Abstract
It is critical for the iron and steel industry to achieve green transformation and development by effectively utilizing abundant biomass resources in blast furnace ironmaking. In this paper, four types of typical biomass were carbonized and upgraded using the hydrothermal carbonization (HTC) method, and the metallurgical performance of the prepared hydrochar for blast furnace injection was systematically tested. The results show that HTC treatment could remove volatile matter and dissolved mineral elements in biomass so that the hydrochar had the characteristics of high fixed carbon and low ash and alkali metal content. Moreover, the hydrochar had good grindability and excellent combustion performance, which meet the requirements of blast furnace injection. Finally, the metallurgical performance of blended coal and wood chip hydrochar was examined. It was observed that when the ratio of hydrochar was less than 15%, it would not affect the blast furnace injection, and the potential safety hazard caused by the explosive hydrochar could be resolved by mixing hydrochar with anthracite. The application of hydrochar in blast furnace injection could not only alleviate the current energy shortage situation, but also be of great significance to realize the “carbon peak” of the steel industry.
1. Introduction
With the increasing emissions of carbon dioxide and greenhouse gases, climate change has gradually become a major global problem faced by mankind. Following the Paris Conference on Climate Change in 2015, China put forward for the first time the medium- and long-term strategic goals of achieving a “carbon peak” by 2030 and “carbon neutrality” by 2060 at important meetings such as the General Debate of the United Nations General Assembly in 2020, which aroused widespread concern in the international community [1,2]. China is a big steel producer. In 2021, China’s crude steel output reached 1.033 billion tons, accounting for 54.0% of the world’s total crude steel output. Along with the high output, there are also higher energy consumption and pollutant emissions. How to reduce steel energy consumption and carbon emissions has become a key issue in realizing peak carbon dioxide emissions and carbon neutrality in the steel industry. In the iron and steel production process, the energy consumption and pollutant emissions of the pre-iron system account for more than 50% of the total energy consumption of iron and steel, which is the focus of energy conservation and emissions reduction in the iron and steel industry. China is a big agricultural country with abundant biomass resources, producing 1.6 billion tons of agricultural and forestry wastes every year, but the actual utilization rate is less than 33%, resulting in serious waste of resources [3,4]. Compared with traditional fossil fuels, biomass resources are renewable and carbon neutral. If abundant biomass resources are applied to blast furnace ironmaking technology, on the one hand, it can effectively reduce the dependence of traditional blast furnace ironmaking technology on fossil energy and reduce carbon dioxide emissions in steel production, and on the other hand, it can also consume a large amount of agricultural and forestry wastes, reduce the environmental pollution caused by open burning, and improve the utilization efficiency and value of agricultural and forestry wastes [5,6,7].
At present, there are many problems in the application of biomass in blast furnace ironmaking. Biomass contains a large amount of lignin and cellulose, which is difficult to crush. Moreover, due to the application of a large amount of fertilizer in the growth process of biomass, the content of alkali metal elements in biomass is too high, which limits the application of biomass in blast furnace ironmaking [1,8], so it is necessary to carbonize and upgrade biomass. Pyrolysis carbonization is the main way to improve the quality of biomass at present, which is to heat the biomass to a certain temperature under the condition of an isolated air or oxygen-deficient atmosphere so as to decompose the biomass and produce pyrolysis gas, bio-oil, and biochar, thus realizing biomass resource utilization and clean utilization [9,10]. However, pyrolysis carbonization cannot remove alkali metal elements from biomass, which will lead to alkali metal enrichment in biochar and affect blast furnace smelting. This is the key factor that limits the application of biochar in blast furnace ironmaking. Therefore, a proper carbonization and upgrading method is needed to treat biomass, which can improve grindability and remove alkali metal elements, such as potassium and sodium.
Hydrothermal carbonization (HTC) is a new technology to improve the quality of biomass. HTC makes use of the special properties of subcritical water to make organic matter undergo a series of complex chemical transformations, such as dehydration, deacidification, demethanization, condensation, aromatization, and physical formation, and finally forms a clean and high-grade solid fuel (hydrochar) [11]. Compared with pyrolysis carbonization, HTC has mild reaction conditions (180–350 °C), no need to dry raw materials, low energy consumption, and fewer pollutant emissions [12]. The application of HTC in biomass can be traced back to 1913. German scientist Bergius first used cellulose as raw material and simulated the natural coalification process by HTC [13]. In the following decades, researchers paid more attention to the preparation of specific gaseous and liquid products by HTC. In the 21st century, with the increasingly prominent problems of energy shortage and environmental pollution in the world, researchers began to focus on the application of hydrochar, a solid product of HTC. Mäkelä et al. [14] found that the organic and inorganic components in biomass can be removed by HTC, which greatly increased the fixed carbon content of the sample. Liu et al. [15] studied the influence of carbonization time on hydrochar and found that the change in reaction time has a very significant influence on the internal structure and combustion characteristics of hydrochar. Wang et al. [16] conducted experiments on corn stalks at different carbonization temperatures and times. The results showed that HTC can improve the properties of hydrochar, making it similar to coal in industrial analysis and elemental analysis. With the increase in carbonization temperature and time, the calorific value of hydrochar increased significantly.
At present, a lot of researchers are focusing on the mechanism of the HTC process in the laboratory, but there is little research on the application of biomass hydrochar in blast furnace ironmaking. In this paper, four types of typical agricultural and forestry wastes in the Beijing–Tianjin–Hebei region, such as wood chips (WC), garden trimmings (GW), maize straw (MS), and wheat stalks (WS), were used to carry out HTC experiments, and the metallurgical performance of hydrochar were analyzed. The above research provides basic data for the research and development of biomass for blast furnace injection technology.
2. Materials and Methods
2.1. Raw Material Preparation
The biomass samples used in this experiment were all obtained from the Beijing–Tianjin–Hebei region, which were four types of typical agricultural and forestry wastes: wood chips (WC), garden trimmings (GW), maize straw (MS), and wheat stalks (WS). Meanwhile, Shenhua bituminous coal (BC) and anthracite (AC) used for blast furnace injection in a steel company were selected as the comparison. First, the collected biomass samples were cut into small pieces less than 2 cm, and then they were placed in an oven at 105 °C to fully dry for 12 h.
