] [CrossRef]
- Sommer, S.G.; Christensen, M.L.; Schmidt, T.; Jensen, L.S. Animal Manure Recycling: Treatment and Management; John and Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Esteves, E.M.M.; Herrera, A.M.N.; Esteves, V.P.P.; Morgado, C.d.R.V. Life cycle assessment of manure biogas production: A review. J. Clean. Prod. 2019, 219, 411–423. [Google Scholar] [CrossRef]




| Animal Category | Dairy Cow Manure | Fattening Pig Manure |
|---|---|---|
| Temperature in loco (°C) | 16.0 | 18.9 |
| EC (mS/cm) | 9.88 | 24.1 |
| DM (in %FM) | 11.74 | 1.68 |
| VS (in %DM) | 86.07 | 58.13 |
| VS (in %FM) | 10.10 | 0.98 |
| pH | 6.61 | 7.79 |
| TAN (in mg/kgFM) | 866.9 | 2578.1 |
| Methanol (in g/kg) | 0.06 | 0.00 |
| Ethanol (in g/kg) | 0.09 | 0.00 |
| Acetic acid (in g/kg) | 4.06 | 2.4 |
| Propionic acid (in g/kg) | 0.89 | 0.34 |
| i-Butyric acid (in g/kg) | 0.05 | 0.04 |
| Butyric acid (in g/kg) | 0.32 | 0.00 |
| i-Valeric acid (in g/kg) | 0.05 | 0.06 |
| Valeric acid (in g/kg) | 0.06 | 0.00 |
| Sum of VFA as acetic acid (in g/kg) | 5.10 | 2.74 |
| COD in mg/kgFM | 111,729.2 | 8400.8 |
| Crude fat (in %DM) | 1.52 | 1.34 |
| NDF (in %DM) | 54.29 | 3.21 |
| ADF (in %DM) | 32.81 | 1.40 |
| ADL (in %DM) | 9.71 | 0.64 |
| N (in %DM) | 2.46 | 2.68 |
| C (in %DM) | 44.2 | 30.54 |
| S (in %DM) | 0.24 | 1.35 |
| H (in %DM) | 3.87 | 2.56 |
| Crude protein (in %DM) | 15.38 | 16.75 |
| Dairy Manure | |||||
| Storage temperature (°C) | 5 | 10 | 15 | 20 | 25 |
| DM (in %FM) | 12.04 ± 0.37 | 12.44 ± 0.36 | 11.74 ± 0.20 | 11.34 ± 0.22 | 11.46 ± 0.37 |
| VS (in %DM) | 85.44 ± 0.26 | 86.10 ± 0.62 | 84.98 ± 0.07 | 84.58 ± 0.56 | 84.72 ± 0.89 |
| VS (in %FM) | 10.29 ± 0.35 | 10.71 ± 0.39 | 9.98 ± 0.16 | 9.59 ± 0.21 | 9.71 ± 0.39 |
| pH-average | 6.52 ± 0.16 a | 6.26 ± 0.08 ab | 5.97 ± 0.08 bc | 5.90 ± 0.11 bc | 5.65 ± 0.20 c |
| Alcohols (in g/kg) | 0.24 ± 0.06 | 0 | 0 | 0 | 0 |
| Acetic acid (in g/kg) | 5.95 ± 0.66 b | 7.95 ± 0.20 ab | 8.63 ± 1.05 ab | 9.81 ± 1.38 ab | 10.31 ± 1.57 a |
| Propionic acid (in g/kg) | 2.31 ± 0.06 b | 2.46 ± 0.12 b | 2.78 ± 0.44 ab | 3.07 ± 0.34 ab | 3.28 ± 0.43 a |
| Butyric acid (in g/kg) | 1.93 ± 0.12 | 2.59 ± 0.30 | 3.07 ± 0.62 | 2.43 ± 0.31 | 2.98 ± 0.72 |
| Valeric acid (in g/kg) | 0.20 ± 0.03 c | 0.46 ± 0.18 bc | 0.62 ± 0.27 abc | 0.85 ± 0.06 ab | 1.01 ± 0.22 a |
| VFA-Sum as acetic acid (in gAAeq/kg) | 9.27 ± 0.58 c | 12.13 ± 0.62 b | 13.65 ± 2.15 abc | 14.76 ± 1.87 abc | 16.23 ± 2.45 a |
| Fattening Pig Manure | |||||
| Storage temperature (°C) | 5 | 10 | 15 | 20 | 25 |
| DM (in %FM) | 1.50 ± 0.01 b | 1.48 ± 0 b | 1.49 ± 0.09 abc | 1.24 ± 0.01 c | 1.11 ± 0.03 d |
| VS (in %DM) | 52.81 ± 0.29 b | 52.66 ± 0.10 b | 51,67 ± 1.40 ab | 42.70 ± 0.92 c | 35.32 ± 1.01 d |
| VS (in %FM) | 0.79 ± 0.01 b | 0.78 ± 0 b | 0.77 ± 0.07 abc | 0.53 ± 0.02 c | 0.39 ± 0.02 d |
| pH-average | 7.69 ± 0.05 c | 7.84 ± 0.10 bc | 7.84 ± 0.08 bc | 8.15 ± 0.03 b | 8.29 ± 0.04 a |
| Acetic acid (in g/kg) | 3.04 ± 0.03 c | 3.40 ± 0.05 b | 3.65 ± 0.02 a | 1.5 ± 0.05 d | 0.33 ± 0 e |
| Propionic acid (in g/kg) | 0.47 ± 0 | 0.48 ± 0 | 0.34 ± 0 | 0.03 ± 0 | 0.0 |
