A Sustainable Approach for the Development of Innovative Products from Fruit and Vegetable By-Products
Abstract
:1. Introduction
2. Materials and Methods
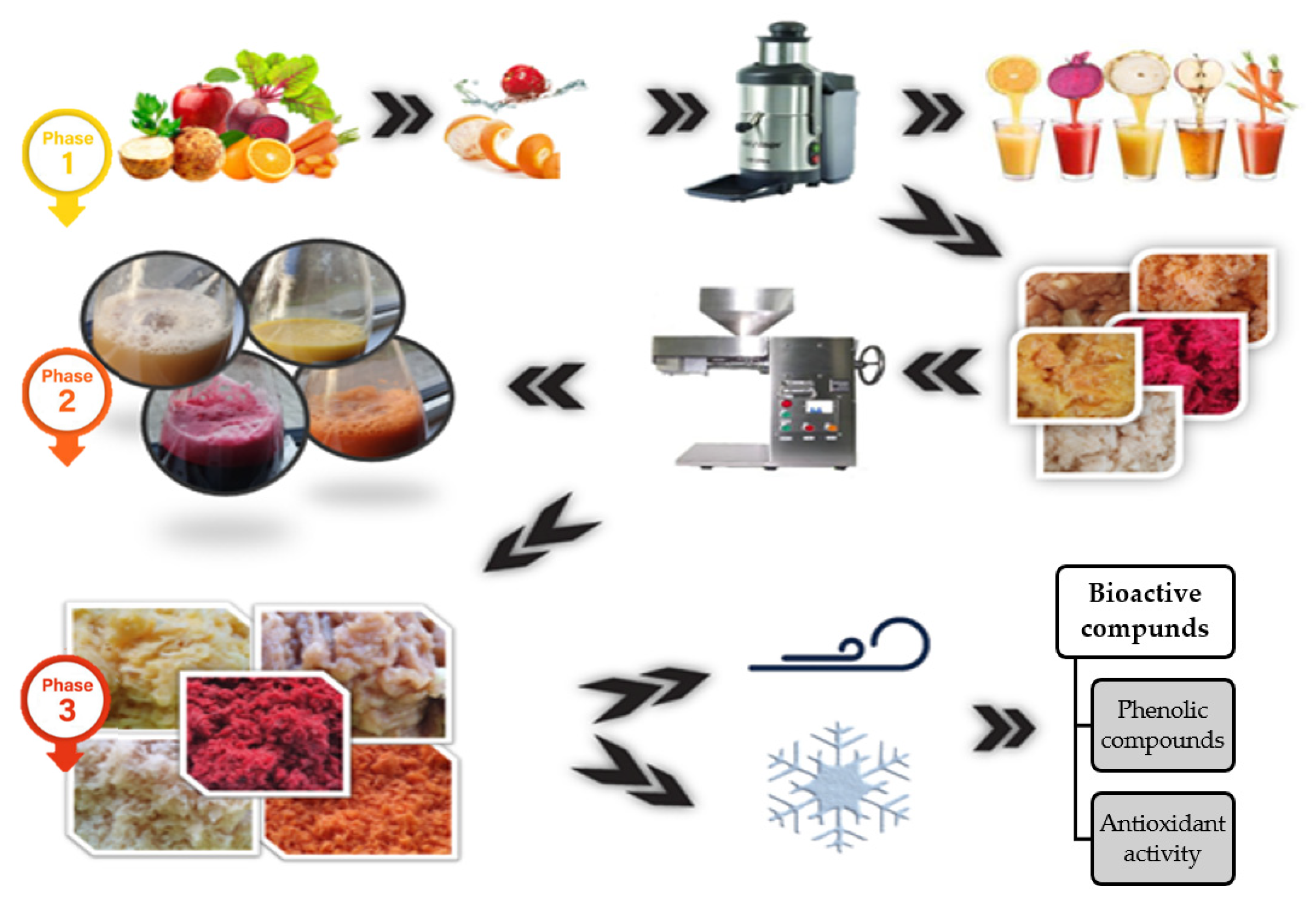
2.1. Samples Processing
2.2. Evaluation of the Antioxidant Activity of Extracts and Quantification of Total Phenolics
2.2.1. Phenolic Compounds
2.2.2. The Total Phenolic Compounds Assay
2.2.3. Determination of DPPH Radical Scavenging Capacity
2.3. Statistical Analysis
3. Results and Discussion
3.1. By-Products Analysis
3.2. Results and Discussion Regarding the Total Phenolics and Antioxidant Activities
3.3. Determination of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Capacity
4. Literature Review for Easy-to-Implement Solutions Using Small Investment for Capitalizing on the By-Products
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WF | whole fruit |
| FJ | fresh fruit |
| FP | fresh pulp |
| PP | pressed pulp |
| PJ | pressed juice |
| FFP | flour from fresh pulp |
| FPP | flour from pressed pulp |
| D.W. | reported as dry weight |
| AA | antioxidant activity |
| GAE | gallic acid equivalents |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
References
- Fruit Juice Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. Available online: https://www.imarcgroup.com/fruit-juice-manufacturing-plant (accessed on 21 March 2022).
