Novel Electrochemical Preparation of N-Doped TiO2/Graphene for Enhanced Stability and Photocatalysis Degradation of Humic Acid
Abstract
:1. Introduction
2. Materials & Methods
2.1. Materials
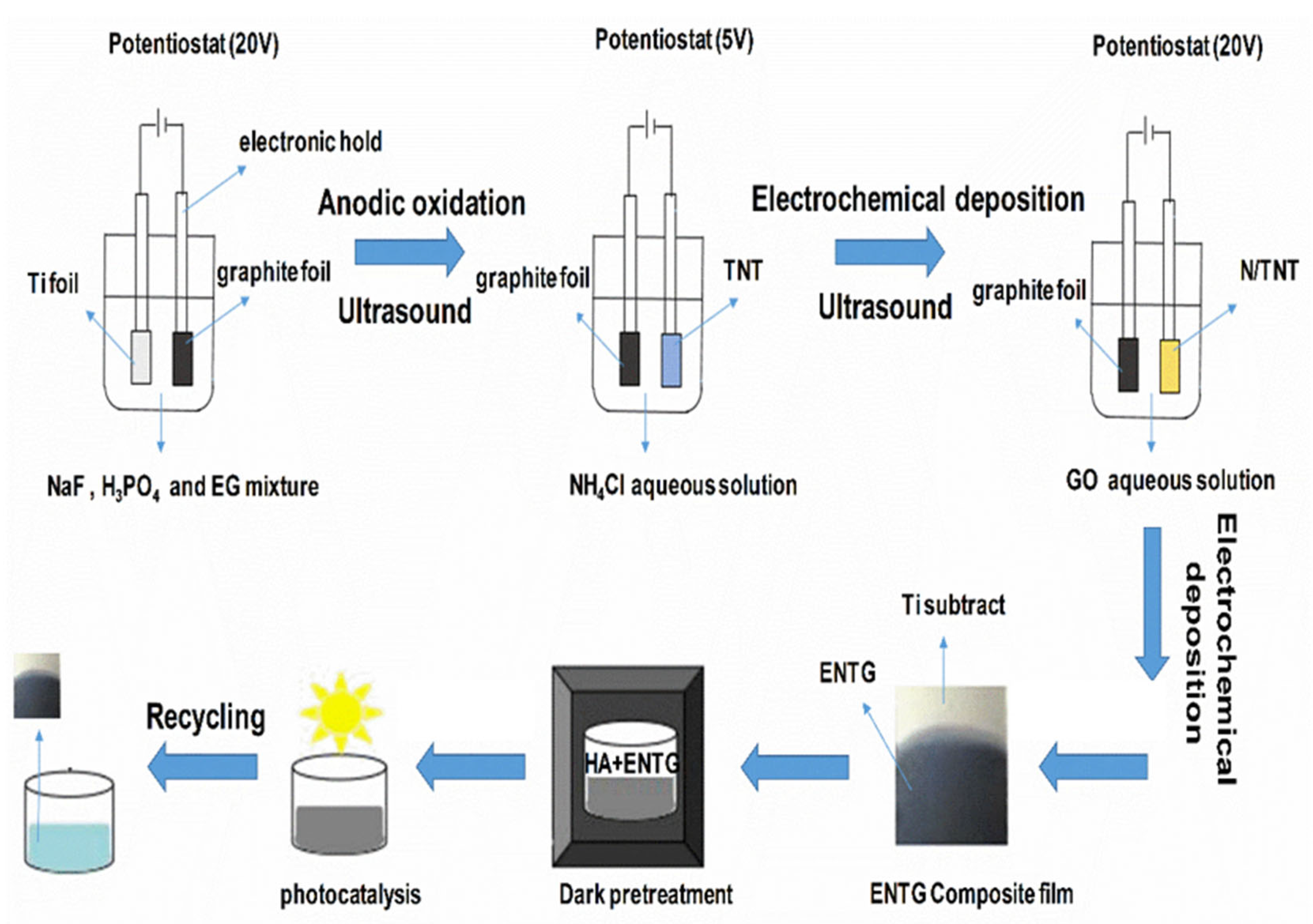
2.2. Preparation of TNT
2.3. Preparation of N/TNT
2.4. Preparation of ENTG
2.5. Preparation of NTG
2.6. Characterization of the Photocatalysts
2.7. Photodegradation Experiments
3. Results and Discussion
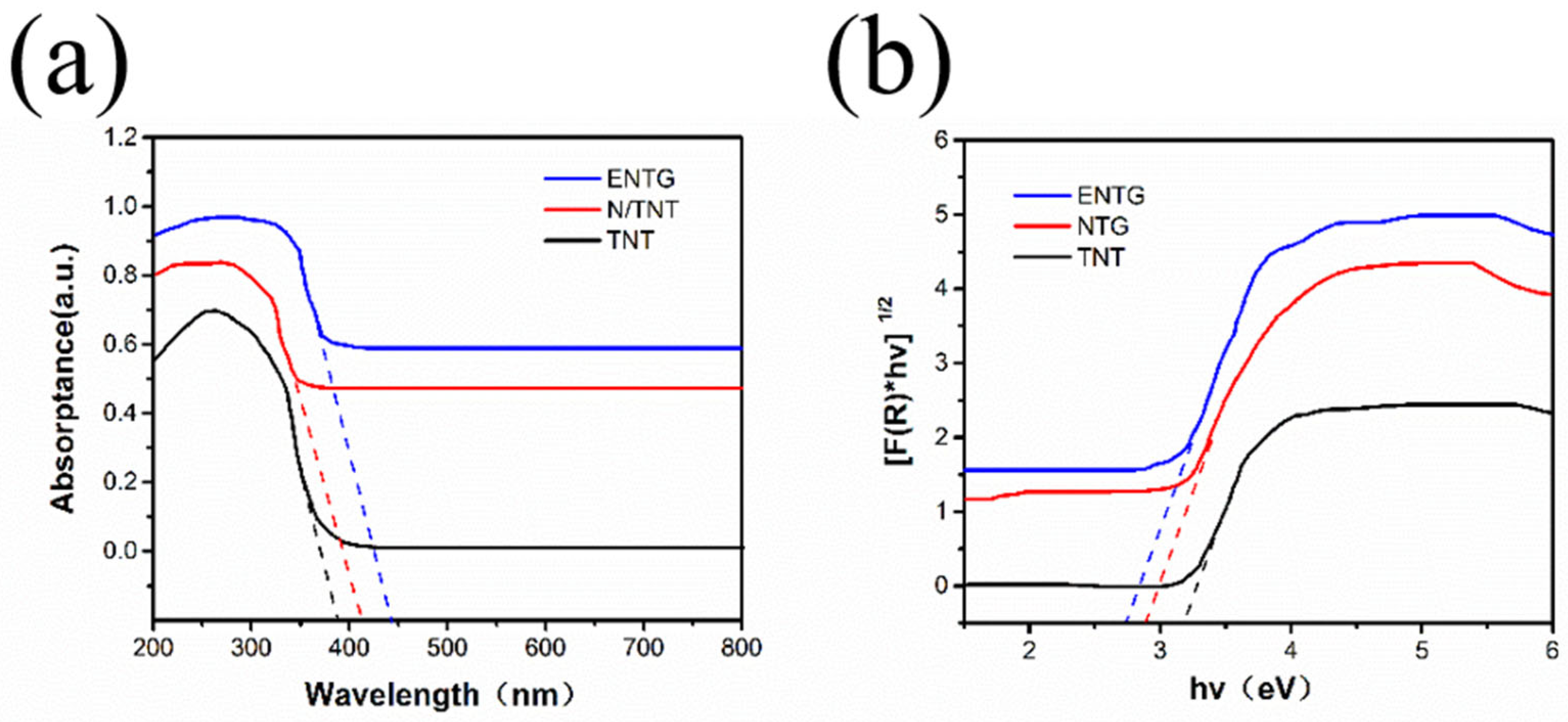
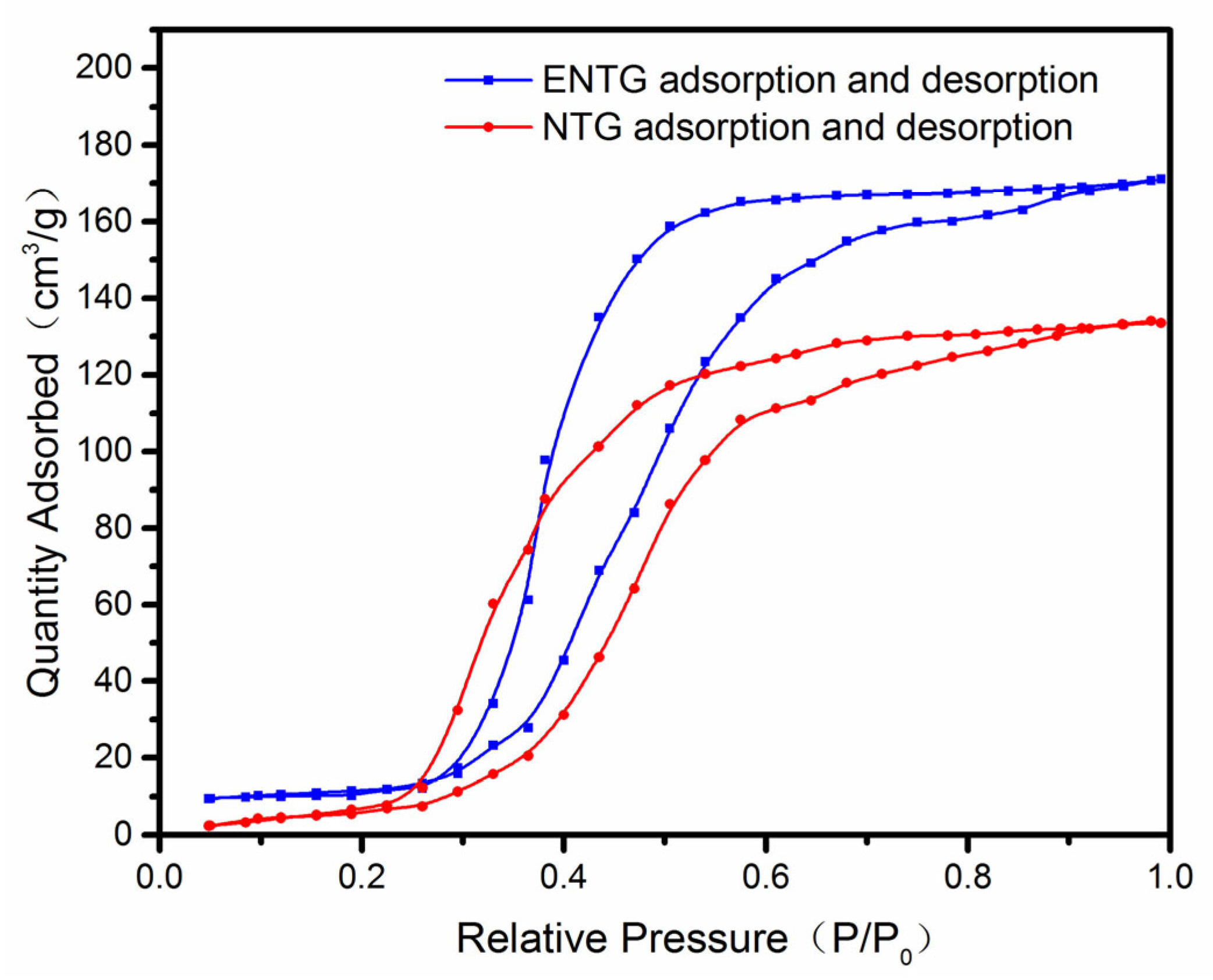
3.1. The Characterization of NTG, TNT, N-TNT and ENTG
3.2. Degradation Performance of NTG, TNT, N-TNT and ENTG
3.3. Influential Factors on ENTG Performance
3.4. Formation Mechanism for ENTG
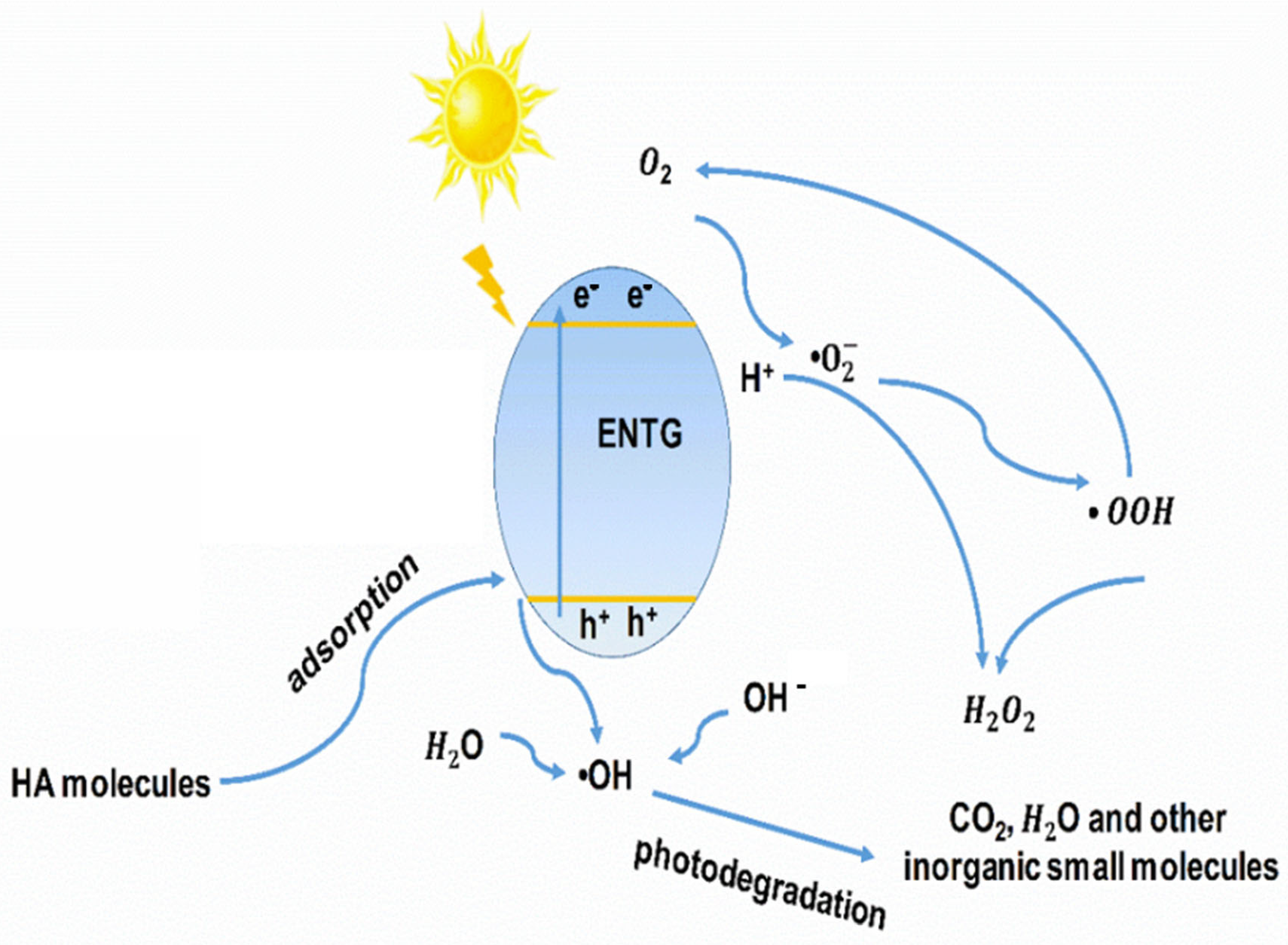
3.5. Degradation Mechanism for ENTG
(i.e., CO2, H2O)
3.6. Recyclability and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ly, Q.V.; He, K.; Maqbool, T.; Sun, M.; Zhang, Z. Exploring the potential application of hybrid permonosulfate/reactive electrochemical ceramic membrane on treating humic acid-dominant wastewater. Sep. Purif. Technol. 2022, 286, 120513. [Google Scholar] [CrossRef]
- Mohsin, M.; Bhatti, I.A.; Ashar, A.; Khan, M.W.; Farooq, M.U.; Khan, H.; Hussain, M.T.; Loomba, S.; Mohiuddin, M.; Zavabeti, A.; et al. Iron-doped zinc oxide for photocatalyzed degradation of humic acid from municipal wastewater. Appl. Mater. Today 2021, 23, 101047. [Google Scholar] [CrossRef]
- Asgharian, F.; Khosravi-Nikou, M.R.; Anvaripour, B.; Danaee, I. Electrocoagulation and ultrasonic removal of humic acid from wastewater. Environ. Prog. Sustain. Energy 2017, 36, 822–829. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Lin, T.; Ding, M.; Wang, Y.; Tao, H.; Hu, K. Preparation of Fe3O4/TiO2-N-GO sonocatalyst and using for humic acid removal with the assist of ultrasound. Mater. Sci. Semicond. Process. 2019, 102, 104593. