Biosynthesis of Nanoparticles Using Endophytes: A Novel Approach for Enhancing Plant Growth and Sustainable Agriculture
Abstract
:1. Introduction
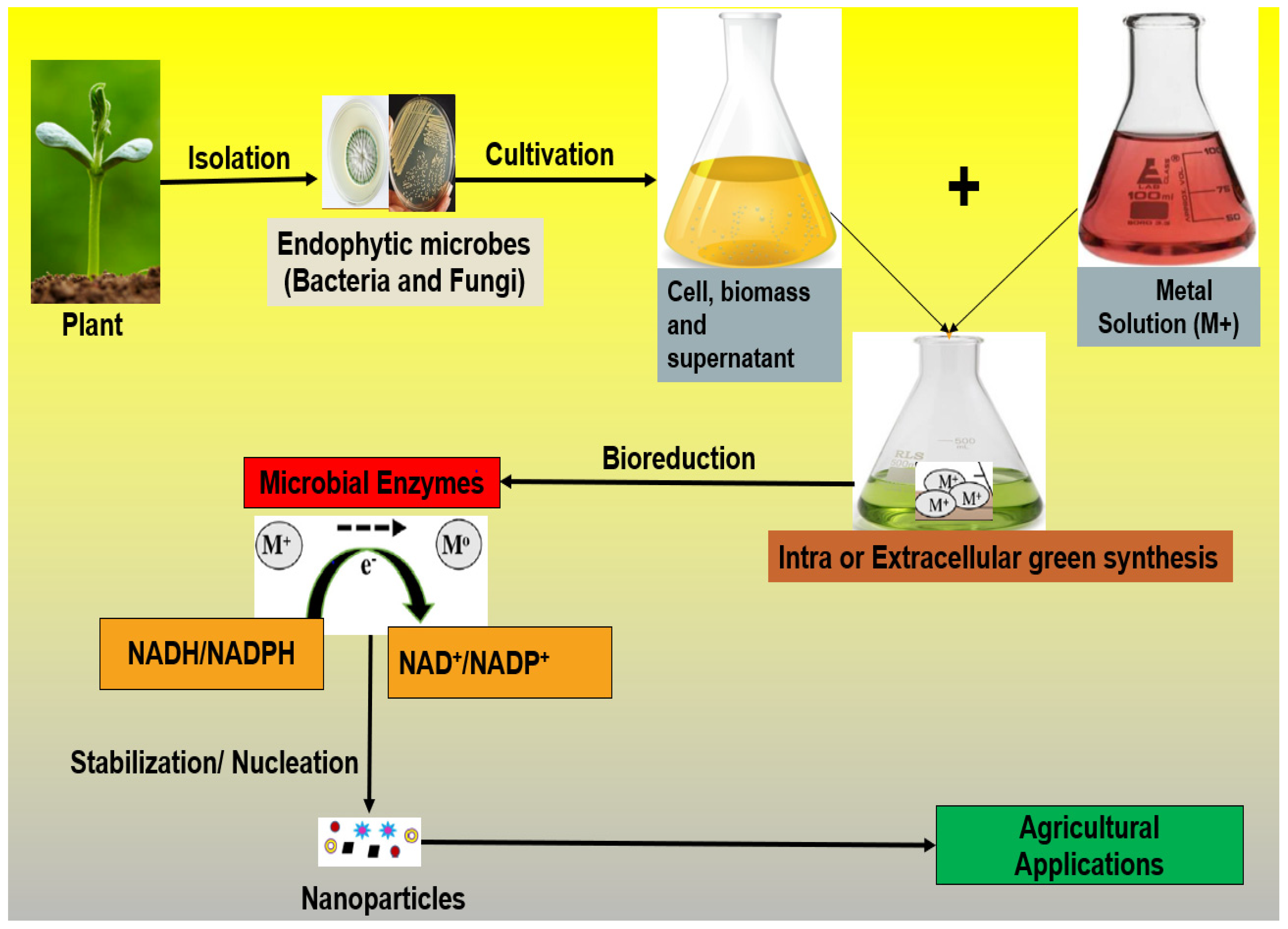
2. Biosynthesis of Nanoparticles: Endophytic Microbiomes as Biological Factories
3. Endophytic Microbes in the Biosynthesis of Nanostructural Materials
3.1. Silver Nanoparticles
3.2. Gold Nanoparticles
3.3. Copper-Based Nanoparticles
3.4. Zinc-Based Nanoparticles
3.5. Titanium-, Cobalt, Nickel, Iron-Based Nanoparticles
4. Applications of NPs from Endophytic Microbiomes for Controlling Disease and Promoting Sustainable Agriculture
4.1. Antimicrobial/Disease Suppression Activities
4.2. Production of Nanopesticides
5. Toxicity Assessment of Endophytic Nanoparticles
6. Challenges with the Biosynthesis of Nanoparticles Using Endophytic Microbiomes
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Nalwa, H.S. Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. J. Biomed. Nanotechnol. 2011, 7, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Imade, E.E.; Ajiboye, T.O.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Green synthesis of zinc oxide nanoparticles using plantain peel extracts and the evaluation of their antibacterial activity. Sci. Afr. 2022, 16, e01152. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Babalola, O.O.; Nikoro, D.O. Nanotechnology as Vehicle for Biocontrol of Plant Diseases in Crop Production. In Food Security and Safety: Africa’s Perspective; Babalola, O.O., Ed.; Springer: Cham, Switzerland, 2021; pp. 709–724, eBook; ISBN1 978-3-030-50672-8. Hardcover; ISBN2 978-3-030-50671-1. Available online: https://www.springer.com/gp/book/9783030506711 (accessed on 20 July 2022). [CrossRef]
- Fadiji, A.E.; Mthiyane, D.M.N.; Onwudiwe, D.C.; Babalola, O.O. Harnessing the known and unknown impact of nanotechnology on enhancing food security and reducing postharvest losses: Constraints and future prospects. Agronomy 2022, 12, 1657. [Google Scholar] [CrossRef]
- Locke, J.M.; Bryce, J.H.; Morris, P.C. Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J. Exp. Bot. 2000, 51, 1843–1849. [Google Scholar] [CrossRef]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Ahmad, F.; Siddiqui, M.A.; Babalola, O.O.; Wu, H.-F. Biofunctionalization of nanoparticle assisted mass spectrometry as biosensors for rapid detection of plant associated bacteria. Biosens. Bioelectron. 2012, 35, 235–242. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Yadav, N.; Yadav, A.N. Endophytic Microbes in Nanotechnology: Current Development, and Potential Biotechnology Applications Eds by Ajay Kumar and Vipin Kumar Singh “in Microbial Endophytes”; Elsevier: Amsterdam, The Netherlands, 2020; pp. 231–262. [Google Scholar] [CrossRef]
- Bogas, A.C.; Saulo, H.R.; Gonçalves, M.O.; De Assis, M.; Longo, E.; Paiva De Sousa, C. Endophytic microorganisms from the tropics as biofactories for the synthesis of metal-based nanoparticles: Healthcare applications. Front. Nanotechnol. 2022, 4, 823236. [Google Scholar] [CrossRef]
