Effects of Bentonite Addition on the Speciation and Mobility of Cu and Ni in Soils from Old Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Separation and Measurements of Heavy Metal Fractions
2.3. Statistical Analysis
3. Results
3.1. Effects of Bentonite on Soil Physicochemical Properties
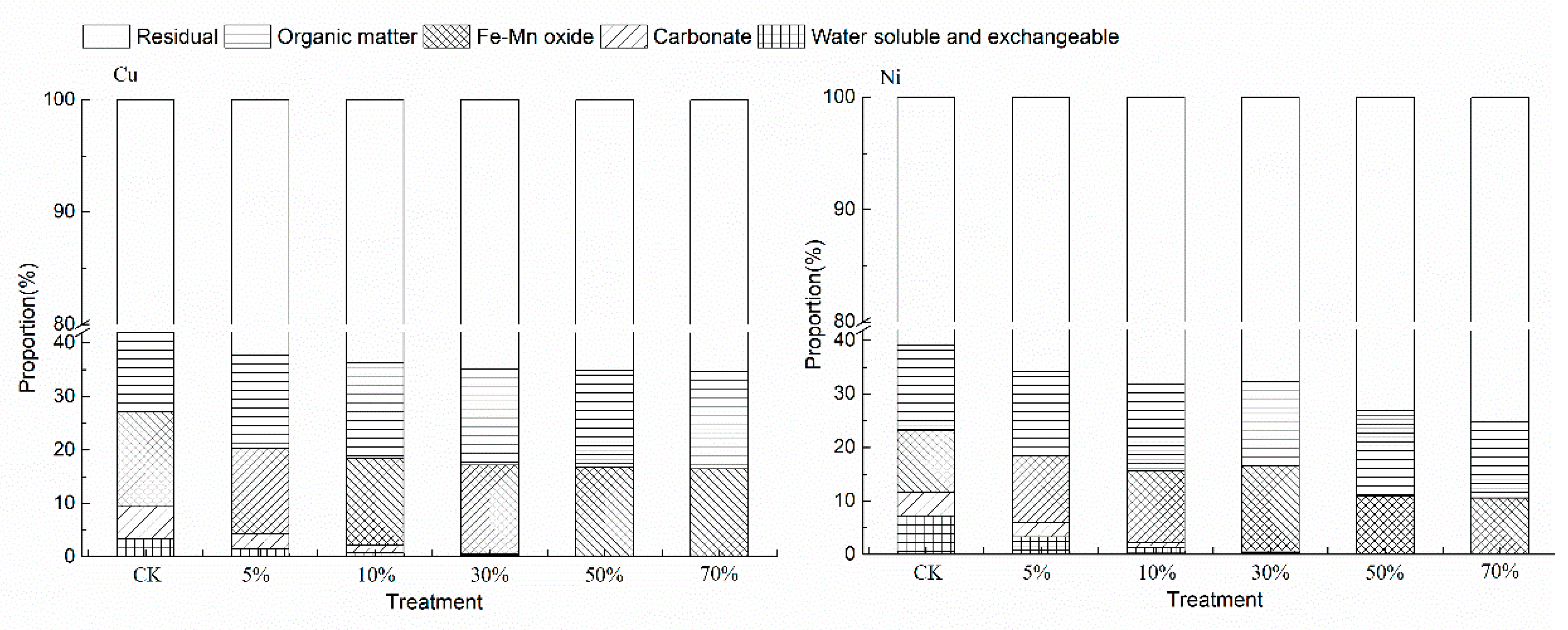
3.2. Effects of Bentonite on the Form of Heavy Metals in Soil
3.3. Effects of Bentonite on Mobility of Heavy Metals in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, Y.K.; Kim, J.W.; Lee, S.P.; Yang, J.E.; Kim, S.C. Heavy metal remediation in soil with chemical amendments and its impact on activity of antioxidant enzymes in Lettuce (Lactuca sativa) and soil enzymes. Appl. Biol. Chem. 2020, 63, 42. [Google Scholar] [CrossRef]
- González Henao, S.; Ghneim-Herrera, T. Heavy Metals in Soils and the Remediation Potential of Bacteria Associated with the Plant Microbiome. Front. Environ. Sci 2021, 9, 604216. [Google Scholar] [CrossRef]
- Xiao, P.; Zhou, Y.; Li, X.; Xu, J.; Zhao, C. Assessment of Heavy Metals in Agricultural Land: A Literature Review Based on Bibliometric Analysis. Sustainability 2021, 13, 4559. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ma, Z.W.; Kuijp, T.J.V.; Yuan, Z.W.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Liao, X.Y.; Chen, T.B.; Wu, B.; Yan, X.L.; Nie, C.J.; Xie, H.; Zhai, L.M.; Xiao, X.Y. Mining urban soil pollution: Concentrations and patterns of heavy metals in the soils of Jinchang. China. Chin. J. Geogr. Res. 2006, 25, 843–852. (In Chinese) [Google Scholar]
- Shang, W.; Tang, Q.; Zheng, L.; Cheng, H. Chemical forms of heavy metals in agricultural soils affected by coal mining in the Linhuan subsidence of Huaibei Coalfield, Anhui Province, China. Environ. Sci. Pollut. Res. 2016, 23, 23683–23693. [Google Scholar] [CrossRef]
- Munir, N.; Jahangeer, M.; Bouyahya, A.; El Omari, N.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Ali Shah, S.M.; et al. Heavy Metal Contamination of Natural Foods Is a Serious Health Issue: A Review. Sustainability 2022, 14, 161. [Google Scholar] [CrossRef]
- Hong-gui, D.; Teng-feng, G.; Ming-Hui, L.; Xu, D. Comprehensive assessment model on heavy metal pollution in soil. Int. J. Electrochem. Sci. 2012, 7, 5286–5296. [Google Scholar]
- Lu, N.; Wei, Y.; Zhang, Z.; Li, Y.; Li, G.; Han, J. The Response of Cd Chemical Fractions to Moisture Conditions and Incubation Time in Arable Land Soil. Sustainability 2022, 14, 6270. [Google Scholar] [CrossRef]
- Sun, Y.B.; Li, Y.; Xu, Y.M.; Liang, X.F.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105, 200–206. [Google Scholar] [CrossRef]