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Li, C.; Hu, X.; Yang, S.; You, X.; Liang, W. Persulfate activation using Co/AC particle electrodes and synergistic effects on humic acid degradation. Appl. Catal. B 2021, 285, 119848. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Algamdi, M.S.; Alsohaimi, I.H.; Lawler, J.; Ali, H.M.; Aldawsari, A.M.; Hassan, H.M.A. Fabrication of graphene oxide incorporated polyethersulfone hybrid ultrafiltration membranes for humic acid removal. Sep. Purif. Technol. 2019, 223, 17–23. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.B.T.; Ha, T.T.L.; Nguyen, T.D.; Le, H.N.; Ha-Thuc, C.N.; Nguyen, T.M.L.; Perre, P.; Nguyen, D.M. Effectiveness of photocatalysis of MMT-supported TiO2 and TiO2 nanotubes for rhodamine B degradation. Chemosphere 2021, 280, 130802. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Brame, J.; Qin, F.; Bao, J.; Li, Q.; Alvarez, P.J. Phosphate Changes Effect of Humic Acids on TiO2 Photocatalysis: From Inhibition to Mitigation of Electron-Hole Recombination. Environ. Sci. Technol. 2017, 51, 514–521. [Google Scholar] [CrossRef]
- Zhong, R.Y.; Zhang, Z.S.; Luo, S.Q.; Zhang, Z.C.; Huang, L.M.; Gu, M. Comparison of TiO2 and g-C3N4 2D/2D nanocomposites from three synthesis protocols for visible-light induced hydrogen evolution. Catal. Sci. Technol. 2019, 9, 75–85. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Hussain, H.; Tocci, G.; Woolcot, T.; Torrelles, X.; Pang, C.L.; Humphrey, D.S.; Yim, C.M.; Grinter, D.C.; Cabailh, G.; Bikondoa, O.; et al. Structure of a model TiO2 photocatalytic interface. Nat. Mater. 2017, 16, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.; Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.P.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef]