- Azmath, P.; Baker, S.; Rakshith, D.; Satish, S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 2016, 24, 140–146. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Brader, G.; Corretto, E.; Sessitsch, A. Metagenomics of plant microbiomes. In Functional Metagenomics: Tools and Applications; Charles, T., Liles, M., Sessitsch, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 179–200. [Google Scholar]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Satish, S. Biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef]
- Alappat, C.; Kannan, K.; Vasanthi, N. Biosynthesis of Au nanoparticles using the endophytic fungi isolated from Bauhinia variegata L. Eng. Sci. Technol. Int. J. 2012, 2, 377–380. [Google Scholar]
- Bala, M.; Arya, V. Biological synthesis of silver nanoparticles from aqueous extract of endophytic fungus Aspergillus fumigatus and its antibacterial action. Int. J. Nanomater. Biostruct. 2013, 3, 37–41. [Google Scholar]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824. [Google Scholar] [CrossRef]
- Verma, V.C.; Anand, S.; Ulrichs, C.; Singh, S.K. Biogenic gold nanotriangles from Saccharomonospora sp., an endophytic actinomycetes of Azadirachta indica A. Juss. Int. Nano Lett. 2013, 3, 21. [Google Scholar] [CrossRef]
- Kaur, P. Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: A review. Biotechnol. Res. Innov. 2018, 2, 63–73. [Google Scholar]
- Sone, B.; Diallo, A.; Fuku, X.; Gurib-Fakim, A.; Maaza, M. Biosynthesized CuO nano-platelets: Physical properties and enhanced thermal conductivity nanofluidics. Arab. J. Chem. 2020, 13, 160–170. [Google Scholar] [CrossRef]
- Bansal, P.; Duhan, J.S.; Gahlawat, S.K. Biogenesis of nanoparticles: A review. Afr. J. Biotechnol. 2014, 13, 2778–2785. [Google Scholar]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Busi, S.; Rajkumari, J. Microbially synthesized nanoparticles as next generation antimicrobials: Scope and applications. In Nanoparticles Pharmacotherapy; Grumezescu, A.M., Ed.; Elsevier: Norwich, NY, USA, 2019; pp. 485–524. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Brock/Springer Series in Contemporary Bioscience; Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar] [CrossRef]
- Xu, R.; Li, T.; Cui, H.; Wang, J.; Yu, X.; Ding, Y.; Wang, C.; Yang, Z.; Zhao, Z. Diversity and characterization of Cd-tolerant dark septate endophytes (DSEs) associated with the roots of Nepal alder (Alnus nepalensis) in a metal mine tailing of southwest China. Appl. Soil Ecol. 2015, 93, 11–18. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Zubair, M.; Almatroudi, A.; Khurshid, M.; Tariq, F.; Mumtaz, R.; Li, B. Bioprospecting a native silver-resistant Bacillus safensis strain for green synthesis and subsequent antibacterial and anticancer activities of silver nanoparticles. J. Adv. Res. 2020, 24, 475–483. [Google Scholar] [CrossRef]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef]