- Sun, Y.B.; Sun, G.H.; Xu, Y.M.; Liu, W.F.; Liang, X.F.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.P.; Campbell, P.G.C.; Bisson, M.X. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Chen, Z.; He, M.; Sakurai, K.; Kang, Y.; Iwasaki, K. Concentrations and chemical forms of heavy metals in urban soils of Shanghai, China. Soil Sci. Plant Nutr. 2007, 53, 517–529. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M. Miscellaneous additives can enhance plant uptake and affect geochemical fractions of copper in a heavily polluted riparian grassland soil. Ecotoxicol. Environ. Saf. 2015, 119, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.; Ng, H.S.; Munawaroh, H.S.H.; Karamang, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Bian, R.J.; Li, L.Q.; Bao, D.D.; Zheng, J.W.; Zhang, X.H.; Zheng, J.F.; Liu, X.Y.; Cheng, K.; Pan, G.X. Cd immobilization in a contaminated rice paddy by inorganic stabilizers of calcium hydroxide and silicon slag and by organic stabilizer of biochar. Environ. Sci. Pollut. Res. 2016, 23, 10028–10036. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, X.F.; Xu, Y.M.; Qin, X.; Huang, Q.Q.; Wang, L.; Sun, Y.B. Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Ling, W.; Shen, Q.; Gao, Y.; Gu, X.; Yang, Z. Use of bentonite to control the release of copper from contaminated soils. Soil Res. 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Lin, H.; Jin, X.N.; Dong, Y.B.; Luo, M.K.; Zhao, Y.M. Effects of Bentonite on Chemical Forms and Bioavailability of Heavy Metals in Different Types of Farmland Soils. Chin. J. Environ. Sci. 2019, 40, 945–952. (In Chinese) [Google Scholar]
- Naseem, R.; Tahir, S.S. Removal of Pb (II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res. 2001, 35, 3982–3986. [Google Scholar] [CrossRef]
- Li, X.H.; Tang, Z.L.; Chu, F.Y.; Yang, L.Y. Characteristics of distribution and chemical speciation of heavy metals in environmental mediums around Jinchang mining city, Northwest China. Environ. Earth Sci. 2011, 64, 1667–1674. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, P.; Hu, Y.G.; Zhao, Y. Vegetation and soil restoration in refuse dumps from open pit coal mines. Ecol. Eng. 2016, 94, 638–646. [Google Scholar]
- Banat, K.M.; Howari, F.M.; Al-Hamad, A.A. Heavy metals in urban soils of central Jordan: Should we worry about their environmental risks? Environ. Res. 2005, 97, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process. Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Alexander, J.A.; Ahmad Zaini, M.A.; Surajudeen, A.; Aliyu, E.N.U.; Omeiza, A.U. Surface modification of low-cost bentonite adsorbents—A review. Part. Sci. Technol. 2019, 37, 538–549. [Google Scholar] [CrossRef]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Yi, N.; Wu, Y.; Fan, L.; Hu, S. Remediating Cd-contaminated soils using natural and chitosan-introduced zeolite, bentonite, and activated carbon. Pol. J. Environ. Stud. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.Q.; Wang, S.A. Distribution of Cadmium and Lead Forms and Its Affecting Factors in Soils of Hebei Province. Acta Pedol. Sin. 2003, 40, 393–401. (In Chinese) [Google Scholar]
- Wang, D.Y.; Qing, C.L.; Guo, T.Y.; Guo, Y.J. Effects of humic acid on transport and transformation of mercury in soil-plant systems. Water Air Soil Pollut. 1997, 95, 35–43. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Shen, Q.R.; Xie, X.J.; Sun, Z.H. Effect of Pig Manure and Rice Straw on Biological Activity of Cd2 contaminated soil. Chin. J. Appl. Ecol. 2003, 14, 1997–2000. (In Chinese) [Google Scholar]