- Chowdhury, P.; Athapaththu, S.; Elkamel, A.; Ray, A.K. Visible-solar-light-driven photo-reduction and removal of cadmium ion with Eosin Y-sensitized TiO2 in aqueous solution of triethanolamine. Sep. Purif. Technol. 2017, 174, 109–115. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Water splitting. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Microwave-assisted acid functionalized carbon nanofibers decorated with Mn doped TNTs nanocomposites: Efficient contenders for lithium adsorption and recovery from aqueous media. J. Ind. Eng. Chem. 2020, 92, 263–277. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Hybrid biochar supported transition metal doped MnO2 composites: Efficient contenders for lithium adsorption and recovery from aqueous solutions. Desalination 2022, 522, 115387. [Google Scholar] [CrossRef]
- Yoshida, T.; Niimi, S.; Yamamoto, M.; Nomoto, T.; Yagi, S. Effective nitrogen doping into TiO2 (N-TiO2) for visible light response photocatalysis. J. Colloid. Interface Sci. 2015, 447, 278–281. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.; Martins, R. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 5934, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nanosci. Technol. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Xia, D.-C.; Zhou, L.; Qiao, S.; Zhang, Y.; Tang, D.; Liu, J.; Huang, H.; Liu, Y.; Kang, Z. Graphene/Ni–Fe layered double-hydroxide composite as highly active electrocatalyst for water oxidation. Mater. Res. Bull. 2016, 74, 441–446. [Google Scholar] [CrossRef]
- De Almeida, G.C.; Della Santina Mohallem, N.; Viana, M.M. Ag/GO/TiO2 nanocomposites: The role of the interfacial charge transfer for application in photocatalysis. Nanotechnology 2021, 33, 035710. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Rhee, K.Y.; Lee, S.Y.; Park, S.J. Innovative progress in graphene derivative-based composite hybrid membranes for the removal of contaminants in wastewater: A review. Chemosphere 2022, 306, 135590. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Hou, J.; Lei, Y.; Ma, X.; Lu, G. Facile one-step hydrothermal synthesis toward strongly coupled TiO2/graphene quantum dots photocatalysts for efficient hydrogen evolution. Appl. Surf. Sci. 2017, 396, 1375–1382. [Google Scholar] [CrossRef]