- Consumer Markets Non-Alcoholic Drinks. Available online: https://www.statista.com/outlook/cmo/non-alcoholic-drinks/juices/worldwide#revenue (accessed on 3 August 2022).
- Available online: https://www.fortunebusinessinsights.com/cold-pressed-juice-market-106647 (accessed on 4 August 2022).
- United Nations. World Population Prospects 2019: Highlights; Report No. ST/ESA/SER.A/423; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Vollset, S.E.; Goren, E.; Yuan, C.-W.; Cao, J.; Smith, A.E.; Hsiao, T.; Murray, C.J.L. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Canudas-Romo, V.; Shen, T.; Payne, C.F. The Components of Change in Population Growth Rates. Demography 2022, 59, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://es.statista.com/outlook/cmo/non-alcoholic-drinks/juices/worldwide (accessed on 21 March 2022).
- Food Waste Index Report. 2021. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/35280/FoodWaste.pdf (accessed on 21 February 2022).
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- 2019 Food Loss Report and Database. Available online: https://www.fao.org/platform-food-loss-waste/flw-data/en/) (accessed on 3 August 2022).
- Available online: https://www.fao.org/hunger/en/ (accessed on 3 August 2022).
- Tracking Progress on Food and Agriculture-Related SDG Indicators 2021. A Report on the Indicators under FAO Custodianship. Available online: https://www.fao.org/sdg-progress-report/en/ (accessed on 3 August 2022).
- Available online: https://www.fao.org/sustainable-development-goals/indicators/232/en/ (accessed on 3 August 2022).
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- De Ancos, B.; Colina-Coca, C.; González-Peña, D.; Sánchez-Moreno, C. Bioactive compounds from vegetable and fruit by-products. Biotechnol. Bioact. Compd. Sources Appl. 2015, 1–36. [Google Scholar] [CrossRef]
- Venkat, K. The climate change and economic impacts of food waste in the United States. Int. J. Food Syst. Dyn. 2011, 2, 431–446. [Google Scholar]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and waste reduction as an integral part of a circular economy. Front. Environ. Sci. 2017, 5, 21. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Kaavya, R.; Kalpana, L.; Kumar, A.A. Microwave methods for the extraction of bioactive components and enzymes from pineapple waste and its application in meat tenderization. Int. J. Agric. Sci. 2017, 9, 4612–4620. [Google Scholar]
- Shengjiu, G.U.; Kaimei, Z.H.U.; Caizhen, L.U.O.; Jiang, Y.; Yourui, X.U. Microwaves-assisted Extraction of Polyphenols from Banana Peel. Med. Plant 2014, 5, 21–24. [Google Scholar]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Maximising recovery of phenolic compounds and antioxidant properties from banana peel using microwave assisted extraction and water. J. Food Sci. Technol. 2019, 56, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-P.; Liu, R.L.; Cui, H.Y.; Zhang, Z.Q. Microwave-assisted extraction and LC/MS analysis of phenolic antioxidants in sweet apricot (Prunus armeniaca L.) kernel skins. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 2182–2195. [Google Scholar] [CrossRef]
- Chaisamlitpol, S.; Hiranvarachat, B.; Srichumpoung, J.; Devahastin, S.; Chiewchan, N. Bioactive compositions of extracts from cabbage outer leaves as affected by drying pretreatment prior to microwave-assisted extraction. Sep. Purif. Technol. 2014, 136, 177–183. [Google Scholar] [CrossRef]