| Butyric acid (in g/kg) | 0.07 ± 0 | 0.05 ± 0 | 0.03 ± 0 | 0.0 | 0.0 |
| Valeric acid (in g/kg) | 0.11 ± 0 | 0.09 ± 0 | 0.06 ± 0 | 0.0 | 0.0 |
| VFA-Sum as acetic acid (in gAAeq/kg) | 3.53 ± 0.02 b | 3.87 ± 0.06 a | 3.98 ± 0.02 a | 1.53 ± 0.05 c | 0.33 ± 0 d |
| Storage Temperature (°C) | Maximum Cumulative Production (LNCH4 kg−1VS) | Maximum Specific Production Rate (LNCH4 kg−1VS d−1) | Half-Life (d) |
|---|---|---|---|
| Dairy Manure | |||
| 5 | 0.210 ± 0.009 c | 0.071 ± 0.004 b | 43.85 ± 4.29 ab |
| 10 | 1.552 ± 0.238 b | 0.048 ± 0.001 c | 42.35 ± 1.98 a |
| 15 | 3.741 ± 0.305 a | 0.071 ± 0.002 ab | 28.47 ± 1.46 b |
| 20 | 4.620 ± 0.562 a | 0.096 ± 0.006 a | 16.65 ± 0.78 c |
| 25 | 4.273 ± 0.270 a | 0.088 ± 0.001 abc | 14.64 ± 2.55 c |
| Fattening Pig Manure | |||
| 15 | 36.145 ± 4.926 b | 0.044 ± 0.004 b | 61.2 ± 4.2 b |
| 20 | 196.530 ± 21.734 a | 0.044 ± 0.003 b | 79.6 ± 5.2 a |
| 25 | 175.933 ± 15.088 a | 0.072 ± 0.002 a | 47.2 ± 1.4 c |
| Temperature Storage (°C) | First-Order Differential Equation | Modified Gompertz Equation | ||
|---|---|---|---|---|
| First-Order Decay (d−1) | Maximum Specific Methane Yield (LN kg−1VS) | Maximum Specific Methane Production Rate (LN kg−1VS d−1) | Lag Phase (d) | |
| Dairy Manure | ||||
| Fresh | 0.10 ± 0.001 a | 216.97 ± 22.096 ab | 17.26 ± 1.222 | 0.952 ± 0.174 b |
| 5 | 0.04 ± 0.006 c | 259.92 ± 35.572 ab | 12.98 ± 1.757 | 2.969 ± 0.459 a |
| 10 | 0.07 ± 0.001 b | 223.00 ± 4.964 a | 14.35 ± 0.355 | 0.841 ± 0.055 ab |
| 15 | 0.07 ± 0.002 b | 253.51 ± 6.665 b | 14.93 ± 0.013 | 0.887 ± 0.107 b |
| 20 | 0.06 ± 0.016 abc | 241.58 ± 8.920 ab | 15.33 ± 1.014 | 2.783 ± 2.908 ab |
| 25 | 0.08 ± 0.002 b | 253.44 ± 2.504 b | 15.60 ± 0.287 | 0.991 ± 0.324 b |
| Fattening Pig Manure | ||||
| Fresh | 0.020 ± 0.002 c | 238.76 ± 8.88 a | 13.42 ± 0.34 b | 7.85 ± 0.27 a |
| 5 | 0.088 ± 0.009 ab | 261.72 ± 22.96 a | 20.91 ± 3.05 abc | 1.84 ± 0.07 c |
| 10 | 0.096 ± 0.004 a | 272.57 ± 23.12 a | 22.63 ± 0.65 a | 1.54 ± 0.16 c |
| 15 | 0.098 ± 0.004 a | 233.02 ± 13.80 a | 23.49 ± 1.25 a | 2.36 ± 0.11 b |
| 20 | 0.076 ± 0.002 b | 149.13 ± 9.03 b | 6.91 ± 0.35 c | - |
| 25 | 0.026 ± 0.006 c | 90.29 ± 19.99 b | 2.70 ± 0.43 d | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilgert, J.E.; Amon, B.; Amon, T.; Belik, V.; Dragoni, F.; Ammon, C.; Cárdenas, A.; Petersen, S.O.; Herrmann, C. Methane Emissions from Livestock Slurry: Effects of Storage Temperature and Changes in Chemical Composition. Sustainability 2022, 14, 9934. https://doi.org/10.3390/su14169934
Hilgert JE, Amon B, Amon T, Belik V, Dragoni F, Ammon C, Cárdenas A, Petersen SO, Herrmann C. Methane Emissions from Livestock Slurry: Effects of Storage Temperature and Changes in Chemical Composition. Sustainability. 2022; 14(16):9934. https://doi.org/10.3390/su14169934
Chicago/Turabian StyleHilgert, Julio E., Barbara Amon, Thomas Amon, Vitaly Belik, Federico Dragoni, Christian Ammon, Aura Cárdenas, Søren O. Petersen, and Christiane Herrmann. 2022. "Methane Emissions from Livestock Slurry: Effects of Storage Temperature and Changes in Chemical Composition" Sustainability 14, no. 16: 9934. https://doi.org/10.3390/su14169934








