Variations of Soil Chemical Properties and Microbial Community around the Acid Reservoir in the Mining Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Soil Properties
2.3. DNA Extraction and Sequencing
3. Results and Discussion
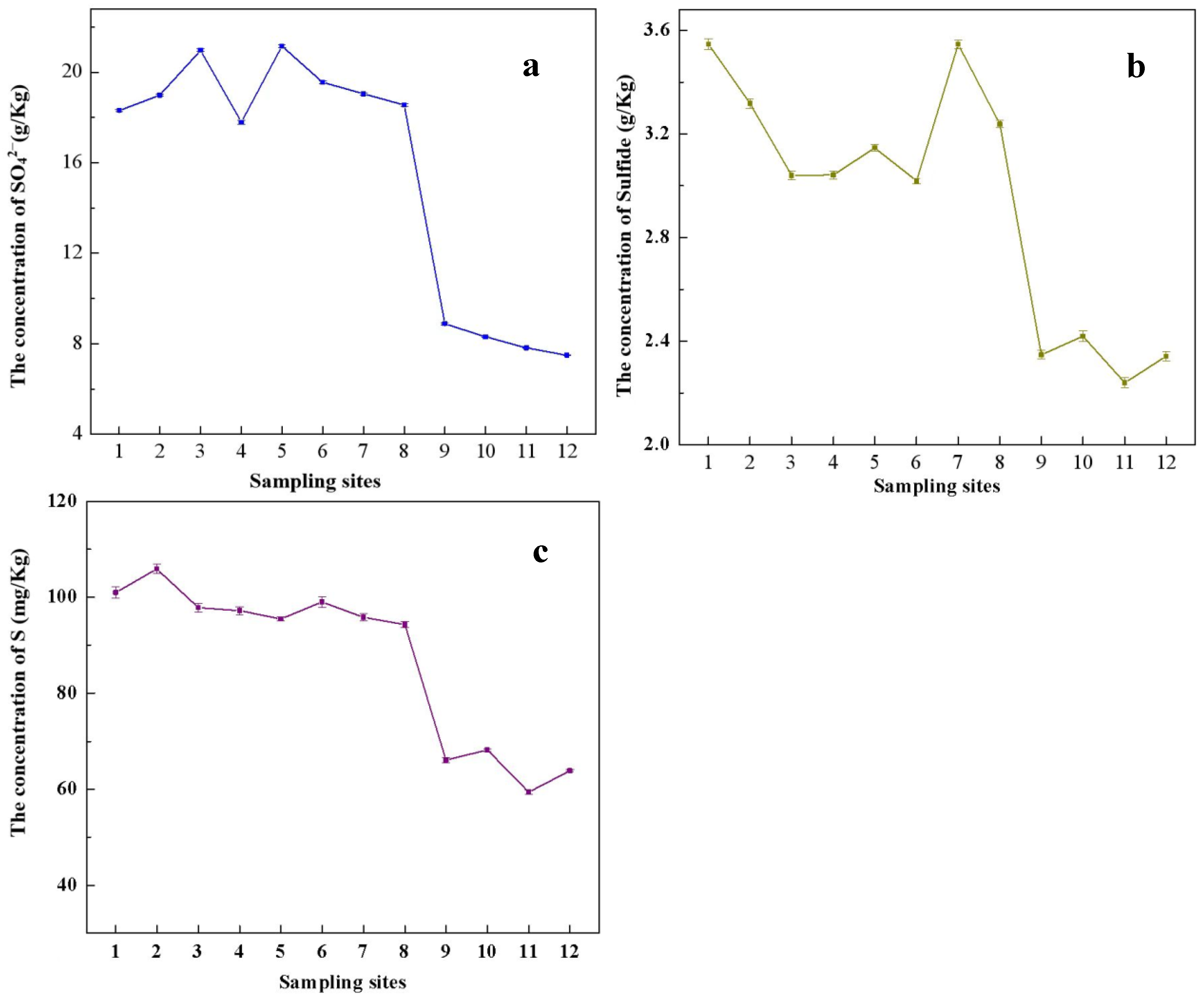
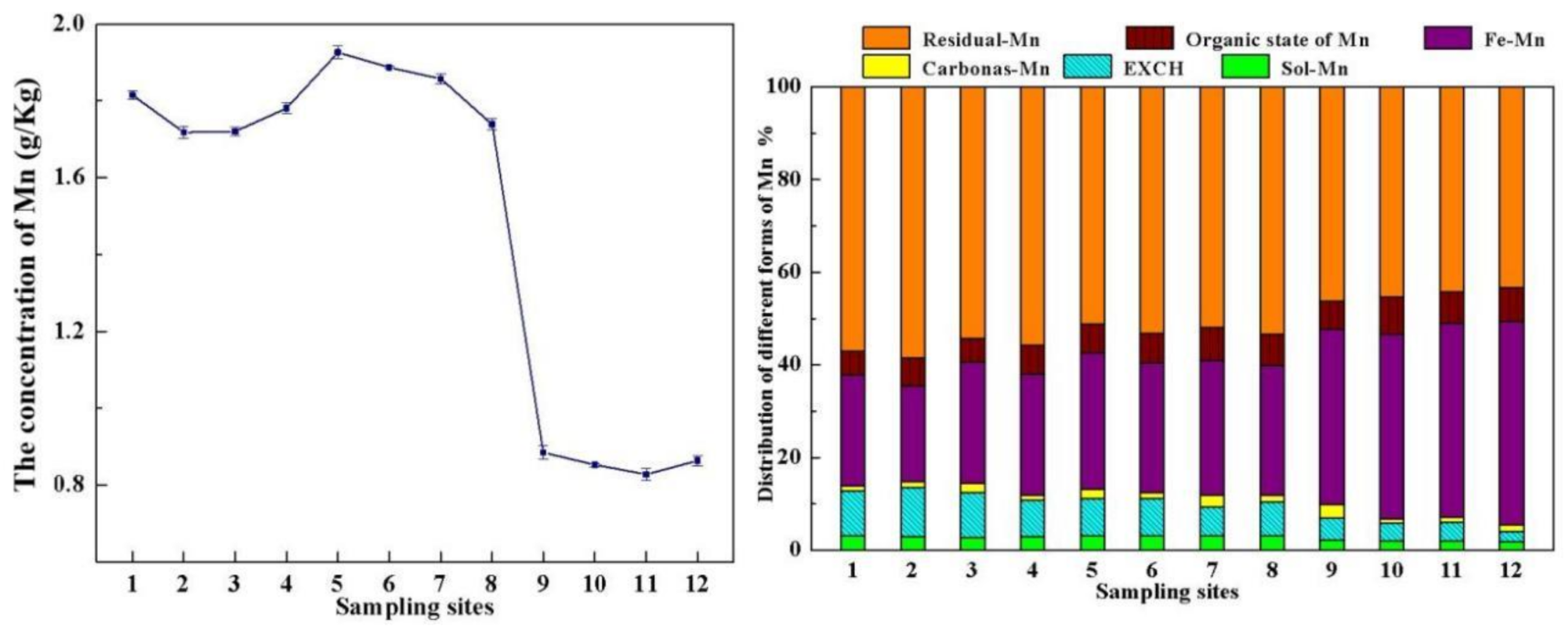
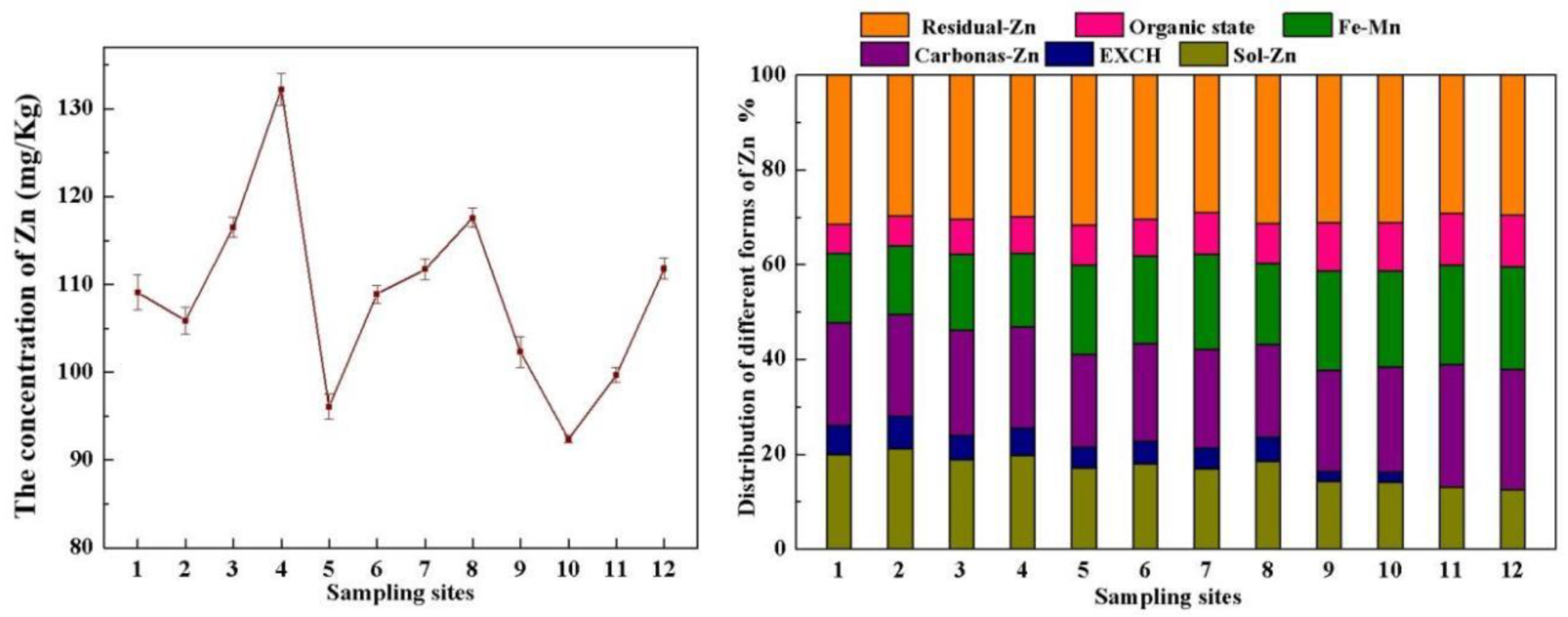
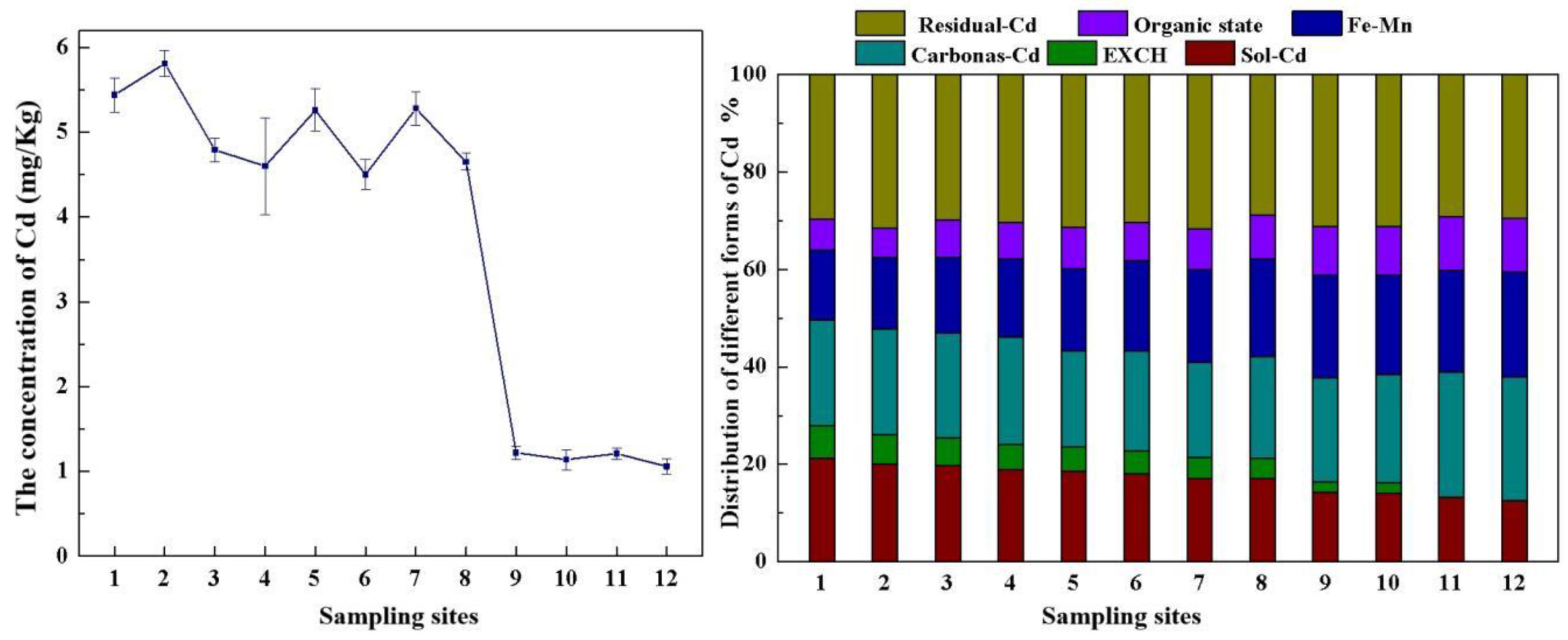
3.1. Chemical Properties of the Acid Reservoir Soil Samples
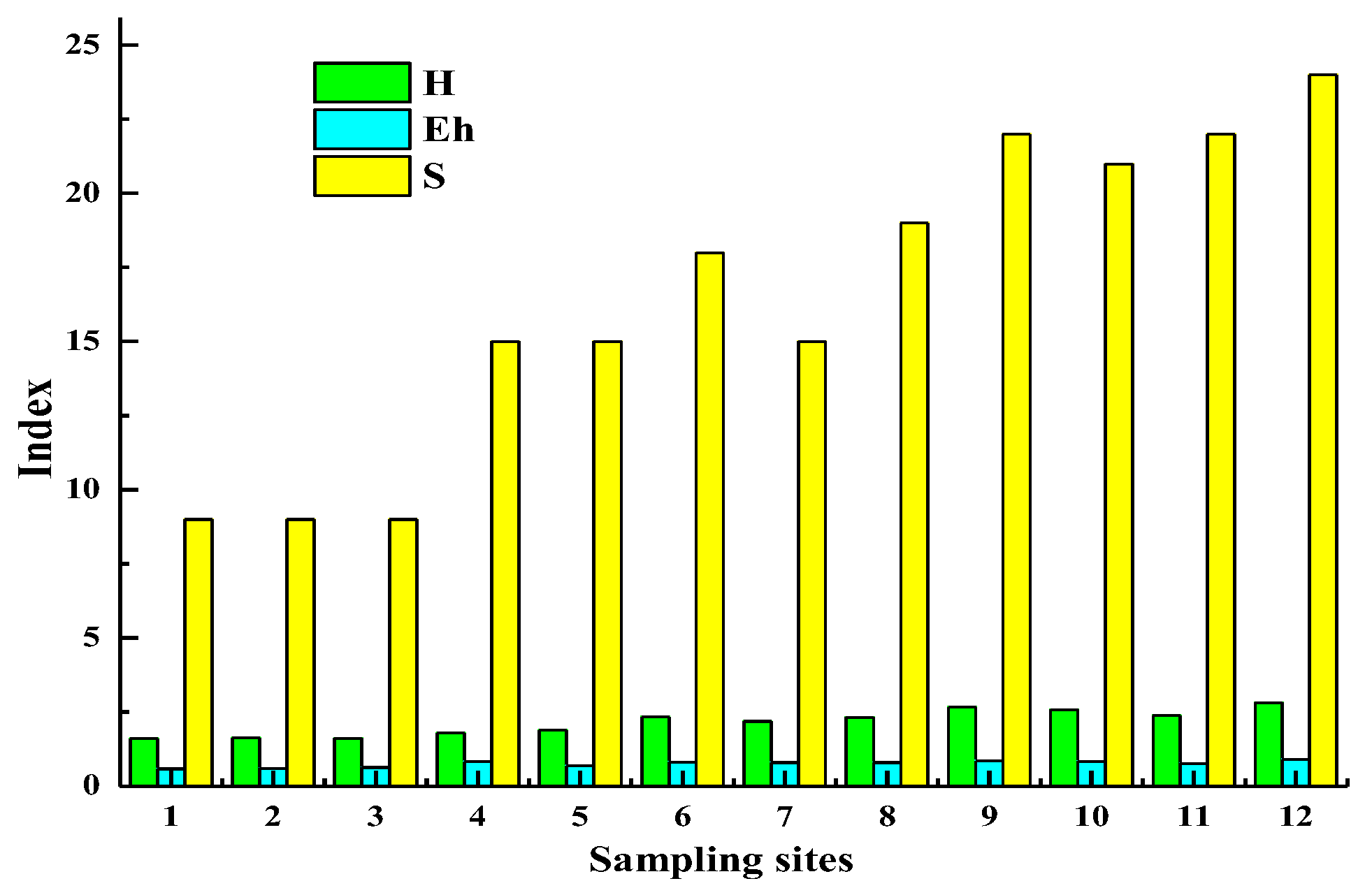
3.2. Characterization of Microbial Communities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boonsrang, A.; Chotpantarat, S.; Sutthirat, C. Factors controlling the release of metals and a metalloid from the tailings of a gold mine in Thailand. Geol. Soc. Lond. Collect. 2017, 18, 109–119. [Google Scholar] [CrossRef]
- Chotpantarat, S. A review of static tests and recent studies. Am. J. Appl. Sci. 2011, 8, 400–406. [Google Scholar] [CrossRef]
- Pakostova, E.; Johnson, D.B.; Bao, Z.; MacKenzie, P.M.; Ptacek, C.J.; Blowes, D.W. Bacterial and archaeal diversity in sulfide-bearing waste rock at faro mine complex, Yukon Territory, Canada. Geomicrobiol. J. 2020, 37, 511–519. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Charuseiam, Y.; Chotpantarat, S.; Sutthirat, C. Acid mine drainage potential of waste rocks in a gold mine (Thailand): Application of a weathering cell test and multivariate statistical analysis. Environ. Geochem. Health 2021, 4, 1049–1079. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Bankar, A.; Patil, S. Microbial cell factories for treatment of soil polluted with heavy metals: A green approach—ScienceDirect. Microbiome Stimul. Crops 2021, 18, 315–332. [Google Scholar]
- Jia, S.; Zhou, Y. Ecological compensation method for soil polluted by heavy metals based on internet of things. Earth Sci. Res. J. 2020, 24, 153–161. [Google Scholar]
- Liu, N.; Liu, H.; Wu, P.; Meng, W.; Li, X.; Chen, X. Distribution characteristics and potential pollution assessment of heavy metals (Cd, Pb, Zn) in reservoir sediments from a historical artisanal zinc smelting area in Southwest China. Environ. Sci. Pollut. R. 2021, 29, 14288–14298. [Google Scholar] [CrossRef]
- Wang, R.; Deng, H.; Jia, Z.M.; Wang, J.B.; Zeng, Q.Q. Spatial distribution characteristics, pollution, and ecological risk assessment of soil heavy metals around mercury mining areas. Huan Jing Ke Xue 2021, 42, 3018–3027. [Google Scholar] [PubMed]