- Gupta, M.; Shukla, K.K. Endophytic Fungi: A Treasure Trove ofNovel Bioactive Compounds. In Bioactive Natural Products in Drug Discovery; Singh, J., Meshram, V., Gupta, M., Eds.; Springer: Singapore, 2020; pp. 427–449. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Metagenomic profiling of the community structure, diversity, and nutrient pathways of bacterial endophytes in maize plant. Antonie Leeuwenhoek 2020, 113, 1559–1571. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Organic Farming enhances the diversity and community structure of endophytic archaea and fungi in maize plant: A shotgun approach. J. Soil Sci. Plant. Nutr. 2020, 20, 2587–2599. [Google Scholar] [CrossRef]
- Messaoudi, O.; Bendahou, M. Biological Synthesis of Nanoparticles Using Endophytic Microorganisms: Current Development. In “BiologicalSynthesis of Nanoparticles Using Endophytic Microorganisms: Current Development” in Nanotechnology and the Environment; Sen, M., Ed.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: A review. J. Fungi 2021, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Correa-Galeote, D.; Bedmar, E.J.; Arone, G.J. Maize endophytic bacterial diversity as affected by soil cultivation history. Front. Microbiol. 2018, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Dai, L.; Xu, G.F.; Wang, H.S. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 2021, 11, 7637. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014, 219. [Google Scholar] [CrossRef]
- Assis, M.; Groppo Filho, F.C.; Pimentel, D.S.; Robeldo, T.; Gouveia, A.F.; Castro, T.F.; Fukushima, H.C.; de Foggi, C.C.; da Costa, J.P.; Borra, R.C. Ag nanoparticles/AgX (X = Cl, Br and I) composites with enhanced photocatalytic activity and low toxicological effects. ChemistrySelect 2020, 5, 4655–4673. [Google Scholar] [CrossRef]
- Salesa, B.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Carbon nanofibers versus silver nanoparticles: Time-dependent cytotoxicity, proliferation, and gene expression. Biomedicines 2021, 9, 1155. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Narsing Rao, M.P.; Xiao, M.; Wang, H.-F.; Hozzein, W.N.; Chen, W.; Li, W.-J. Antibacterial activity of silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 2017, 8, 1090. [Google Scholar] [CrossRef] [Green Version]
- Balakumaran, M.; Ramachandran, R.; Kalaichelvan, P. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31–39. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A.M. Antimicrobial activity of green silver nanoparticles from endophytic fungi isolated from Calotropis procera (Ait) latex. Microbiology 2019, 165, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bagur, H.; Poojari, C.C.; Melappa, G.; Rangappa, R.; Chandrasekhar, N.; Somu, P. Biogenically synthesized silver nanoparticles using endophyte fungal extract of Ocimum tenuiflorum and evaluation of biomedical properties. J. Clust. Sci. 2020, 31, 1241–1255. [Google Scholar] [CrossRef]
- Bagur, H.; Medidi, R.S.; Somu, P.; Choudhury, P.J.; Karua, C.S.; Guttula, P.K.; Melappa, G.; Poojari, C.C. Endophyte fungal isolate mediated biogenic synthesis and evaluation of biomedical applications of silver nanoparticles. Mater. Technol. 2022, 37, 167–178. [Google Scholar] [CrossRef]