- Tabaraki, R.; Shahrbanoo, R. Comparison between conventional and ultrasound-assisted extractions of natural antioxidants from walnut green husk. Korean J. Chem. Eng. 2014, 31, 676–683. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Effect of ultrasounds and high pressure homogenization on the extraction of antioxidant polyphenols from lettuce waste. Innov. Food Sci. Emerg. Technol. 2018, 50, 11–19. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Pedreira Nogueira, J.; Narain, N. Comparison and optimization of conventional and ultrasound assisted extraction for bioactive compounds and antioxidant activity from agro-industrial acerola (Malpighia emarginata DC) residue. LWT-Food Sci. Technol. 2017, 85, 158–169. [Google Scholar] [CrossRef]
- Liang, J.L.; Yeow, C.C.; Teo, K.C.; Gnanaraj, C.; Chang, Y.P. Valorizing cabbage (Brassica oleracea L. var. capitata) and capsicum (Capsicum annuum L.) wastes: In vitro health-promoting activities. J. Food Sci. Technol. 2019, 56, 4696–4704. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted extraction for recovery of polyphenolic antioxidants from ripe mango (Mangifera indica L.) peel using lactic acid/sodium acetate deep eutectic mixtures. Food Sci. Technol. Int. 2020, 26, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of vegetable fresh-processing residues as functional powdered ingredients. A review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Deepa, P.; Sujathamma, P. Information source and consultancy pattern of different sericultural technologies at field level and technology adoption in the semi-arid conditions of Chittoor District in Andhra Pradesh. Indian J. Seric. 2007, 46, 86–88. [Google Scholar]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Phenolic compound contents in edible parts of broccoli inflorescences after domestic cooking. J. Sci. Food Agric. 2003, 83, 1511–1516. [Google Scholar] [CrossRef]
- Ho, C.-T. Phenolic Compounds in Food: An Overview; ACS Publications: Washington, DC, USA, 1992; pp. 2–7. [Google Scholar]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef]
- Polat, S.; Guclu, G.; Kelebek, H.; Keskin, M.; Selli, S. Comparative elucidation of colour, volatile and phenolic profiles of black carrot (Daucus carota L.) pomace and powders prepared by five different drying methods. Food Chem. 2022, 369, 130941. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Brito, T.B.N.; Ferreira, M.S.L.; Fai, A.E.C. Utilization of agricultural by-products: Bioactive properties and technological applications. Food Rev. Int. 2020, 38, 1305–1329. [Google Scholar] [CrossRef]
- Urbina, L.; Eceiza, A.; Gabilondo, N.; Corcuera, M.Á.; Retegi, A. Valorization of apple waste for active packaging: Multicomponent polyhydroxyalkanoate coated nanopapers with improved hydrophobicity and antioxidant capacity. Food Packag. Shelf Life 2019, 21, 100356. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef] [Green Version]
- Ribarova, F.; Atanassova, M.; Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Metal 2005, 40, 255–260. [Google Scholar]