- Chen, M.; Li, Y.; Jiang, X.; Zhao, D.; Liu, X.; Zhou, J. Study on soil physical structure after the bioremediation of pb pollution using microbial-induced carbonate precipitation methodology. J. Hazard. Mater. 2021, 411, 125103. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; Mcdonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies-ScienceDirect. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Almeida, W.I.; Vieira, R.P.; Cardoso, A.M.; Silveira, C.B.; Costa, R.G.; Gonzalez, A.M.; Paranhos, R. Archaeal and bacterial communities of heavy metal contaminated acidic waters from zinc mine residues in Sepetiba Bay. Extremophiles 2009, 13, 263–271. [Google Scholar] [CrossRef]
- Mohanty, D.K.; Matty, D.J. Method of Using a Crosslinked Polymer-carbon Sorbent for the Removal of Heavy Metals, Toxic Materials and Carbon Dioxide. US Patent US8840855B2, 23 September 2014. [Google Scholar]
- Zhu, S.; Rong, Y.; Kiang, T.K.L. Effects of p-Cresol on oxidative stress, glutathione depletion, and necrosis in HepaRG Cells: Comparisons to other uremic toxins and the role of p-Cresol glucuronide formation. Pharmaceutics 2021, 13, 857. [Google Scholar] [CrossRef]