- Chandankere, R.; Chelliah, J.; Subban, K.; Shanadrahalli, V.C.; Parvez, A.; Zabed, H.M.; Sharma, Y.C.; Qi, X. Pleiotropic functions and biological potentials of silver nanoparticles synthesized by an endophytic fungus. Front. Bioeng. Biotechnol. 2020, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.M.; dos Morais, E.S.; da Sena, I.S.; Lima, A.L.; de Oliveira, F.R.; de Freitas, C.M.; Fernandes, C.P.; de Carvalho, J.C.T.; Ferreira, I.M. Silver nanoparticle from whole cells of the fungi Trichoderma spp. isolated from Brazilian Amazon. Biotechnol. Lett. 2020, 42, 833–843. [Google Scholar] [CrossRef]
- Hulikere, M.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Neethu, S.; Midhun, S.J.; Radhakrishnan, E.; Jyothis, M. Green synthesized silver nanoparticles by marine endophytic fungus Penicillium polonicum and its antibacterial efficacy against biofilm forming, multidrug-resistant Acinetobacter baumanii. Microb. Pathog. 2018, 116, 263–272. [Google Scholar] [CrossRef]
- Kavitha, K.; Baker, S.; Rakshith, D.; Kavitha, H.; Yashwantha Rao, H.; Harini, B.; Satish, S. Plants as green source towards synthesis of nanoparticles. Res. J. Biol. Sci. 2013, 2, 66–76. [Google Scholar]
- Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Munawer, U.; Raghavendra, V.B.; Ningaraju, S.; Krishna, K.L.; Ghosh, A.R.; Melappa, G.; Pugazhendhi, A. Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: Photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 2020, 588, 119729. [Google Scholar] [CrossRef]
- Hemashekhar, B.; Chandrappa, C.; Govindappa, M.; Chandrashekar, N. Endophytic fungus Alternaria spp isolated from Rauvolfia tetraphylla root arbitrate synthesis of gold nanoparticles and evaluation of their antibacterial, antioxidant and antimitotic activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 035010. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Ratti, R.; Serrano, N.; Hokka, C.; Sousa, C. Antagonistic properties of some microorganisms isolated from Brazilian tropical savannah plants against Staphylococcus coagulase-positive strain. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 294–302. [Google Scholar] [CrossRef]
- Romano, L.H. Bioprospecção de Microrganismos Endofíticos Isolados de Tabebuia spp. e Hymenaea Courbaril e Identificação da Produção de Metabólitos de Interesse Biotecnológico. Ph.D. Thesis, Federal University of São Carlos, São Carlos, Brazil, 2015; pp. 1–136. [Google Scholar]
- Mousa, S.A.; El-Sayed, E.-S.R.; Mohamed, S.S.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Abdou, D.A. Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic Aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl. Microbiol. Biotechnol. 2021, 105, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Rasool, U.; Hemalatha, S. Marine endophytic actinomycetes assisted synthesis of copper nanoparticles (CuNPs): Characterization and antibacterial efficacy against human pathogens. Mater. Lett. 2017, 194, 176–180. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Uddandarao, P. ZnS semiconductor quantum dots production by an endophytic fungus Aspergillus flavus. Mater. Sci. Eng. B 2016, 207, 26–32. [Google Scholar] [CrossRef]