2.2. HTC Experiment
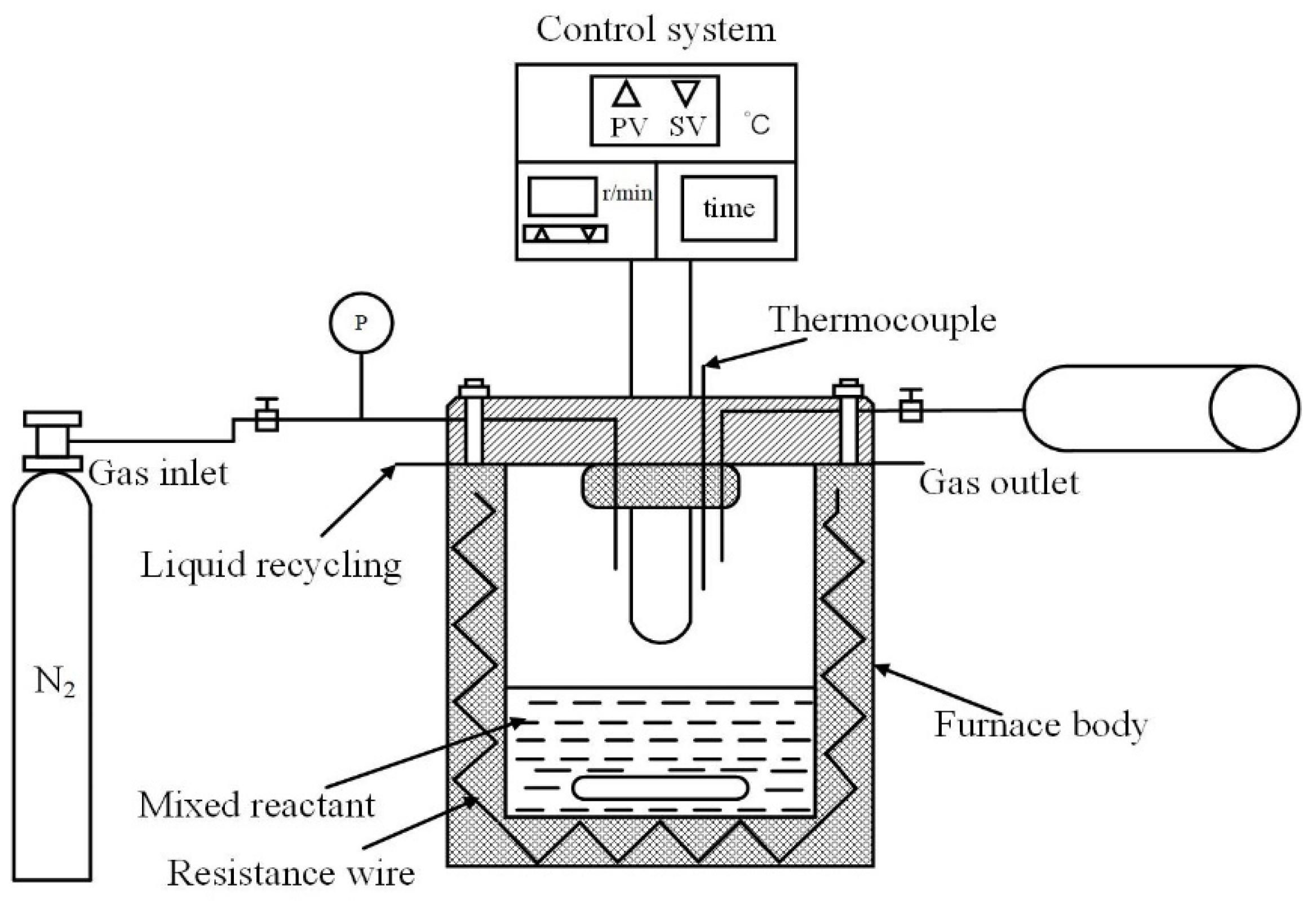
The HTC biomass experiment was carried out in a 250 mL high-pressure reactor (HT-250J0, HTLAB, Beijing, China). The maximum allowable pressure was 30 MPa and the maximum allowable temperature was 350 °C. The schematic diagram is shown in Figure 1. In each experiment, a 30 g biomass sample was mixed with 90 mL deionized water to ensure a solid–liquid ratio of 1:3. Before each experiment, nitrogen gas (N2) with a purity of 99.9% was continuously injected for five minutes to ensure that the reaction process was in an oxygen-free environment. In the HTC experiment, the stirring speed of the reaction kettle was set at 300 rpm/min, the reaction temperature was set at 240 °C, and the reaction time was kept at 60 min. After the reaction, the hydrochar was filtered out from the liquid-phase product and dried in an oven at 105 °C for 12 h, which was labeled “XX-HTC.” “XX” is the biomass sample for short. The dried hydrochar was crushed and sieved to below 0.074 μm and placed in a sealed bag for subsequent testing.
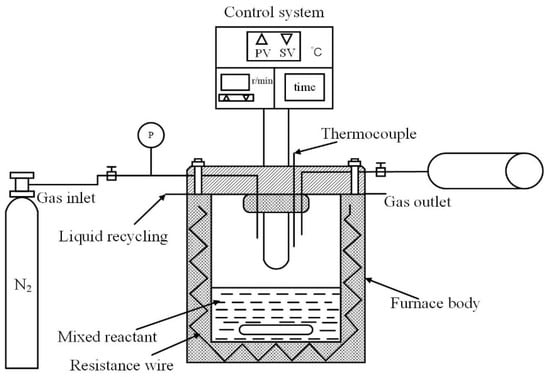
Figure 1.
Lab-scale autoclave reactor.
2.3. Basic Performance Analysis
The metallurgical performance of a pulverized coal injection into a blast furnace included proximate analysis, ultimate analysis, calorific value, and grindability. In addition, with the deepening of the knowledge of alkali metal elements such as potassium and sodium to blast furnace smelting, the alkali metal content has become an important evaluation index. Proximate and ultimate analyses of the different samples were carried out in accordance with the Industrial Methods of Analysis for Coal GB/T212 and the Method for the Determination of Carbon and Hydrogen in Coal GB-T476, respectively, and the oxygen content was calculated using the difference method. The calculation equations were as follows (1):
O, C, H, N, S, and Ash in Equation (1) represent oxygen, carbon, hydrogen, nitrogen, sulfur, and ash content, respectively.
The alkali metal content of biomass samples and their hydrochar was measured by inductively coupled plasma atomic emission spectrometry (ICP-OES). The grindability of hydrochar was measured by a Hargreaves grindometer. Under the conditions specified in the Determination Method of Coal Grindability Index GB-2565 (Hardgrove method), the prepared coal samples were ground, sieved, and weighed, and the Hardgrove Grindability Index was found from the calibration chart drawn by standard coal samples. Figure 2 is a picture of the Hargreaves grindometer. The greater the index, the easier it is to grind [17].

Figure 2.
The Hargreaves grindometer.
The higher heating value (HHV), mass yield, and energy yield of the samples were calculated using the following equations [18,19]:
2.4. Technological Property Analysis
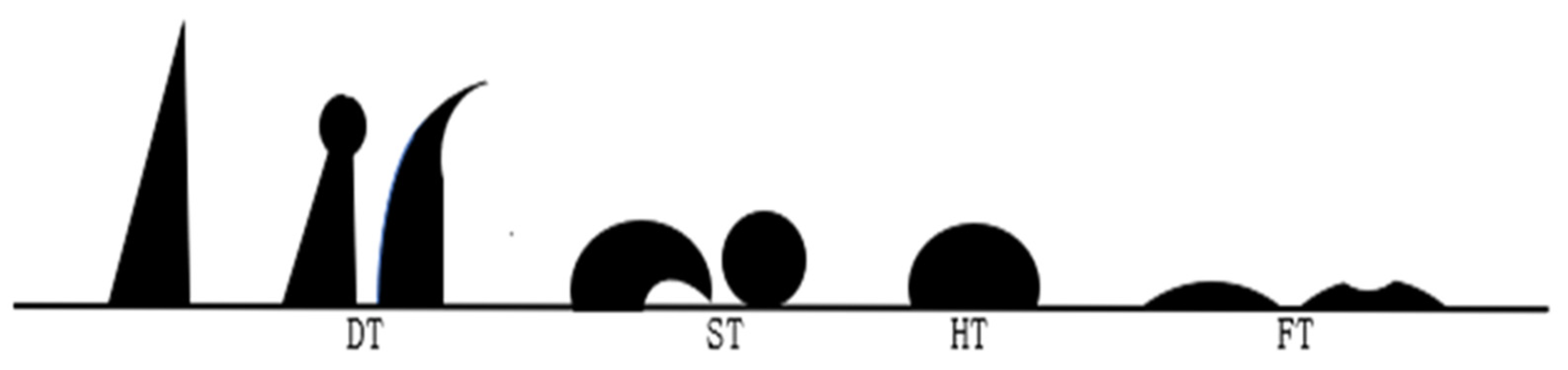
The indexes for evaluating the technological performance of blast furnace fuel injection included ignition point, explosiveness, combustibility, fluidity, spouting property, ash composition, and ash fusion characteristics. The methods used to determine ignition and explosivity are known as the solid oxidant method and the long tube test apparatus, respectively, and the national standard was the Method for Determination of Ignition Temperature of Coal GB/T 8511-2001. An X-ray fluorescence spectrum analyzer (XRF) was used to measure the ash composition of the samples, and the Determination Method of Ash Fusibility of Pulverized Coal GB/T219-2008 national standard was used to measure the ash fusion characteristics of hydrochar samples, including deformation temperature (DT), softening temperature (ST), hemisphere temperature (HT), and flow temperature (FT). The specific shapes of ash cones at each characteristic temperature are shown in Figure 3 [20].
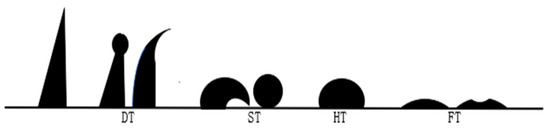
Figure 3.
Schematic diagram of different characteristic temperatures of ash fusion.