- Gharib, F.A.; Mansour, K.H.; Ahmed, E.Z.; Galal, T.M. Heavy metals concentration, and antioxidant activity of the essential oil of the wild mint (Mentha longifolia L.) in the Egyptian watercourses. Int. J. Phytoremediat. 2020, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Rafique, S. Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci. Rep. 2021, 1, 17006–17017. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.A.; Valente, T.; Torre, M.L.; Santisteban, M.; Ceron, J.C.; Perez-Ostale, E. Characterization of acid mine drainage sources in the Iberian Pyrite Belt: Base methodology for quantifying affected areas and for environmental management. Environ. Earth Sci. 2014, 71, 2729–2738. [Google Scholar] [CrossRef]
- Nieto, J.; Sarmineto, A.; Olias, M.; Avora, C. Acid mine drainage in the Iberian Pyrite Belt: 1. Hydrochemical characteristics and pollutant load of the Tinto and Odiel rivers. Environ. Sci. Pollut. R. 2013, 20, 7509–7519. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS. Microbiol. Eco. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Niu, S.; Fang, Q.; Yu, J.; Wu, J. Heavy metals present in the soils from extremely large opencast iron mine pit. Bull. Environ. Contam. Toxicol. 2021, 107, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.M.; Chen, Z.L.; Lei, G.J.; Fang, X.H. Vertical pollution characteristic and ecological risk assessment of heavy metal of soil profiles in polymetallic ore mine. Ecol. Environ. Sci. 2016, 25, 130–134. [Google Scholar]
- Zhang, C.; Pan, W.; Tang, C. Assessment of arsenic distribution in paddy soil and rice plants of a typical karst basin affected by acid mine drainage in Southwest China. Environ. Pollut. 2013, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Kim, J.; Chung, J.O.; Roh, J.H.; Hong, Y.D. Variations in the composition of tea leaves and soil microbial community. Biol. Fert. Soils 2022, 58, 167–179. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W. Study on an extraction method of pathogenic fungal DNA from farmland soil. J. ZhengZhou Univ. Light Ind. (Nat. Sci.) 2013, 4, 54–59. [Google Scholar]
- Voelkner, A.; Holthusen, D.; Horn, R. Determination of soil dispersion caused by anaerobic digestates: Interferences of pH and soil charge with regard to soil texture and water content. J. Soil Sediment 2015, 15, 1491–1499. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of soil analysis: Mineralogical, organic and inorganic methods. Int. J. Cancer 2006. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar]