- Jiang, Y.F.; Yuan, J.M.; Lu, Z.Y.; Wang, A.P.; Chen, H. The Effect of Humic Acid on Species of Cu, Cd, Pb, Zn in Sewage Farm. J. Northwest Norm. Univ. 2005, 41, 42–46. (In Chinese) [Google Scholar]
- McBride, M.B. Reactions Controlling Heavy Metal Solubility in Soil. In Advances in Soil Science; Springer: New York, NY, USA, 1989; Volume 10, pp. 1–56. [Google Scholar]

| Components | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Na2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 59.8 | 19.6 | 6.0 | 2.3 | 1.4 | 1.3 | 0.7 | 8.9 |
| Soil Particle Size Distribution (%) | Available Nutrients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coarse (>0.25 mm) | Fine (0.25–0.05 mm) | Clay + Silt (<0.05 mm) | Bulk Density (g/cm3) | pH | SOM (mg/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | Total Cu (mg/kg) | Total Ni (mg/kg) |
| 44.5 | 23.7 | 31.8 | 1.2 | 7.2 | 0.9 | 2.4 | 0.6 | 12.4 | 3122 | 1886 |
| Treatment | pH | SOM % | AN mg/kg | AP mg/kg | AK mg/kg |
|---|---|---|---|---|---|
| CK | 7.2 | 0.9 | 2.4 | 0.6 | 12.4 |
| 5% | 7.3 | 0.8 | 2.0 | 0.5 | 12.7 |
| 10% | 7.9 | 0.8 | 1.9 | 0.5 | 12.9 |
| 30% | 8.1 | 0.7 | 1.8 | 0.4 | 13.2 |
| 50% | 9.0 | 0.6 | 1.5 | 0.3 | 14.4 |
| 70% | 9.1 | 0.5 | 1.4 | 0.2 | 15.8 |
| Treatment | Total Amount | Exchangeable | Bound to Carbonates | Bound to Fe-Mn Oxides | Bound to Organic Matter | Residual | Absorptivity (%) |
|---|---|---|---|---|---|---|---|
| CK | 480 | 16 | 28 | 85 | 95 | 252 | 25 |
| 5% | 628 | 9 | 17 | 97 | 106 | 378 | 32 |
| 10% | 911 | 7 | 13 | 147 | 161 | 577 | 47 |
| 30% | 1305 | 3 | 4 | 209 | 229 | 824 | 66 |
| 50% | 1683 | 1 | 2 | 271 | 297 | 1068 | 86 |
| 70% | 1818 | 1 | 1 | 289 | 320 | 1080 | 89 |
| Treatment | Total Amount | Exchangeable | Bound to Carbonates | Bound to Fe-Mn Oxides | Bound to Organic Matter | Residual | Absorptivity (%) |
|---|---|---|---|---|---|---|---|
| CK | 1067 | 41 | 68 | 109 | 151 | 576 | 30 |
| 5% | 1089 | 29 | 35 | 133 | 169 | 706 | 34 |
| 10% | 1574 | 15 | 19 | 207 | 253 | 1057 | 49 |
| 30% | 2141 | 8 | 7 | 251 | 346 | 1492 | 66 |
| 50% | 2893 | 2 | 3 | 301 | 448 | 2052 | 89 |
| 70% | 2995 | 1 | 1 | 307 | 418 | 2104 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, X. Effects of Bentonite Addition on the Speciation and Mobility of Cu and Ni in Soils from Old Mine Tailings. Sustainability 2022, 14, 10878. https://doi.org/10.3390/su141710878
Gao Y, Li X. Effects of Bentonite Addition on the Speciation and Mobility of Cu and Ni in Soils from Old Mine Tailings. Sustainability. 2022; 14(17):10878. https://doi.org/10.3390/su141710878
Chicago/Turabian StyleGao, Yongping, and Xiaojun Li. 2022. "Effects of Bentonite Addition on the Speciation and Mobility of Cu and Ni in Soils from Old Mine Tailings" Sustainability 14, no. 17: 10878. https://doi.org/10.3390/su141710878
APA StyleGao, Y., & Li, X. (2022). Effects of Bentonite Addition on the Speciation and Mobility of Cu and Ni in Soils from Old Mine Tailings. Sustainability, 14(17), 10878. https://doi.org/10.3390/su141710878