- Uddandarao, P.; Balakrishnan, R.M.; Ashok, A.; Swarup, S.; Sinha, P. Bioinspired ZnS: Gd nanoparticles synthesized from an endophytic fungi Aspergillus flavus for fluorescence-based metal detection. Biomimetics 2019, 4, 11. [Google Scholar] [CrossRef]
- Arya, S.; Sonawane, H.; Math, S.; Tambade, P.; Chaskar, M.; Shinde, D. Biogenic titanium nanoparticles (TiO2NPs) from Tricoderma citrinoviride extract: Synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int. Nano Lett. 2021, 11, 35–42. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Kim, T.; Feng, Q.L.; Kim, J.; Wu, J.; Wang, H.; Chen, G.; Cui, F. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Raheman, F.; Deshmukh, S.; Ingle, A.; Gade, A.; Rai, M. Silver nanoparticles: Novel antimicrobial agent synthesized from an endophytic fungus Pestalotia sp. isolated from leaves of Syzygium cumini (L.). Nano Med. Eng. 2011, 3, 174–178. [Google Scholar] [CrossRef]
- Devi, L.S.; Joshi, S. Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. J. Microsc. Ultrastruct. 2015, 3, 29–37. [Google Scholar] [PubMed]
- Verma, V.C.; Kharwar, R.N.; Gange, A.C. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 2010, 5, 33–40. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, H.; He, D.; Yang, H.; Wang, W.; Wan, X.; Wang, L. Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess Biosyst. Eng. 2013, 36, 1613–1619. [Google Scholar] [CrossRef]
- Netala, V.R.; Bethu, M.S.; Pushpalatha, B.; Baki, V.B.; Aishwarya, S.; Rao, J.V.; Tartte, V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016, 11, 5683. [Google Scholar] [CrossRef]
- Hossain, A.; Hong, X.; Ibrahim, E.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeya dadantii. Molecules 2019, 24, 2303. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef]
- Hemashekhar, B.; Chandrappa, C.; Govindappa, M.; Chandrasekhar, N.; Ganganagappa, N.; Ramachandra, Y. Green synthesis of silver nanoparticles from Endophytic fungus Aspergillus niger isolated from Simarouba glauca leaf and its Antibacterial and Antioxidant activity. Int. J. Eng. Res. Appl. 2017, 7, 17–24. [Google Scholar]
- Singh, D.; Rathod, V.; Ninganagouda, S.; Herimath, J.; Kulkarni, P. Biosynthesis of silver nanoparticle by endophytic fungi Pencillium sp. isolated from Curcuma longa (turmeric) and its antibacterial activity against pathogenic gram negative bacteria. J. Pharm. Res. 2013, 7, 448–453. [Google Scholar] [CrossRef]
- Farsi, M.; Farokhi, S. Biosynthesis of antibacterial silver nanoparticles by endophytic fungus Nemania sp. Isolated From Taxus baccata L.(Iranian Yew). Zahedan J. Res. Med. Sci. 2018, 20, e57916. [Google Scholar] [CrossRef] [Green Version]
- Ranjani, S.; Ahmed, S.M.; Adnan, M.; Kumar, S.N.; Ruckmani, K.; Hemalatha, S. Synthesis, characterization and applications of endophytic fungal nanoparticles. Norganic Nano-Met. Chem. 2020, 51, 380–387. [Google Scholar] [CrossRef]