- Muyzer, G.; Dewaal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Daghio, M.; Vaiopoulou, E.; Patil, S.A.; Suárez-Suárez, A.; Head, I.M.; Franzetti, A.; Rabaey, K. Anodes stimulate anaerobic toluene degradation via sulfur cycling in marine sediments. Appl. Environ. Microbiol. 2015, 23, 297–307. [Google Scholar]
- Olefeldt, D.; Roulet, N.T. Effects of permafrost and hydrology on the composition and transport of dissolved organic carbon in a subarctic peatland complex. J. Geophys. Res. Biogeosciences 2012, 21, 117–125. [Google Scholar]
- Wu, Q.B.; Xiao, K.; Zhi, Y. soil organic carbon and its fractions across vegetation types: Effects of soil mineral surface area and microaggregates. Pedosphere 2009, 19, 258–264. [Google Scholar]
- Gilliam, F.S.; Billmyer, J.H.; Walter, C.A. Effects of excess nitrogen on biogeochemistry of a temperate hardwood forest: Evidence of nutrient redistribution by a forest understory species. Atmos. Environ. 2016, 146, 261–270. [Google Scholar]
- Chen, H.M. Environmental Soil Science; Science: Bejing, China, 2010. [Google Scholar]
- NY/T 391-2000; China Green Food-Standard of Environmental and Technical Conditions of Origin. China Standard Press: Beijing, China, 2000.