Thermogravimetric experimentation was one of the important means to study the combustibility of pulverized coal [21,22,23]. The combustibility was analyzed and studied by thermogravimetric equipment produced by Beijing Hengjiu Optical Instrument Company (HCT-3, Henven Scientific Instrument Factory, Beijing, China). The combustion performance experiment used an alumina crucible with a φ (diameter) of 5 mm and h (height) of 3 mm to hold the sample. The sample mass was 5 ± 0.2 mg and the reaction atmosphere was air. The heating rate was 20 °C/min, and the final temperature was 800 °C. The conversion rate curve was drawn by the collected weightless data, and the equation for calculating the conversion rate (x) of the sample was shown in Equation (6):
where m0 is the initial mass of the sample, mt is the quality of the sample when the reaction goes to T, and m∞ is the final mass at the end of the test.
where Rmax and Rmean are the maximum and the average weight loss rate, respectively; Ti is the ignition temperature K; and Tf is the burn out temperature K.
2.5. Study on Optimization of Biomass Carbon/Pulverized Coal Blending
Based on the analysis of metallurgical performance of a single hydrochar injection in a blast furnace, a biomass hydrochar with better comprehensive performance was selected to be mixed with pulverized coal. The composition analysis, combustion performance, safety performance detection, and alkali load change of each scheme of blended coal were carried out, and the feasibility of hydrochar blended coal used in the blast furnace injection was analyzed.
3. Results and Discussion
3.1. Performance Test of Hydrochar Blast Furnace
3.1.1. Proximate and Ultimate Analyses
The above four kinds of biomass were subjected to HTC (carbonization temperature is 240 °C, heat preservation is 60 min) to prepare biomass hydrochar. The appearance after HTC is shown in Figure 4. It can be seen from the Figure that the color of the hydrochar obtained from the carbonization of four kinds of biomass turned black. This is because cellulose, hemicellulose, and lignin undergo thermal decomposition and polycondensation reaction during HTC to generate carbonized materials. In addition, due to the stirring effect of the paddle during carbonization, it was impossible to clearly distinguish the types of the original biomass. The composition and calorific value of the original and hydrochar were detected, and the results are shown in Table 1. From the table, it can be seen that the fixed carbon of the original biomass was relatively low, not exceeding 20%, and the calorific value was also low. Only the calorific value of garden trimmings exceeded 20 MJ/kg, and the calorific values of the other three kinds of biomass did not exceed 20 MJ/kg, and the volatile content was high, at about 80%. After HTC, the calorific value and fixed carbon content of biomass hydrochar were greatly improved compared with the original one. The higher heating value of four kinds of hydrochar all exceeded 24.67 MJ/kg, among which the higher heating value of corn stalk reached 27.73 MJ/kg, reaching the level of injecting bituminous coal into the blast furnace, and the volatile content also dropped to 50~60%. From the fixed carbon and volatile content, biomass hydrochar was closer to lignite. According to the yield data, the mass yield of four kinds of biomass hydrochar ranged from 45.40% to 59.51%, and the energy yield ranged from 60.07% to 76.49%, all of which were at a high level. Higher mass yield and energy yield are conducive to reducing the preparation cost and increasing the yield of biomass hydrochar. It is worth noting that water-soluble mineral elements can be partially removed during HTC. Except for wood chips, the ash content of the other three biomass hydrochars decreased to different degrees, and this change was more obvious for straw elements with high alkali metal content. Although the fixed carbon content of biomass hydrochar was lower than that of bituminous coal injected into the blast furnace, it still had a higher heating value due to its low ash content. Extremely low ash content and higher heating value are beneficial to reducing the fuel consumption of blast furnace ironmaking [24]. In addition, from the results of ultimate analysis, it can be seen that the content of C increased to different degrees after HTC, and the sulfur content of hydrochar was much lower than that of pulverized coal injected into the blast furnace. Its application in blast furnace injection is conducive to reducing the sulfur load, and has positive significance for improving the quality of molten iron and reducing the desulfurization cost.

Figure 4.
Image of changes in appearance of the samples. (a) WC, (A) WC-HTC; (b) GW, (B) GW-HTC; (c) MS, (C) MS-HTC; (d) WS, (D) WS-HTC.

Table 1.
Proximate and ultimate analyses of hydrochars.
3.1.2. Alkali Metal Content
The high alkali metal content is an important factor that limits the application of biomass in blast furnaces, especially straw biomass. Due to the addition of potassium fertilizer in the growth process, the alkali metal content of straw biomass exceeds the standard seriously, so it cannot be directly applied to a blast furnace injection. In this study, the alkali metal content of biomass and hydrochar was detected, and the alkali metal removal rate during HTC treatment was calculated. The results are shown in Table 2.

Table 2.
Content of alkali metals and removal rate in carbonization process of different samples.
As can be seen from Table 2, the alkali metal content in the original biomass was generally high, and the alkali metal content of MS was as high as 1.15%, which belongs to the level of high basicity coal. Because of the phenomenon of circulating enrichment of alkali metals in the blast furnace, it would have a serious negative impact on the metallurgical properties of coke and ore in a blast furnace and the life of refractories. At the same time, the formed low-melting-point compounds would also easily cause nodulation of the blast furnace, affect the furnace type, and cause abnormal conditions of the blast furnace. Therefore, the alkali metal content of the raw materials in the furnace must be strictly controlled [25]. As alkali metal compounds are easily soluble in water, the alkali metal elements in the samples can be removed well in the process of HTC and upgrading. It can be seen from the table that the alkali metal removal rates of four kinds of hydrochar were all above 74.90%, and the alkali metal removal rates of garden trimmings and corn stalks were above 90%. The alkali metal content of MS after carbonization and upgrading was only 0.11%, which belonged to low-alkali coal. The alkali metal content of WC-HTC and GW-HTC were both less than 0.10%, which belonged to ultra-low-alkali coal, which was lower than that of bituminous coal injected into the blast furnace. The content of alkali metal potassium and sodium in biomass was greatly reduced after HTC and upgrading, reaching the level of low-alkalinity coal/ultra-low-alkali coal, which can be used as alternative fuel for injecting bituminous coal into a blast furnace.
3.1.3. Ash Composition and Ash Fusion Characteristics
The ash composition and ash fusion characteristics of injected fuel in blast furnaces are another important factor affecting the quality of injected fuel in blast furnaces. The ash composition has great influence on the fluidity, desulfurization ability, and melting characteristics of blast furnace slag [16,26,27]. Coal/biomass ash is the combustion product of minerals in hydrochar at high temperature, mainly containing silicate, carbonate, sulfate and sulfide of silicon, aluminum, iron, calcium, magnesium, potassium and sodium, kaolin and quartz, etc. Most of the combustion products are oxidized or decomposed after high-temperature burning. The content and properties of these products determine the fusibility of ash. The ash components of the original biomass, hydrochar, and bituminous coal were detected, and the results are shown in Table 3.

Table 3.
Ash composition of different samples (wt %).
From the ash composition in Table 3, it can be seen that the ash of the original biomass and hydrochar was mainly composed of CaO, K2O, SiO2, and Al2O3. Compared with the original biomass and bituminous coal injected into blast furnace, the content of SiO2 and Al2O3 in the hydrochar was higher, and SiO2 and Al2O3 are acidic oxides. The higher the content of SiO2 and Al2O3, the higher the ash melting point of biomass ash. A higher ash melting point can avoid the problems of gun blocking and slagging at tuyere during biomass injection, but additional flux is needed for SiO2 and Al2O3. In addition, it can be seen that the content of MgO, K2O, and Na2O in the ash of the hydrochar was obviously lower than that of the original. This is because HTC treatment has an obvious alkali metal removal effect, and the harm of alkali metal to blast furnace smelting is evident. Ash with a higher alkali metal content tends to have a lower ash melting point, which easily leads to gun blockage and slag formation in tuyere, thus affecting the normal production of blast furnace smelting.