- Vijayan, S.; Koilaparambil, D.; George, T.K.; Manakulam Shaikmoideen, J. Antibacterial and cytotoxicity studies of silver nanoparticles synthesized by endophytic Fusarium solani isolated from Withania somnifera (L.). J. Water Environ. Nanotechnol. 2016, 1, 91–103. [Google Scholar]
- Muhsin, T.; Hachim, A. Antitumor and antibacterial efficacy of mycofabricated silver nanoparticles by the endophytic fungus Papulaspora pallidula. Am. J. Biosci. Bioeng. 2016, 2, 24–38. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-H.; Kim, H.-J.; Choi, S.-H. A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol. J. 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef]
- Nirmala, C.; Sridevi, M. Characterization, antimicrobial and antioxidant evaluation of biofabricated silver nanoparticles from Endophytic Pantoea anthophila. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3711–3725. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Cambre, M.; Lee, H.-J. The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Kai, W.; Xiaojun, X.; Ximing, P.; Zhenqing, H.; Qiqing, Z. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 2011, 6, 480. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Kim, M.; Cho, H.; Lee, J.; Yu, J.; Chung, H.; Choi, S. Factors influencing the cytotoxicity of zinc oxide nanoparticles: Particle size and surface charge. J. Phys. Conf. Ser. 2011, 304, v012044. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Lee, H.-J.; Tolliver, L.M.; Aronstam, R.S. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: Opportunities and challenges. BioMed Res. Int. 2015, 2015, 834079. [Google Scholar] [CrossRef] [PubMed]
- Chusuei, C.C.; Wu, C.-H.; Mallavarapu, S.; Hou, F.Y.S.; Hsu, C.-M.; Winiarz, J.G.; Aronstam, R.S.; Huang, Y.-W. Cytotoxicity in the age of nano: The role of fourth period transition metal oxide nanoparticle physicochemical properties. Chem.-Biol. Interact. 2013, 206, 319–326. [Google Scholar] [CrossRef]
- Ranjani, S.; Matheen, A.; Jenish, A.A.; Hemalatha, S. Nanotechnology derived natural poly bio-silver nanoparticles as a potential alternate biomaterial to protect against human pathogens. Mater. Lett. 2021, 304, 130555. [Google Scholar] [CrossRef]
- Antisari, L.V.; Carbone, S.; Fabrizi, A.; Gatti, A.; Vianello, G. Response of soil microbial biomass to CeO2 nanoparticles. EQA-Int. J. Environ. Qual. 2011, 7, 1–16. [Google Scholar]
- Lee, S.; Kim, S.; Kim, S.; Lee, I. Effects of soil-plant interactive system on response to exposure to ZnO nanoparticles. Microbiol. Biotechnol. 2012, 22, 1264–1270. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef]
- Lee, W.-M.; Kwak, J.I.; An, Y.-J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef]
- Nafeh, A.; Mazhar, S. Endophytic nanoparticles: Towards a new therapeutic future. J. Microbiol. Mol. Genet. 2021, 2, 19–30. [Google Scholar] [CrossRef]

| Endophytic Species | Activity | Pathogens Active Against | References |
|---|---|---|---|
| Silver nanoparticles | |||
| Aspergillus niger | Antibacterial | K. pneumoniae | Hemashekhar et al. [77] |