- Zhang, J.; Wu, X.; Shi, Y.; Jin, C.; Wang, J. A slight increase in soil pH benefits soil organic carbon and nitrogen storage in a semi-arid grassland. Ecol. Indic. 2021, 130, 108037. [Google Scholar]
- Zhang, W.; Zhang, I.; Deng, J. Comparison of retention and output of sulfur in limestone soil and yellow soil and their responses to acid deposition in a small karst catchment of Guizhou Province, Southwest China. Environ. Sci. Pollut. R. 2021, 28, 60993–61007. [Google Scholar]
- Shamshuddin, J.; Muhrizal, S.; Fauziah, I.; Husni, M.H.A. Effects of adding organic materials to an acid sulfate soil on the growth of cocoa (Theobroma cacao L.) seedlings. Sci. Total. Environ. 2004, 323, 33–45. [Google Scholar]
- Nystrand, M.I.; Hadzic, M.; Postila, H.; Wichmann, A.; Karppinen, A.; Ihme, R.; Österholm, P. Characteristics of sulfide bearing soil materials in peat extraction areas in N-Finland. J. Geochem. Explor. 2021, 220, 106640. [Google Scholar]
- Vlachy, N.; Jagoda-Cwiklik, B.; Vácha, R.; Touraud, D.; Jungwirth, P.; Kunz, W. Hofmeister series and specific interactions of charged headgroups with aqueous ions. Adv. Colloid Interface Sci. 2009, 146, 42–47. [Google Scholar]
- Zahra, N.; Hafeez, M.B.; Shaukat, K.; Wahid, A.; Hasanuzzaman, M. Iron toxicity in plants: Impacts and remediation. Physiol. Plant 2021, 173, 201–222. [Google Scholar]
- Li, Y.F.; Yang, Y.F.; Wang, Y.Y.; Liu, W.J.; Zhang, P.J. Characteristics of soil iron forms in wetlands with hvarious restoration ages around the Caizi lake, Anhui Province. Acta Sci. Circumstantiae 2015, 35, 3234–3241. [Google Scholar]
- Martinez, C.E.; Motto, H.L. Solubility of lead, zinc and copper added to mineral soils. Environ. Pollut. 2000, 107, 153–158. [Google Scholar]
- Lucas, B.; Seavey, K.C.; Liu, Y.A. Steady-state and dynamic modeling for new product design for the solid-state polymerization of Poly(Ethylene Terephthalate). Ind. Eng. Chem. Res. 2006, 46, 190–202. [Google Scholar]
- Liu, C.; Liu, W.S.; Ent, A.; Morel, J.L.; Qiu, R.L. Simultaneous hyperaccumulation of rare earth elements, manganese and aluminum in Phytolacca americana in response to soil properties. Chemosphere 2021, 282, 131096. [Google Scholar]
- Sirous, S.; Mahboub, S. The status of chemical forms of iron and manganese in various orders of calcareous soils and their relationship with some physicochemical and mineralogical properties. Commun. Soil. Sci. Plan. 2020, 15, 2054–2068. [Google Scholar]
- Sinha, M.K.; Purcell, W. Reducing agents in the leaching of manganese ores: A comprehensive review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar]
- Cui, J.; Li, X.; Muhammad, Y.; Shi, C.Y.; Li, H.B.; Su, H. Residual organics removal from manganese electrochemical solution using combined Fenton oxidation process with adsorption over activated carbon. Environ. Sci. Pollut. Res. 2020, 27, 44240–44248. [Google Scholar]
- GB 15618-2008; Soil Environmental Quality Standards in China (Revised). State Environmental Protection Administration (SEPA): Beijing, China, 2008.
- Arao, T.; Ishikawa, S.; Murakami, M.; Abe, K.; Maejima, Y.; Makino, T. Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ. 2010, 8, 247–257. [Google Scholar]
- Wang, G.X.; Zeng, C.; Zhang, F. Traffic-related trace elements in soils along six highway segments on the Tibetan Plateau: Influence factors and spatial variation. Sci. Total Environ. 2017, 581–582, 811–821. [Google Scholar]
- Zitoun, R.; Clearwater, S.J.; Hassler, C.; Thompson, K.J.; Albert, A.; Sander, S.G. Copper toxicity to blue mussel embryos (Mytilus galloprovincialis): The effect of natural dissolved organic matter on copper toxicity in estuarine waters. Sci. Total. Environ. 2019, 25, 300–314. [Google Scholar]
- Liu, X.; Zhang, X.; Li, R.; Wang, G.L.; Jin, Y.; Xu, W.; Wang, H.; Qu, J. Organic amendment improves rhizosphere environment and shapes soil bacterial community in black and red soil under lead stress. J. Hazard. Mater. 2021, 416, 125805. [Google Scholar]