The results of ash melting characteristics are shown in Table 4. It can be seen from the table that the softening temperature (ST) of biomass hydrochar was between 1279 °C and 1401 °C, which is in the range of moderate softening temperature ash and medium–high softening temperature ash. The sum of CaO, MgO, and K2O in wood chip hydrochar (WW-HTC) and garden trimming hydrochar (GW-HTC) reached 80%. CaO, MgO, and K2O have dual effects on ash melting point. When their content is low, they can be used as cosolvents of biomass ash, which reduces the melting point of biomass ash. However, when their content increases beyond the combination with other components, a large number of crystals of CaO, MgO, and K2O appear. Because of its high melting point, it increases the ash melting point, which is the main reason for the high ash melting point of WW-HTC and GW-HTC. Ash with higher alkali metal content tends to have a lower ash melting point. The contents of MgO, K2O, and Na2O in the ash of the corn stalk hydrochar (MS-HTC) and wheat stalk hydrochar (WS-HTC) were obviously lower than the original ones, which made the ash melting points of the corn stalk hydrochar (MS-HTC) and wheat stalk hydrochar (WS-HTC) higher. The higher ash melting point can avoid gun blockage and tuyere formation during biomass injection.

Table 4.
Ash melting characteristics of different samples.
3.1.4. Grindability
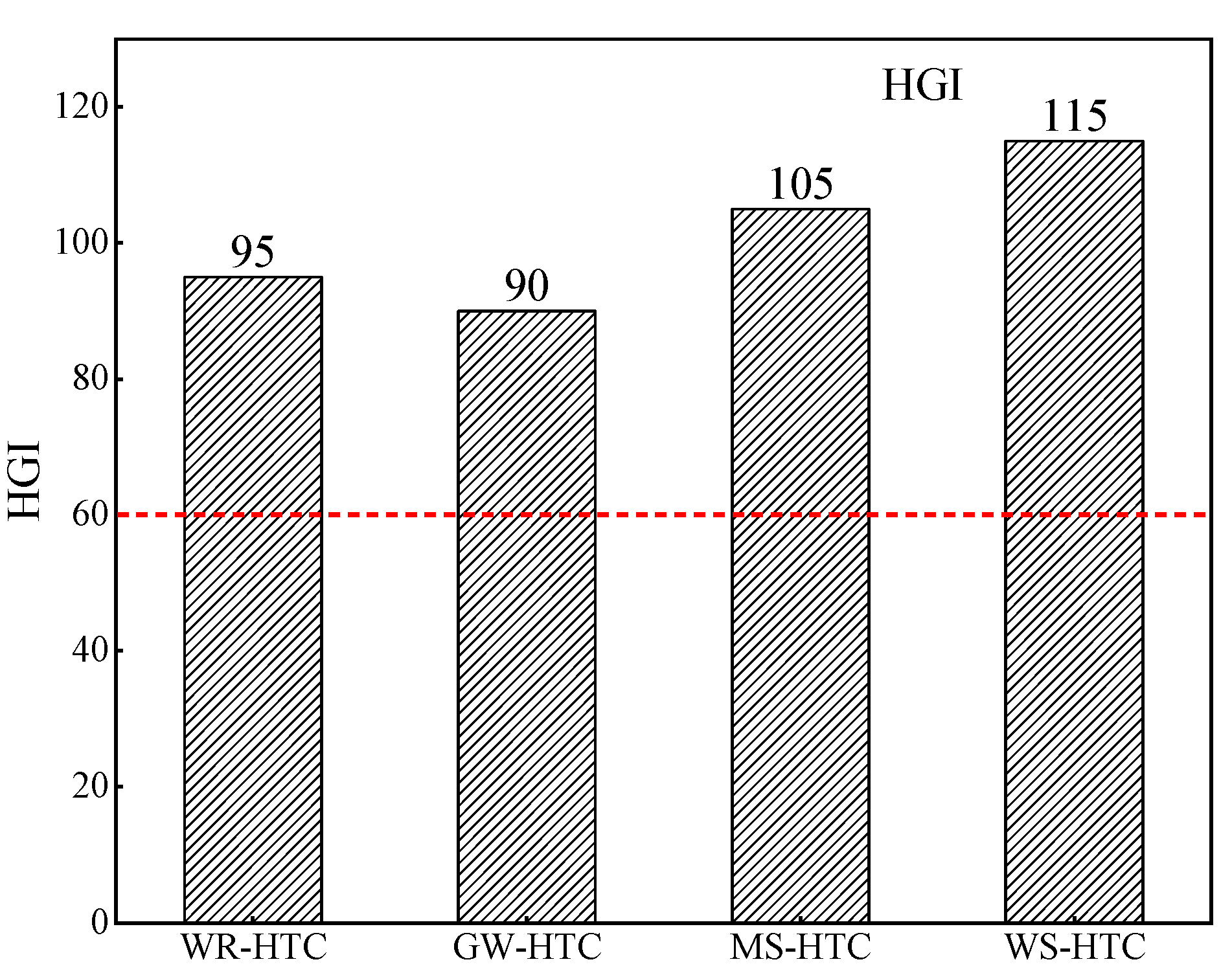
The grindability index indicates the difficulty of sample pulverizing. Biomass is different than pulverized coal in that it has strong brittleness and toughness, so it is difficult to directly crush it using milling equipment such as ball mills in iron and steel enterprises. Therefore, the HGI of blast furnace fuel injection is generally required to be higher than 60. In the process of biomass hydrocharization, black carbonized material, namely, hydrochar, is produced. As shown in Figure 3, compared with the original one, the hardness of hydrochar was reduced, the brittleness was increased, and it was easy to be broken under external force extrusion, so the grindability was greatly improved. The grindability index of four kinds of biomass hydrochar was measured by a Hargreaves grindometer, and the results are shown in Figure 5. As can be seen from Figure 4, the HGI of four kinds of biomass hydrochar was much higher than the requirement of steel enterprises that the HGI of injected fuel be higher than 60. The existing medium-speed mill or ball mill in steel enterprises can be directly used to crush the hydrochar. Thus, the investment cost of equipment transformation can be reduced and the even mixing of biomass carbon and pulverized coal into blast furnace can be promoted during crushing.
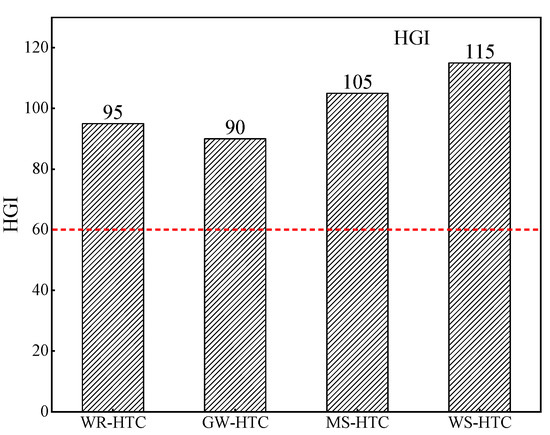
Figure 5.
Grindability index distribution of different biomass char samples.