| Epicoccum nigrum | Antifungal | C. tropicalis | Qian et al. [73] |
| Penicillium sp. | Antibacterial | K. pneumoniae | Singh et al. [78] |
| Bacillus cereus | Antibacterial | K. pneumoniae | Sunkar and Nachiyar [18] |
| Epicoccum nigrum | Antifungal | A. fumigatus | Qian et al. [73] |
| Nemania sp. | Antibacterial | P. aureginosa | Farsi and Farokhi [79] |
| Epicoccum nigrum | Antifungal | S. schenckii | Qian et al. [73] |
| Epicoccum. nigrum | Antifungal | C. neoformans | Qian et al. [73], |
| Rana et al. [9] | |||
| Penicillium polonicum | Antibacterial | A. baumanii | Neethu et al. [54] |
| Epicoccum nigrum | Antifungal | C. tropicalis | Qian et al. [73] |
| Lasiodiplodia theobromae | Antibacterial | P. aeruginosa | Ranjani et al. [80] |
| Penicillium sp. | Antibacterial | E. aerogenes | Singh et al. [78] |
| Penicillium oxalicum | Antibacterial | B. subtilis | Balakumaran et al. [46] |
| Epicoccum nigrum | Antifungal | A. flavus | Qian et al. [73] |
| Fusarium oxysporum | Antibacterial | C. cladosporioides | Vijayan et al. [81] |
| Pheidole pallidula | Antibacterial | P. mirabilis | Muhsin and Hachim [82] |
| Bacillus cereus | Antibacterial | P. aureginosa | Sunkar and Nachiyar [18] |
| Pestalotia sp. | Antibacterial | S. aureus | Raheman et al. [70] |
| Gold nanoparticles | |||
| Cladosporium cladosporioides | Antifungal | A. niger | Joshi et al. [56] |
| Cladosporium cladosporioides | Antibacterial | P. aureginosa | Joshi et al. [56] |
| Copper nanoparticles | |||
| Streptomyces capillispiralis | Antibacterial | P. aureginosa | Hassan et al. [64] |
| Streptomyces capillispiralis | Antifungal | A. brasiliensi | Hassan et al. [64] |
| Actinobacteria | Antibacterial | E. coli | Rasool and Hemalatha [63] |
| Streptomyces capillispiralis | Antibacterial | B. dimenuta | Hassan et al. [64] |
| Actinobacteria | Antibacterial | P. mirabilis | Rasool and Hemalatha [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadiji, A.E.; Mortimer, P.E.; Xu, J.; Ebenso, E.E.; Babalola, O.O. Biosynthesis of Nanoparticles Using Endophytes: A Novel Approach for Enhancing Plant Growth and Sustainable Agriculture. Sustainability 2022, 14, 10839. https://doi.org/10.3390/su141710839
Fadiji AE, Mortimer PE, Xu J, Ebenso EE, Babalola OO. Biosynthesis of Nanoparticles Using Endophytes: A Novel Approach for Enhancing Plant Growth and Sustainable Agriculture. Sustainability. 2022; 14(17):10839. https://doi.org/10.3390/su141710839
Chicago/Turabian StyleFadiji, Ayomide Emmanuel, Peter Edward Mortimer, Jianchu Xu, Eno E. Ebenso, and Olubukola Oluranti Babalola. 2022. "Biosynthesis of Nanoparticles Using Endophytes: A Novel Approach for Enhancing Plant Growth and Sustainable Agriculture" Sustainability 14, no. 17: 10839. https://doi.org/10.3390/su141710839
APA StyleFadiji, A. E., Mortimer, P. E., Xu, J., Ebenso, E. E., & Babalola, O. O. (2022). Biosynthesis of Nanoparticles Using Endophytes: A Novel Approach for Enhancing Plant Growth and Sustainable Agriculture. Sustainability, 14(17), 10839. https://doi.org/10.3390/su141710839