- Kumar, M.; Gholamvand, Z.; Morrissey, A.; Nolan, K.; Ulbricht, M.; Lawler, J. Preparation and characterization of low fouling novel hybrid ultrafiltration membranes based on the blends of GO−TiO2 nanocomposite and polysulfone for humic acid removal. J. Membr. Sci. 2016, 506, 38–49. [Google Scholar] [CrossRef]
- Ye, T.; Chen, W.; Xu, H.; Geng, N.; Cai, Y. Preparation of TiO2/graphene composite with appropriate N-doping ratio for humic acid removal. J. Mater. Sci. 2017, 53, 613–625. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, H.; Sun, H.; Zeng, R.-C. Hydrothermal synthesis and photoelectrochemical performance enhancement of TiO2/graphene composite in photo-generated cathodic protection. Appl. Surf. Sci. 2016, 382, 128–134. [Google Scholar] [CrossRef]
- Anjusree, G.S.; Nair, A.S.; Nair, S.V.; Vadukumpully, S. One-pot hydrothermal synthesis of TiO2/graphene nanocomposites for enhanced visible light photocatalysis and photovoltaics. RSC Adv. 2013, 3, 12933–12938. [Google Scholar] [CrossRef]
- Liu, C.; Teng, Y.; Liu, R.; Luo, S.; Tang, Y.; Chen, L.; Cai, Q. Fabrication of graphene films on TiO2 nanotube arrays for photocatalytic application. Carbon 2011, 49, 5312–5320. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Xu, H.; Chen, T.; Geng, N.; Gao, X. An ultrafiltration membrane with enhanced photocatalytic performance from grafted N–TiO2/graphene oxide. RSC Adv. 2017, 7, 9880–9887. [Google Scholar] [CrossRef]
- Mak, C.H.; Han, X.; Du, M.; Kai, J.-J.; Tsang, K.F.; Jia, G.; Cheng, K.-C.; Shen, H.-H.; Hsu, H.-Y. Heterogenization of homogeneous photocatalysts utilizing synthetic and natural support materials. J. Mater. Chem. A 2021, 9, 4454–4504. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Liu, S.; Li, Y.; Shen, Z.; Wang, K. Preparation and characterization of novel polysulphone hybrid ultrafiltration membranes blended with N-doped GO/ TiO2 nanocomposites. Polymer 2017, 117, 198–207. [Google Scholar] [CrossRef]