- Kays, S.E.; Barton, F.E.; Windham, W.R.; Himmelsbach, D.S. Prediction of Total Dietary Fibre by Near-Infrared Reflectance Spectroscopy in Cereal Products Containing High Sugar and Crystalline Sugar. J. Agric. Food Chem. 1997, 45, 3944–3951. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Saltveit, M.E. Physical and physiological changes in minimally processed fruits and vegetables. In Proceedings-Phytochemical Society of Europe; Oxford University Press Inc.: Oxford, UK, 1996; Volume 41. [Google Scholar]
- Sáyago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goñi, I. Dietary fibre content and associated antioxidant compounds in roselle flower (Hibiscus sabdariffa L.) beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef]
- Reyes, L.F.; Villarreal, J.E.; Cisneros-Zevallos, L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Özgen, M.; Torun, A.A.; Ercisli, S.; Serçe, S. Changes in chemical composition, antioxidant activities and total phenolic content of Arbutus andrachne fruit at different maturation stages. Ital. J. Food Sci. 2009, 21, 65–72. [Google Scholar]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Bianchi, F.; Tolve, R.; Rainero, G.; Bordiga, M.; Brennan, C.S.; Simonato, B. Technological, nutritional and sensory properties of pasta fortified with agro-industrial by-products: A review. Int. J. Food Sci. Technol. 2021, 56, 4356–4366. [Google Scholar] [CrossRef]
- Göksel Saraç, M.; Dogan, M. Incorporation of dietary fibre concentrates from fruit and vegetable wastes in butter: Effects on physicochemical, textural, and sensory properties. Eur. Food Res. Technol. 2016, 242, 1331–1342. [Google Scholar] [CrossRef]
- Kumari, S.; Grewal, R.B. Nutritional evaluation and utilization of carrot pomace powder for preparation of high fibre biscuits. J. Food Sci. Technol. 2007, 44, 56–58. [Google Scholar]
- Wirkijowska, A.; Zarzycki, P.; Sobota, A.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. The possibility of using by-products from the flaxseed industry for functional bread production. LWT 2020, 118, 108860. [Google Scholar] [CrossRef]
- Bielig, H.J.; Fischer-Ayloff-Cook, K.P. Bedeutung der Rheologie in der Lebensmitteltechnologie. Z. Lebensm. Unters. Forsch. 1984, 179, 364–370. [Google Scholar] [CrossRef]
- Wang, H.J.; Thomas, R.L. Direct use of apple pomace in bakery products. J. Food Sci. 1989, 54, 618–620. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Santos, M.C.; Moro, T.; Basto, G.J.; Andrade, R.; Gonçalves, É.C. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, Y.; Chao, H.; Zhang, M.; Zhou, Y.; Wang, M. Effects of celery powder on wheat dough properties and textural, antioxidant and starch digestibility properties of bread. J. Food Sci. Technol. 2020, 57, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.W.; Cobb, J.B.; Carson, C.S. The Green National Product: A Proposed Index of Sustainable Economic Welfare; University Press of America: Lanham, MD, USA, 1994; p. 343. [Google Scholar]
- Younis, K.; Ahmad, S. Waste utilization of apple pomace as a source of functional ingredient in buffalo meat sausage. Cogent Food Agric. 2015, 1, 1119397. [Google Scholar] [CrossRef]
- Thebaudin, J.Y.; Lefebvre, A.C.; Harrington, M.; Bourgeois, C.M. Dietary fibres: Nutritional and technological interest. Trends Food Sci. Technol. 1997, 8, 41–48. [Google Scholar] [CrossRef]
- Crizel, T.D.M.; Rios, A.D.O.; Thys, R.C.S.; Flôres, S.H. Effects of orange by-product fibre incorporation on the functional and technological properties of pasta. Food Sci. Technol. 2015, 35, 546–551. [Google Scholar] [CrossRef]