- Wu, S.C.; Luo, Y.M.; Cheung, K.C.; Wong, M.H. Influence of bacteria on Pb and Zn speciation, mobility and bioavailability in soil: A laboratory study. Environ. Pollut. 2006, 144, 765–773. [Google Scholar]
- Brallier, S.; Harrison, R.B.; Henry, C.L.; Xue, D.S. Liming effects on availability of Cd, Cu, Ni and Zn in a soil amended with sewage sludge 16 years previously. Water Air Soil Pollut. 1996, 86, 195–206. [Google Scholar]
- Lin, A.; Zhang, X.; Yang, X. Glomus mosseae enhances root growth and Cu and Pb acquisition of upland rice (Oryza sativa L.) in contaminated soils. Ecotoxicology 2014, 23, 2053–2061. [Google Scholar]
- Wang, D.F.; Liu, Y.G.; Tan, X.F.; Liu, H.Y.; Zeng, G.M.; Hu, X.J.; Jian, H.; Gu, J.L. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 3489–3497. [Google Scholar]
- Lukovic, J.; Merkulov, L.; Pajevic, S.; Zoric, L.; Nikolic, N.; Borisev, M. Quantitative Assessment of Effects of Cadmium on the Histological Structure of Poplar and Willow Leaves. Water Air Soil Pollut. 2012, 223, 2979–2993. [Google Scholar]
- Lan, H.; Liu, H.; Ye, Y.P.; Yin, Z.N. The role of surface properties on protein aggregation behavior in aqueous solution of different pH values. AAPS Pharm. Sci. Tech. 2020, 21, 13. [Google Scholar]
- Kazuto, S.; Tomohiro, S.; Hideki, K. High-heat effects on the physical and chemical properties of soil organic matter and its water-soluble components in Japan’s forests: A comprehensive approach using multiple analytical methods. Anal. Sci. 2020, 36, 601–605. [Google Scholar]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.-M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. New Biotechnol. 2020, 56, 130–139. [Google Scholar]
- Gao, Y.; Miao, C.; Xia, J.; Mao, L.; Wang, Y.; Zhou, P. Plant diversity reduces the effect of multiple heavy metal pollution on soil enzyme activities and microbial community structure. Front. Environ. Sci. Eng. 2012, 6, 213–223. [Google Scholar]
- Casalta, E.; Montel, M.C. Safety assessment of dairy microorganisms: The Lactococcus genus. Int. J. Food Microbiol. 2008, 126, 271–273. [Google Scholar]
- Majumder, P.; Palit, D. Isolation, Identification and Characterization of Bacteria of Coal Mine Soil at Sonepur Bazari of Raniganj Coalfield, West Bengal. Int. J. Appl. Environ. Sci. 2017, 12, 1131–1140. [Google Scholar]
- Kelly, D.P.; Mcdonald, I.R.; Wood, A.P. The Family Methylobacteriaceae. Prokaryotes 2014, 11, 313–340. [Google Scholar]
- Thuy, T.A.; Flynn, W.P. DesuLfuromonas carbonis sp. nov., an Fe(III)-, S0- and Mn(IV)-reducing bacteriμm isolated from an active coalbed methane gas well. Int. J. Syst. Evol. Microbiol. 2015, 65, 1686–1693. [Google Scholar]









| Project | Mn | Fe | Cu | Zn | Cd | |
|---|---|---|---|---|---|---|
| Ma’anshan City | / | / | 32.367 | 84.733 | 0.264 | |
| Anhui Province | 530 | 3.14 × 104 | 20.4 | 62 | 0.097 | |
| National Standard (I) | / | / | 35 | 100 | 0.20 | |
| pH < 6.5 | / | / | 50 | 200 | 0.30 | |
| National Standard (II) | pH: 6.5–7.5 | / | / | 100 | 250 | 0.30 |
| pH > 7.5 | / | / | 100 | 300 | 0.60 | |
| National Standard (III) | 583 | / | 400 | 500 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Xuan, F.; Li, D.; Wang, J.; Zhang, B. Variations of Soil Chemical Properties and Microbial Community around the Acid Reservoir in the Mining Area. Sustainability 2022, 14, 10746. https://doi.org/10.3390/su141710746
Guo J, Xuan F, Li D, Wang J, Zhang B. Variations of Soil Chemical Properties and Microbial Community around the Acid Reservoir in the Mining Area. Sustainability. 2022; 14(17):10746. https://doi.org/10.3390/su141710746
Chicago/Turabian StyleGuo, Jing, Fengqin Xuan, Deming Li, Jiaquan Wang, and Baichuan Zhang. 2022. "Variations of Soil Chemical Properties and Microbial Community around the Acid Reservoir in the Mining Area" Sustainability 14, no. 17: 10746. https://doi.org/10.3390/su141710746
APA StyleGuo, J., Xuan, F., Li, D., Wang, J., & Zhang, B. (2022). Variations of Soil Chemical Properties and Microbial Community around the Acid Reservoir in the Mining Area. Sustainability, 14(17), 10746. https://doi.org/10.3390/su141710746