- Elouali, S.; Mills, A.; Parkin, I.P.; Bailey, E.; McMillan, P.F.; Darr, J.A. Photocatalytic evolution of hydrogen and oxygen from ceramic wafers of commercial titanias. J. Photochem. Photobiol. A 2010, 216, 110–114. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Fernandez, A.; Lassaletta, G.; Jimenez, V.M.; Justo, A.; González-Elipe, A.R.; Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Pruna, A.; Wu, Z.; Zapien, J.A.; Li, Y.Y.; Ruotolo, A. Enhanced photocatalytic performance of ZnO nanostructures by electrochemical hybridization with graphene oxide. Appl. Surf. Sci. 2018, 441, 936–944. [Google Scholar]
- Samran, B.; Krongkitsiri, P.; Pimmongkol, S.; Budngam, S.; Tipparach, U. Preparation and Microstructure of Titania (TiO2) Nanotube Arrays by Anodization Method. Adv. Mater. Res. 2013, 802, 104–108. [Google Scholar] [CrossRef]
- Huo, K.; Gao, B.; Fu, J.; Zhao, L.; Chu, P.K. Fabrication, modification, and biomedical applications of anodized TiO2 nanotube arrays. RSC Adv. 2014, 4, 17300–17324. [Google Scholar]
- Lin, C.; Chen, S.; Cao, L. Anodic formation of aligned and bamboo-type TiO2 nanotubes at constant low voltages. Mater. Sci. Semicond. Process. 2013, 16, 154–159. [Google Scholar] [CrossRef]
- Xian, J.; Li, D.; Chen, J.; Li, X.; He, M.; Shao, Y.; Yu, L.; Fang, J. A large-area smooth graphene film on a TiO2 nanotube array via a one-step electrochemical process. J. Mater. Chem. A 2014, 2, 5187–5192. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Jiang, H.; Jiang, W.; Su, R.; Luo, S.; Luo, Y. Photoelectrocatalytic oxidation of bisphenol A over mesh of TiO2 /graphene/Cu2O. Appl. Catal. B 2016, 183, 75–85. [Google Scholar] [CrossRef]