3.1.5. Safety Properties
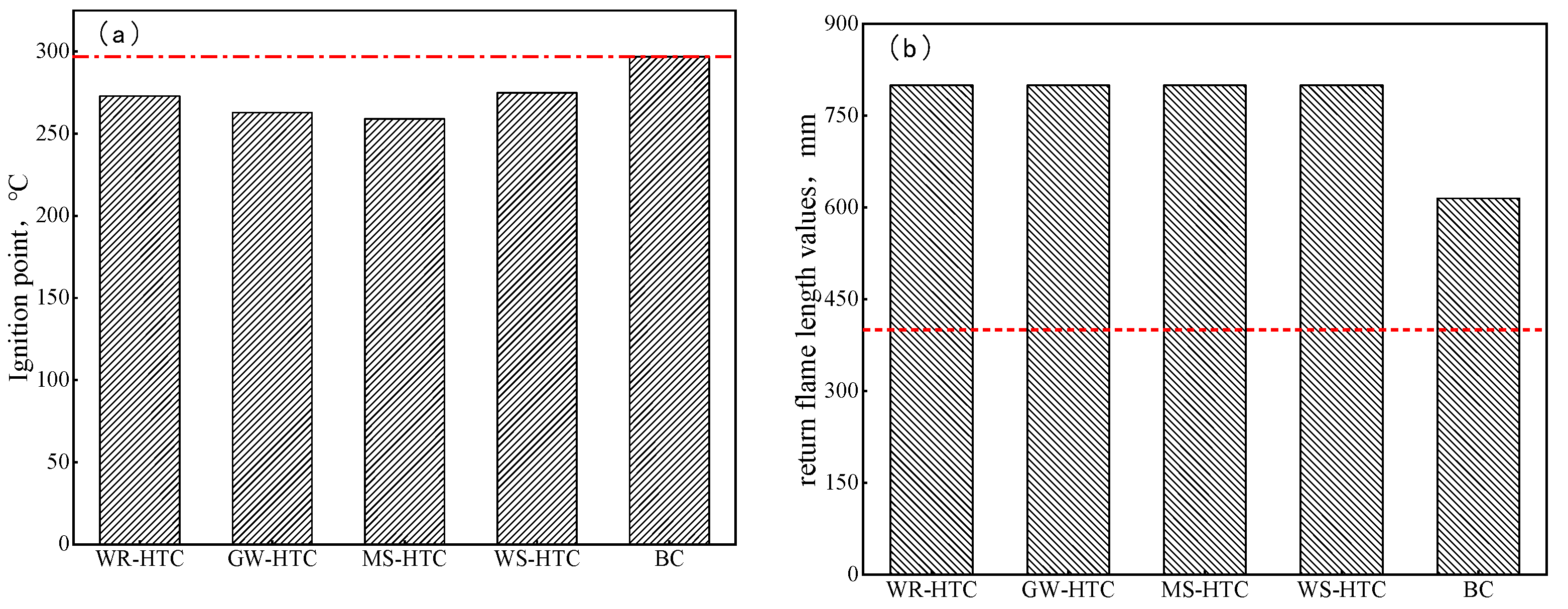
Safety has always been the core tenet of enterprises. Modern iron and steel enterprises need to inject a large amount of pulverized coal every day. These pulverized coal fuels have great potential safety hazards in the process of transportation and long-term stacking, so it is required that pulverized coal fuels have high safety performance. Ignition and explosiveness are important indexes to evaluate the safety performance of fuel injected into blast furnaces. If the ignition temperature of fuel injected into a blast furnace is too low, it is easy to spontaneously ignite during preparation, storage, and transportation, which is one of the main reasons for safety accidents [28,29,30]. In order to ensure the production safety of enterprises, the fuel injected into a blast furnace is required to have a high ignition point and weak explosion performance. The ignition point and explosion performance of four kinds of biomass hydrochar and bituminous coal injected into a blast furnace were tested, and the results are shown in Figure 6. It can be seen from Figure 6a that the ignition point of four biomass hydrochars was around 280 °C, which is lower than that of bituminous coal injected into the blast furnace. The lower ignition point had better combustion performance, so the fuel injected into the blast furnace can be ignited and burn quickly before the tuyere. However, the low ignition point also has its drawbacks, and there are certain potential safety hazards in the process of transportation and long-term stacking. Therefore, countermeasures should be taken in the process of storage and transportation to avoid spontaneous combustion caused by long-term stacking of biomass hydrochar.
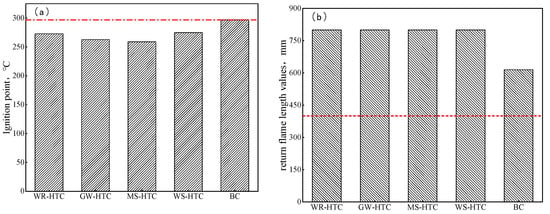
Figure 6.
Ignition point and return flame-length values of different samples. (a) Ignition point, (b) return flame-length values.
Judging from the length of the return flame, the return flame length of less than 400 mm was weakly explosive, and the return flame length of greater than 400 mm was strongly explosive. When only a small number of sparks was present at the fire source, it can be seen from Figure 6b that the return flame lengths of four kinds of biomass hydrochar were all 800 mm. Like bituminous coal injected into blast furnace, biomass hydrochar was also strongly explosive, and the safety performance of the blast furnace injection was lower than that of anthracite. At present, the blast furnace injection system of domestic and foreign iron and steel enterprises can meet the safety requirements of all bituminous coal injections. In the actual production process, it is still necessary to strengthen the inspection, strictly implement safety production index control standards, and ensure safe production in the implementation process of biomass hydrochar technology. In addition, the safe operation of the pulverizing and injection system can be ensured by optimizing the coal mix, relaxing the particle size of injected pulverized coal, and mixing and injecting pulverized coal flux. Due to its low ignition point and strong explosiveness, biomass hydrochar needs to be optimally used with anthracite in blast furnace injection production. On the one hand, the characteristics of biomass hydrochar that is easy to ignite and burn are utilized to promote the ignition and combustion of anthracite in the front section of blast furnace tuyere. Therefore, the combustion efficiency of injected fuel in front of tuyere will be improved, the content of unburned powder entering the furnace burden with gas will be reduced, and the stability and smooth operation of blast furnace smelting will be promoted. On the other hand, the safety performance of biomass hydrochar in the pulverizing and injection system is improved by using the non-explosive characteristic of anthracite. The efficient application of biomass hydrochar in the blast furnace fuel injection technology can be realized.
3.1.6. Thermogravimetric Analysis
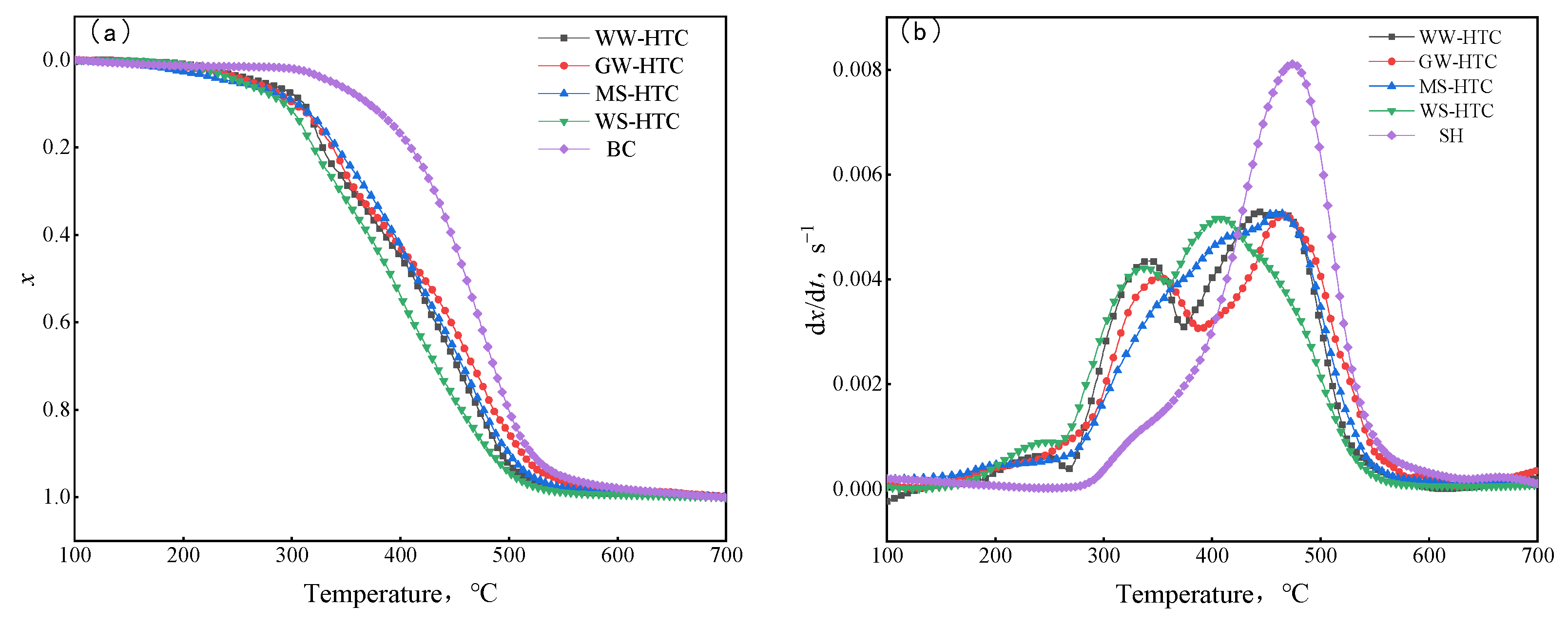
The curves of the combustion conversion rate and reaction rate of biomass hydrochar are shown in Figure 7. It can be seen from Figure 6 that the combustion process can be roughly divided into three stages. In the low temperature zone, the hydrochar sample was gradually heated, water and some adsorbed gases were slowly separated out, the weight loss of the sample was small, and the reaction curve was straight. As the temperature rose, the sample started to burn, the combustible substance reacted violently with the oxygen in the air, and the sample lost weight quickly. The third stage is the burn-out stage, in which the combustible substances were exhausted, the curves of the combustion conversion rate and conversion rate tended to straighten out gradually, and the residual substances were ash in the sample. In the research of gas–solid combustion reaction, the second-stage reaction process was the research object. The similarities and differences of the second-stage reaction of different samples were also compared and analyzed.