- Fissore, E.N.; Rojas, A.M.; Gerschenson, L.N.; Williams, P.A. Butternut and beetroot pectins: Characterization and functional properties. Food Hydrocoll. 2013, 31, 172–182. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Chauhan, S.; Rajput, H. Production of gluten free and high fibre cookies using beetrot waste powder and wheat flour husk. The Pharma Innov. J. 2018, 7, 556. [Google Scholar]
- Pilnik, W.; Rombouts, F.M. Polysaccharides and food processing. Carbohydr. Res. 1985, 142, 93–105. [Google Scholar] [CrossRef]


| Abbreviations | U.m. | Oranges | Celery Root | Carrots | Beetroot | Apples | U.m. | Oranges | Celery Root | Carrots | Beetroot | Apples | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial probe mass | kg | 5.030 ± 0.25 | 5.013 ± 0.25 | 5.036 ± 0.25 | 5.020 ± 0.25 | 5.065 ± 0.25 | Initial probe mass | % | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| Sample Preparation | ||||||||||||||
| Cleaned and peeled probe | kg | 3.56 ± 0.14 | 3.838 ± 0.12 | 3.785 ± 0.15 | 4.359 ± 0.04 | 4.0 ± 0.16 | Cleaned and peeled probe | % | 70.78 | 76.56 | 75.16 | 86.84 | 78.97 | |
| Husks | kg | 1.445 ± 0.03 | 0.936 ± 0.037 | 1.215 ± 0.02 | 0.614 ± 0.025 | 1.055 ± 0.04 | Husks | % | 28.73 | 18.67 | 24.13 | 12.22 | 20.83 | |
| Impurities | kg | 0.025 ± 0.001 | 0.239 ± 0.007 | 0.036 ± 0.002 | 0.047 ± 0.001 | 0.01 ± 0.001 | Impurities | % | 0.5 | 4.77 | 0.71 | 0.94 | 0.2 | |
| Sample to be analyzed | kg | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | Sample to be analyzed | % | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |
| Sample processed as fresh | kg | 3.55 ± 0.14 | 3.828 ± 0.19 | 3.775 ± 0.15 | 4.349 ± 0.17 | 3.99 ± 0.20 | Sample processed as fresh | % | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| Squeezing process | ||||||||||||||
| Resulting juice (fresh) | FJ | kg | 2.1120.11 | 1.2910.01 | 1.5010.06 | 2.4940.10 | 2.4450.12 | Resulting juice (fresh) | % | 59.48 | 33.72 | 39.77 | 57.35 | 61.28 |
| Fresh pulp | kg | 1.438 ± 0.01 | 2.537 ± 0.05 | 2.274 ± 0.09 | 1.855 ± 0.07 | 1.545 ± 0.08 | Fresh pulp | % | 40.52 | 66.28 | 60.23 | 42.65 | 38.72 | |
| Sample to be analyzed | kg | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | Sample to be analyzed | % | 0.28 | 0.26 | 0.26 | 0.23 | 0.25 | |
| Marc to be pressed | kg | 1 | 1 | 1 | 1 | 1 | Marc to be pressed | % | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| Pressing process | ||||||||||||||
| Press juice | PJ | kg | 0.33 ± 0.01 | 0.462 ± 0.02 | 0.514 ± 0.02 | 0.447 ± 0.02 | 0.04 ± 0.02 | Press juice | % | 33.00 | 46.20 | 51.40 | 44.70 | 3.98 |
| Pressed pulp | PP | kg | 0.546 ± 0.03 | 0.427 ± 0.02 | 0.404 ± 0.02 | 0.469 ± 0.02 | 0.82 ± 0.04 | Pressed Marc | % | 54.60 | 42.70 | 40.40 | 46.90 | 81.96 |
| Process losses | kg | 0.124 ± 0.006 | 0.111 ± 0.005 | 0.082 ± 0.004 | 0.084 ± 0.004 | 0.82 ± 0.04 | Process losses | % | 12.40 | 11.10 | 8.20 | 8.40 | 14.06 | |
| Pressing Time | ′, ″ | 23′15″ ±60″ | 14′11″ ±39″ | 6′40″ ±17″ | 6′30″ ±15″ | 6′4″ ±16″ | Pressing Time | % | 23′15″ | 14′11″ | 6′40″ | 6′30″ | 6′4″ | |
| Raw Material | Waste Form | Domain | Benefits | Ref |
|---|---|---|---|---|
| Carrots | Pomace (FP) powder 2–10% w/w | Pasta | Better cooking loss and firmness | [58] |
| Carrots | Pomace (FP) powder 3, 6, 9% w/w | Biscuits | Gluten-free rice crackers with higher dietary fibre and minerals | [62] |