- Sharma, M.; Behl, K.; Nigam, S.; Joshi, M. TiO2-GO nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum 2018, 156, 434–439. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M. Preparation and characterization of Pt, N- TiO2-graphene nanocomposites for hydrogen production. Ceram. Int. 2019, 45, 6058–6065. [Google Scholar] [CrossRef]
- Stefanov, P.; Shipochka, M.; Stefchev, P.; Raicheva, Z.; Lazarova, V.; Spassov, L. XPS characterization of TiO2 layers deposited on quartz plates. J. Phys. Conf. Ser. 2008, 100, 012039. [Google Scholar]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2 Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Shen, J.; Zou, W.; Chen, J.; Li, C. Enriched graphitic N-doped carbon-supported Fe3O4 nanoparticles as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7281–7287. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. Review on modified N–TiO2 for green energy applications under UV/visible light: Selected results and reaction mechanisms. RSC Adv. 2014, 4, 28265–28299. [Google Scholar]
- Liu, C.; Zhang, L.; Liu, R.; Gao, Z.; Yang, X.; Tu, Z.; Yang, F.; Ye, Z.; Cui, L.; Xu, C.; et al. Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J. Alloy. Compd. 2016, 656, 24–32. [Google Scholar] [CrossRef]
- Qu, X.; Cao, L.; Du, F. Fabrication of ordered arrays of CNT/ TiO2 nanotubes and their photocatalytic properties. RSC Adv. 2015, 5, 20976–20980. [Google Scholar] [CrossRef]
- Fujihara, K.; Izumi, S.; Ohno, T.; Matsumura, M. Time-resolved photoluminescence of particulate TiO2 photocatalysts suspended in aqueous solutions. J. Photochem. Photobiol. A 2000, 132, 99–104. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Xu, S.; Cao, S. Evident improvement of nitrogen-doped graphene on visible light photocatalytic activity of N-TiO2/N-graphene nanocomposites. Mater. Res. Bull. 2015, 65, 27–35. [Google Scholar] [CrossRef]
- Su, Z.; Zhou, W. Formation Mechanism of Porous Anodic Aluminium and Titanium Oxides. Adv. Mater. 2008, 20, 3663–3667. [Google Scholar] [CrossRef]