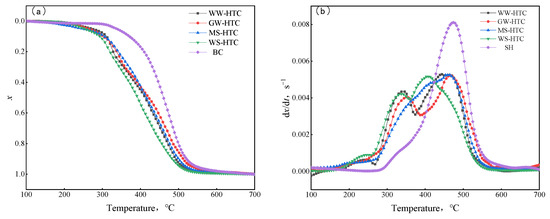
Figure 7.
Combustion curves of different samples: (a) conversion rate, (b) reaction rate.
Comparing the TG curves (a) of four kinds of biomass hydrochar and bituminous coal, the temperature range corresponding to the combustion curve of hydrochar was low. The biomass hydrochar began to lose weight rapidly at about 300 °C, whereas the bituminous coal began to lose weight rapidly at about 340 °C. In addition, from the DTG curve (b), the hydrochar samples had two obvious combustion weight loss peaks during combustion, whereas the bituminous coal had only one strong combustion weight loss peak. This is because hydrochar has higher volatile content and relatively lower fixed carbon content than bituminous coal. The first peak was that the rapid heating of samples released a large amount of volatile matter, which led to a faster combustion rate, resulting in slightly different combustion curves between hydrochar and bituminous coal [26]. In order to quantitatively compare the combustion performance of hydrochar and bituminous coal, the combustion characteristic temperature parameters were extracted, and the results are shown in Table 5. As can be seen from Table 5, the starting combustion temperature of hydrochar was obviously lower than that of bituminous coal, which was maintained at about 300 °C. The lower starting combustion temperature was conducive to rapid ignition and combustion during blast furnace injection, which could fully burn in the tuyere and the raceway of tuyere for a limited time. Thus, the combustion efficiency of injected fuel in front of tuyere and the utilization rate in the furnace can be improved. However, the lower starting combustion temperature means a lower ignition point and a strong explosion, which would have a negative impact on the safety performance of storage, transportation, pulverizing, and transportation. The S value of the comprehensive combustion characteristic index can be used to evaluate the combustion performance. It can be seen that the S values of four kinds of hydrochars were very close to bituminous coal, and they can be used instead of bituminous coal for blast furnace injection.

Table 5.
Combustion characteristic parameters of different samples.
3.2. Performance Test of Blended Coal
3.2.1. Coal-Blending Scheme and Composition Analysis
This coal injection was based on the coal injection scheme in use by steel companies. WC-HTC was selected for coal powder mixing, and the proportions of hydrochar were 5%, 10%, 15%, and 20%. The volatile matter of blended sample was consistent with the original scheme by adjusting the proportions of hydrochar, bituminous coal, and anthracite coal. The results of the specific coal-blending scheme and component analysis are shown in Table 6 and Table 7.

Table 6.
Hydrochar and coal dust mixing scheme.

Table 7.
Proximate analysis and higher heating value of each coal-blending scheme.
As can be seen from Table 7, the volatile matter of blended coal was basically unchanged from scheme No. 1 to scheme No. 3, which was 17.56~17.87%, which is not much different from the original scheme and can ensure the safety performance of injected coal. In addition, ash is a harmful component in the injected coal. If the ash content of pulverized coal injected into the blast furnace is high, it is necessary to improve the basicity of the blast furnace slag, which will increase the coke ratio and not be conducive to the blast furnace production. Therefore, it is necessary to strictly control the ash content of blended coal. Although the proportion of anthracite increased, the ash content of blended coal was basically unchanged due to the extremely low ash content of hydrochar, which was the same as the original scheme. At the same time, the S content also decreased from 0.34% to 0.32%, which is of great significance for reducing the sulfur load of the blast furnace. As the higher heating value of hydrochar was lower than that of bituminous coal and anthracite, the higher heating value of blended coal would decrease slightly with the increase in the hydrochar ratio. However, due to the increase in the anthracite ratio, the change of higher heating value of blended coal was not obvious, which had no great influence on blast furnace ironmaking production. In the scheme, the minimum volatile matter of the four blended coals could only be controlled at 20.05%, so it is suggested that the ratio of hydrochar in actual production not exceed 15%.
3.2.2. Alkali Load Analysis
When hydrochar blended coal is applied to the blast furnace injection process, its influence on blast furnace smelting needs to be analyzed in combination with the change in alkali load of the blended sample. The results shown in Table 8 show that the alkali load of each blended coal scheme changed when the coal ratio was 160 kg/tHM (compared with the initial scheme). It can be seen from Table 8 that although the alkali metal content of hydrochar was extremely low, the alkali load of blended coal increased slightly with the increase in the hydrochar ratio. Because the proportion of anthracite was higher, the alkali metal content of anthracite was higher than that of hydrochar and anthracite. When the proportion of hydrochar reached 20%, the alkali load of blast furnace increased by 0.023 kg/tHM when 160 kg/tHM blended coal was injected, and by 1.15% compared with the current level of 2 kg/tHM. Considering the harmful effect of alkali metals circulating and enriching in blast furnaces, it is necessary to strictly check the alkali metal content of hydrochar when it is injected into a blast furnace.

Table 8.
Change of alkali load in the blast furnace.
3.2.3. Ash Composition and Ash Fusion Characteristics
Ash-melting characteristics of blended samples are also one of the key parameters that affect its safe and efficient injection. A too-low ash-melting point of blended coal can easily lead to gun blockage of the spray gun and small looping of the tuyere, and affect the efficient combustion of pulverized coal in the raceway of the tuyere. The results of ash fusion characteristics of four coal-blending schemes and the original scheme are shown in Table 9. It can be seen from Table 9 that the addition of hydrochar had a certain influence on the ash melting point of blended coal. With the increase in the hydrochar ratio, the deformation temperature (DT) of blended coal gradually increased, whereas the softening temperature (ST), hemisphere temperature (HT), and flow temperature (FT) gradually decreased.

Table 9.
Ash melting characteristics of different schemes (°C).
The ash melting reaction is a complex process, and the mineral types in ash have obvious influence on the ash melting point. It is generally considered that the increase in alkaline oxide content is the main reason for the decrease in the ash melting point of pulverized coal, but excessive alkaline oxide content will cause an increase in the ash melting point temperature. CaO, MgO, and K2O have double effects on the melting point of ash; that is, they can be used as cosolvents and can also increase the melting point of ash. When its content is low, it can be used as a cosolvent of pulverized coal ash, which lowers the melting point of ash. It can be seen from Table 10 that the content of alkaline oxide in the four coal-blending schemes was at a low level. With the increase in the hydrochar ratio, the content of alkaline oxide gradually increases, which lowers the melting point of biomass ash, resulting in a gradual decrease in softening temperature (ST).

Table 10.
Ash composition of different schemes (wt %).