| Carrots | Pomace (FP) powder 10, 20% w/w | Biscuits | Biscuits with a reduced glycemic index | [60] |
| Carrots | Pomace (FP) powder 3, 6, 9% w/w | Dairy | Yogurt with increased gelatinization pH and shortened fermentation time | [63] |
| Carrots | Pomace (FP) powder 3–5% w/w | Dairy | Butter with enhanced physicochemical, textural, and sensory properties | [63] |
| Carrots | Pomace (FP) powder 10, 20, 30% w/w | Biscuits | High fibre biscuits with no negative sensory characteristics | [62] |
| Carrots | Pomace (FP) powder 6.5% carrot | Beverages | Isotonic beverage | [42] |
| Orange | 5.5% orange | Beverages | Isotonic beverage | [42] |
| Orange | powder 25–30% w/w | Biscuits | Biscuits rich in fibre and minerals. | [42] |
| Orange/ carrots | powder 75% w/w | Snacks | Cereal bars rich in fibre and minerals. | [42] |
| Orange | Pomace (FP) powder 3–5% w/w | Dairy | Butter with enhanced physicochemical, textural, and sensory properties | [63] |
| Celery root | Pomace (FP) powder 3–5% w/w | Dairy | Butter with enhanced color, textural, and sensory properties and higher dietary fibre | [63] |
| Celery root | Pomace (FP) powder 1–5% w/w | Bakery | Dough with increased water absorption and significant improvement of its antioxidant properties | [64] |
| Beetroot | Pomace (FP) powder 5% w/w | Bakery | Gluten-free and high fibre cookies | [65] |
| Beetroot | Pomace (FP) powder 2%, 4%, 6% and 8% w/w | Pasta | Pasta with higher dietary fibre content | [66] |
| Apple | Pomace (FP) powder up to 20% w/w | Confectionary | Bakery products with reduced energy content and increased fibre content | [67] |
| Apple | Pomace (FP) powder 5–10% w/w | Bakery | Bread (wheat, rye and mixed) with reduced energy content and manufacturing costs | [68,69] |
| Apple | Pomace (FP) powder 50% w/w | Bakery | Wheat bran muffin with better sensorial characteristics. | [68,70] |
| Apple | Pomace (FP) flakes 40% w/w | Confectionary | Cookies with better sensorial characteristics. | [71] |
| Apple | Pomace (FP) powder 50% w/w | Bakery | Pie fillings and oatmeal cookies with reduced manufacturing costs | [72] |
| Apple | Pomace (FP) powder 2, 4, 6, and 8% w/w | Meat | Buffalo Sausage with higher fibre content | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, M.V.; Fărcaş, A.C.; Medeleanu, M.; Salanţă, L.C.; Borşa, A. A Sustainable Approach for the Development of Innovative Products from Fruit and Vegetable By-Products. Sustainability 2022, 14, 10862. https://doi.org/10.3390/su141710862
Muntean MV, Fărcaş AC, Medeleanu M, Salanţă LC, Borşa A. A Sustainable Approach for the Development of Innovative Products from Fruit and Vegetable By-Products. Sustainability. 2022; 14(17):10862. https://doi.org/10.3390/su141710862
Chicago/Turabian StyleMuntean, Mircea Valentin, Anca Corina Fărcaş, Mădălina Medeleanu, Liana Claudia Salanţă, and Andrei Borşa. 2022. "A Sustainable Approach for the Development of Innovative Products from Fruit and Vegetable By-Products" Sustainability 14, no. 17: 10862. https://doi.org/10.3390/su141710862
APA StyleMuntean, M. V., Fărcaş, A. C., Medeleanu, M., Salanţă, L. C., & Borşa, A. (2022). A Sustainable Approach for the Development of Innovative Products from Fruit and Vegetable By-Products. Sustainability, 14(17), 10862. https://doi.org/10.3390/su141710862