- Hazra, A.; Bhowmik, B.; Dutta, K. Formation Mechanism of Anodically Grown Free-Standing TiO2 Nanotube Array Under the Influence of Mixed Electrolytes. Sci. Adv. Mater. 2014, 6, 714–719. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Jiang, Y.; Chen, K.; Yan, X.; Zhang, D.; Li, R.; Tang, H. Fabrication of P25/Ag3PO4/graphene oxide heterostructures for enhanced solar photocatalytic degradation of organic pollutants and bacteria. Appl. Catal. B 2015, 166, 231–240. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Ding, M.; Liu, Z.; Shen, Z. A sono-photocatalyst for humic acid removal from water: Operational parameters, kinetics and mechanism. Ultrason. Sonochem. 2019, 57, 242–252. [Google Scholar] [PubMed]
- Wang, T.; Qu, G.; Ren, J.; Yan, Q.; Sun, Q.; Liang, D.; Hu, S. Evaluation of the potentials of humic acid removal in water by gas phase surface discharge plasma. Water Res. 2016, 89, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, Z.; Yang, J.; Cizmas, L.; Lichtfouse, E.; Sharma, V.K. Efficient microwave degradation of humic acids in water using persulfate and activated carbon. Environ. Chem. Lett. 2018, 16, 1069–1075. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Hua, D.; Shi, C. Enhanced photocatalytic degradation of humic acids using Al and Fe co-doped TiO2 nanotubes under UV/ozonation for drinking water purification. J. Hazard. Mater. 2013, 262, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkesh, M.; Spanjers, H.; Toran, M.J.; Blanquez, P.; van Lier, J.B. Bioremoval of humic acid from water by white rot fungi: Exploring the removal mechanisms. AMB Express 2016, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhai, Z.; Zhu, E.; Liu, H.; Jiao, T.; Tang, S. Humic Acid Removal in Water via UV Activated Sodium Perborate Process. Coatings 2022, 12, 885. [Google Scholar] [CrossRef]