3.2.4. Safety Properties
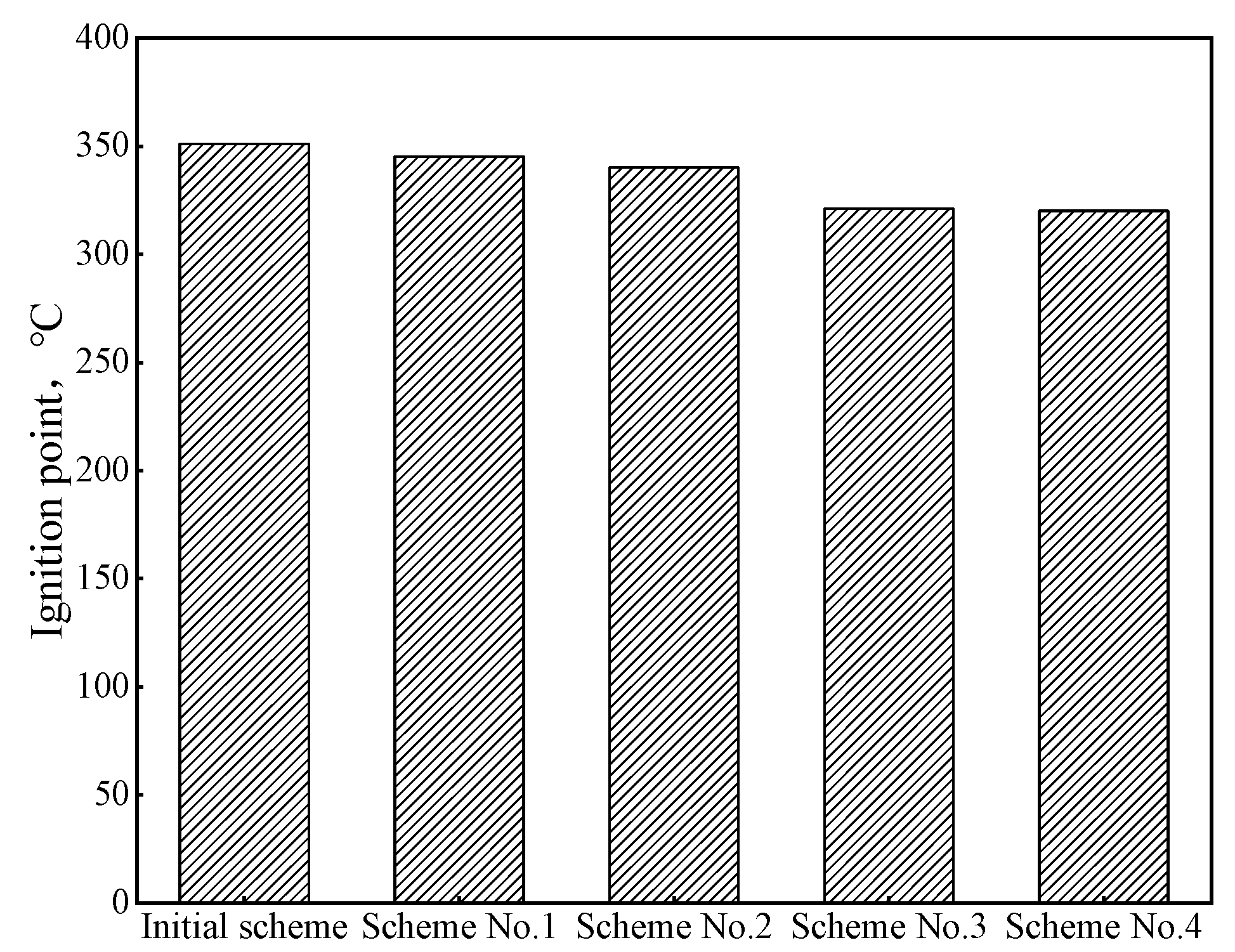
Compared with coal injected into blast furnaces, biomass hydrochar has the problems of low ignition point and strong explosion. By mixing hydrochar with anthracite, the safety performance of hydrochar injected into blast furnaces can be improved. In order to clarify the change rule of safety performance after mixing hydrochar with pulverized coal injected into blast furnaces, the ignition point and explosion of mixed samples obtained by different schemes were analyzed, and the results are shown in Figure 8. The ignition points of the four coal-blending schemes were lower than those of the original scheme. With the increase in the hydrochar ratio, the ignition points gradually decreased, and the lower ignition points could make the fuel injected into the blast furnace be ignited and burn quickly before the tuyere. Due to the existence of anthracite, the volatile content of the four coal-blending schemes was relatively low, so the flame back length was 0 mm, which means there was no explosiveness and the safety hazard caused by the strong explosion of hydrochar was resolved. Therefore, considering the safety performance of blast furnace injection, the proportion of hydrochar should be strictly controlled in actual production.
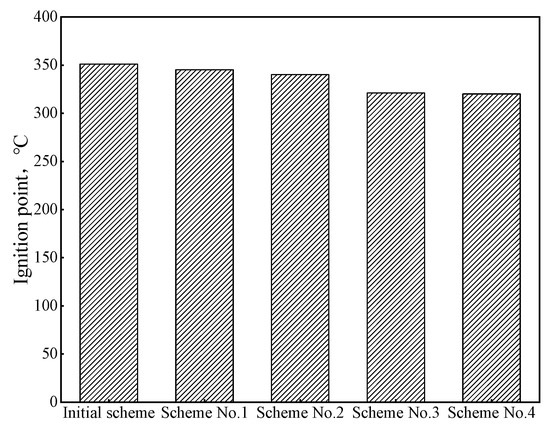
Figure 8.
Ignition point of different schemes.
3.2.5. Thermogravimetric Analysis
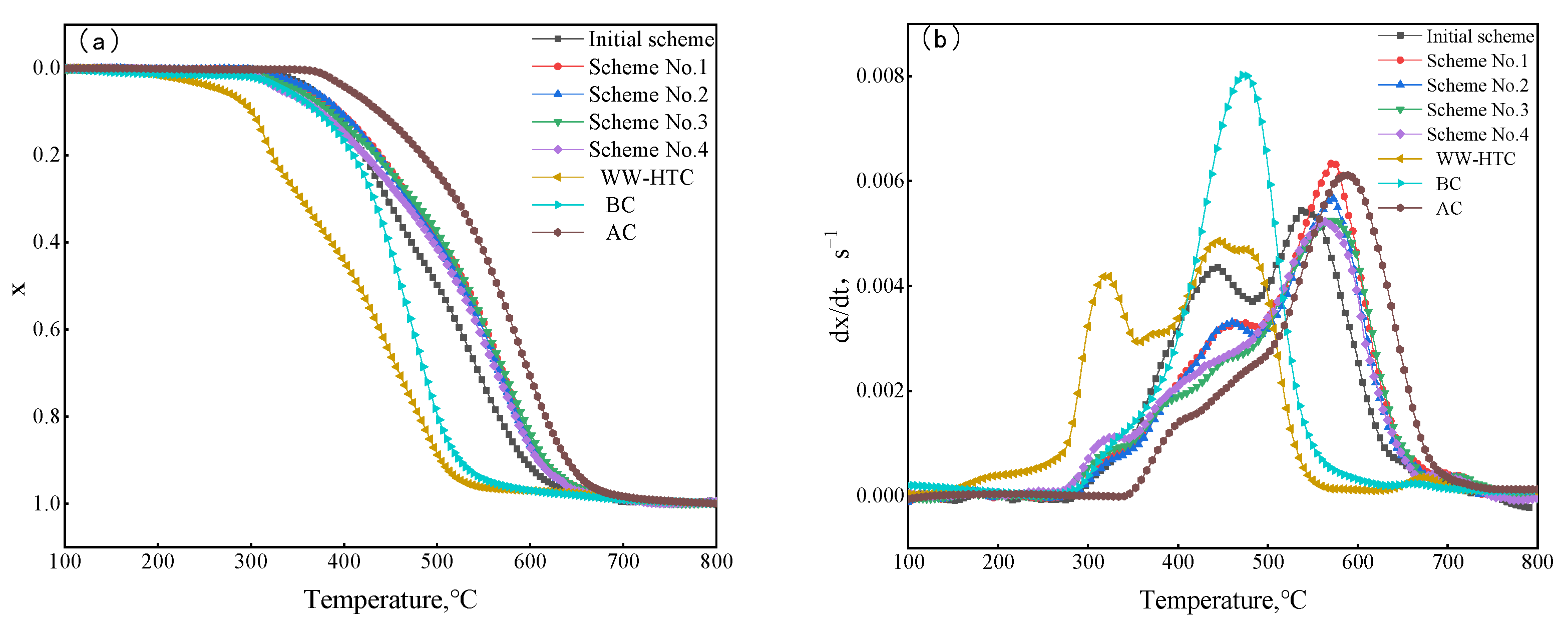
In order to discover the change law of combustion performance of hydrochar mixed with pulverized coal, the combustion performance of the original scheme and four schemes of blends were tested. The results are shown in Figure 8. As can be seen from Figure 9, the combustion process of pulverized coal with different hydrochar ratios was similar to that of single coal, and the combustion process was divided into three stages. The similarities and differences of the second stage were mainly analyzed in the combustion process.
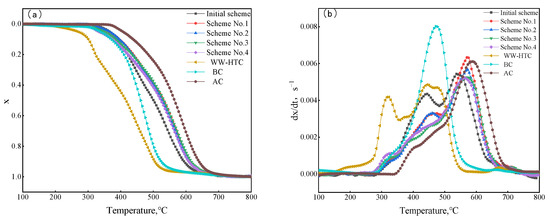
Figure 9.
Combustion curves of coal blends: (a) conversion rate, (b) reaction rate.
It can be seen from Figure 9a that the combustion performance of the four coal-blending schemes was between the original scheme and anthracite. This is because the proportion of anthracite in the four coal-blending schemes increased compared with the original scheme, which led to the movement of the whole combustion weightlessness zone like a high-temperature zone. In order to quantitatively characterize the combustion reactivity of different coal-blending schemes, the combustion characteristic temperature parameters were extracted, and the results are shown in Table 11. As can be seen from Table 11, due to the increase in the hydrochar ratio, the initial combustion temperature Ti of blended coal decreased gradually in scheme No. 1 to scheme No. 4. The lower initial combustion temperature was conducive to rapid ignition and combustion during blast furnace injection, which can improve the comprehensive combustion performance of pulverized coal. The value of comprehensive combustion characteristic index S can be used to evaluate the combustion performance. From scheme No. 1 to scheme No. 3, the comprehensive combustion index S gradually decreased with the increase in the anthracite ratio. Overall, the change of blended coal ratio still had a certain influence on combustion performance.

Table 11.
Combustion characteristic parameters of different blended coal samples.
4. Conclusions
In this study, the characteristics of blast furnace pulverized coal injection technology and the aptitude of biomass resources were combined to prepare high-quality biomass hydrochar for blast furnace injection by treating low-quality biomass raw materials with hydrothermal carbonization. The results showed that the ash content of the hydrochar was low and the HHV was improved with the HHV of all four types of hydrochar higher than 24.67 MJ/kg. HTC treatment could remove the alkali metal content, the alkali metal content of hydrochar was all below 0.33%, and the HGI was all above 90. The ash melting point of hydrochar was significantly higher than that of coal, and the higher ash melting point could avoid the problems of gun blocking and slagging at the air outlet. However, the hydrochar also had a low ignition point and was highly explosive, which needed to be paid attention to regarding safety issues during its storage, transportation, powder production, and transportation. The metallurgical performance of coal and hydrochar blends was also investigated. It was observed that when the proportion of hydrochar in blends was less than 15%, it had little effect on the blast furnace injection process. Through the safety performance test of blends, it was found that all four blends were non-explosive (the return flame length was 0 mm), which improved the safety performance of hydrochar in the blast furnace injection system. The above analysis shows that hydrochar has the potential to be used as a blast furnace injection fuel, which makes up for the blank of the predecessors in the field of biomass application in blast furnace ironmaking, and can help ironmaking production to achieve the peak carbon dioxide emissions and carbon neutrality goals as soon as possible.
Author Contributions
Writing—original draft, K.W., J.Z., S.W., J.W., K.X., J.L., X.N. and G.W.; Writing—review & editing, K.W., J.Z., S.W., J.W., K.X., J.L., X.N. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China: No. 52174295 and No. 52074029.
Institutional Review Board Statement
This study did not involve humans.
Informed Consent Statement
This study did not involve humans.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Xu, Z.X.; Shan, Y.Q.; Zhang, Z.; Deng, X.Q.; Yang, Y.; Luque, R.; Duan, P.G. Hydrothermal carbonization of sewage sludge: Effect of inorganic salts on hydrochar’s physicochemical properties. Green Chem. 2020, 22, 7010–7022. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, G.Q.; Zhou, S.X. Comparison of energy consumption and environmental impact of replacement of coal with straw injection into blast furnace. Environ. Sci. Technol. 2013, 36, 137–140. [Google Scholar]
- Chen, D.F.; Hu, X.Y.; Li, X.J. Biomass resource’s application in iron and steel enterprises of China. Res Iron. Steel 2015, 43, 60–62. [Google Scholar]
- Shan, Y.Q.; Deng, X.Q.; Luque, R.; Xu, Z.X.; Yan, L.; Duan, P.G. Hydrothermal carbonization of activated sewage sludge over ammonia-treated Fenton sludge to produce hydrochar for clean fuel use. Green Chem. 2020, 22, 5077–5083. [Google Scholar] [CrossRef]
- Xu, Z.X.; Song, H.; Li, P.J.; He, Z.X.; Wang, Q.; Wang, K.; Duan, P.G. Hydrothermal carbonization of sewage sludge: Effect of aqueous phase recycling. Chem. Eng. J. 2020, 387, 123410. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, W.Y.; Lu, K.M.; Huang, Y.P. An evaluation on improvement of pulverized biomass property for solid fuel through torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as blast furnace injectant–Considering availability, pretreatment and deployment in the Swedish steel industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, L.; Cang, D.; Li, J.; Li, X.; Xu, C.C. Current status and potential of biomass utilization in ferrous metallurgical industry. Renew. Sustain. Energy Rev. 2017, 68, 511–524. [Google Scholar] [CrossRef]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Bergius, F. Production of hydrogen from water and coal from cellulose at high temperatures and pressures. J. Soc. Chem. Ind. 1913, 32, 462–467. [Google Scholar] [CrossRef]
- Mäkelä, M.; Volpe, M.; Volpe, R.; Fiori, L.; Dahl, O. Spatially resolved spectral determination of polysaccharides in hydrothermally carbonized biomass. Green Chem. 2018, 20, 1114–1120. [Google Scholar] [CrossRef]
- Liu, F.; Yu, R.; Ji, X.; Guo, M. Hydrothermal carbonization of holocellulose into hydrochar: Structural, chemical characteristics, and combustion behavior. Bioresour. Technol. 2018, 263, 508–516. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lee, J.Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal carbonization of maize straw for hydrochar production and its injection for blast furnace. Appl. Energy 2020, 266, 114818. [Google Scholar] [CrossRef]
- Wu, M.R.; Schott, D.L.; Lodewijks, G. Physical properties of solid biomass. Biomass Bioenergy 2011, 35, 2093–2105. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, L.; Gao, Y.; Xu, J.; Chen, H.; Zhu, Y.; Wang, Y.; Liao, C.; Lu, C.; Zhu, C. Characterization and pelletization of cotton stalk hydrochar from HTC and combustion kinetics of hydrochar pellets by TGA. Fuel 2019, 244, 479–491. [Google Scholar] [CrossRef]
- Xu, Z.X.; Liu, P.; Xu, G.S.; Liu, Q.; He, Z.X.; Wang, Q. Bio-fuel oil characteristic from catalytic cracking of hydrogenated palm oil. Energy 2017, 133, 666–675. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Ren, S. Characterisation and model fitting kinetic analysis of coal/biomass co-combustion. Thermochim. Acta 2014, 591, 68–74. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, G.W.; Shao, J.G.; Chen, Y.X.; Yang, T.J. Pulverized coal combustion of nitrogen free blast furnace. J. Iron Steel Res. Int. 2013, 20, 1–5. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Hou, X.; Shao, J.; Geng, W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour. Technol. 2015, 177, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, J.; Adegbite, S.; Gao, X.; Liu, H.; Wu, T. Catalytic and non-catalytic synergistic effects and their individual contributions to improved combustion performance of coal/biomass blends. Appl. Energy 2018, 211, 334–345. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Processing Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Dang, H.; Wang, G.; Wang, C.; Ning, X.; Zhang, J.; Mao, X.; Zhang, N.; Wang, C. Comprehensive Study on the Feasibility of Pyrolysis Biomass Char Applied to Blast Furnace Injection and Tuyere Simulation Combustion. ACS Omega 2021, 6, 20166–20180. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Li, B.; Mbeugang, C.F.M.; Liu, D.; Zhang, S.; Wang, S.; Wang, Q.; Xu, Z.; Hu, X. Simulation of sorption enhanced staged gasification of biomass for hydrogen production in the presence of calcium oxide. Int. J. Hydrog. Energy 2020, 45, 26855–26864. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, P.; Zhou, C.Q. Evaluation of pulverized coal utilization in a blast furnace by numerical simulation and grey relational analysis. Appl. Energy 2019, 250, 1686–1695. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).