- Sarangapani, C.; Lu, P.; Behan, P.; Bourke, P.; Cullen, P.J. Humic acid and trihalomethane breakdown with potential by-product formations for atmospheric air plasma water treatment. J. Ind. Eng. Chem. 2018, 59, 350–361. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, G.; Wang, T.; Li, C.; Qiang, H.; Sun, Q.; Liang, D.; Hu, S. Humic acid removal from micro-polluted source water in the presence of inorganic salts in a gas-phase surface discharge plasma system. Sep. Purif. Technol. 2017, 187, 334–342. [Google Scholar]
- Wang, G.S.; Liao, C.H.; Chen, H.W.; Yang, H.C. Characteristics of natural organic matter degradation in water by UV/H2O2 treatment. Environ. Technol. 2006, 27, 277–287. [Google Scholar] [CrossRef]






| Photocatalyst | R2 | |
|---|---|---|
| TNT | 0.00477 | 0.99889 |
| N/TNT | 0.01734 | 0.99557 |
| NTG | 0.01614 | 0.99346 |
| ENTG | 0.01968 | 0.99824 |
| No. of Cycles | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery rate (%) | 98.8 | 94.6 | 92.3 | 85.2 | 78.6 | 72.6 | 67.0 | 61.8 | 57.1 | 52.7 |
| No. of Cycles | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery rate (%) | 99.2 | 98.6 | 97.5 | 96.0 | 95.2 | 94.6 | 93.1 | 92.8 | 91.1 | 90.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Chen, W.; Geng, N.; Lan, X.; Liu, M.; Wu, X. Novel Electrochemical Preparation of N-Doped TiO2/Graphene for Enhanced Stability and Photocatalysis Degradation of Humic Acid. Sustainability 2022, 14, 10614. https://doi.org/10.3390/su141710614
Wang A, Chen W, Geng N, Lan X, Liu M, Wu X. Novel Electrochemical Preparation of N-Doped TiO2/Graphene for Enhanced Stability and Photocatalysis Degradation of Humic Acid. Sustainability. 2022; 14(17):10614. https://doi.org/10.3390/su141710614
Chicago/Turabian StyleWang, Anqi, Wei Chen, Nannan Geng, Xiaomeng Lan, Mingxiang Liu, and Xing Wu. 2022. "Novel Electrochemical Preparation of N-Doped TiO2/Graphene for Enhanced Stability and Photocatalysis Degradation of Humic Acid" Sustainability 14, no. 17: 10614. https://doi.org/10.3390/su141710614





