Study of the Physical Behaviour and the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues
Abstract
:1. Introduction
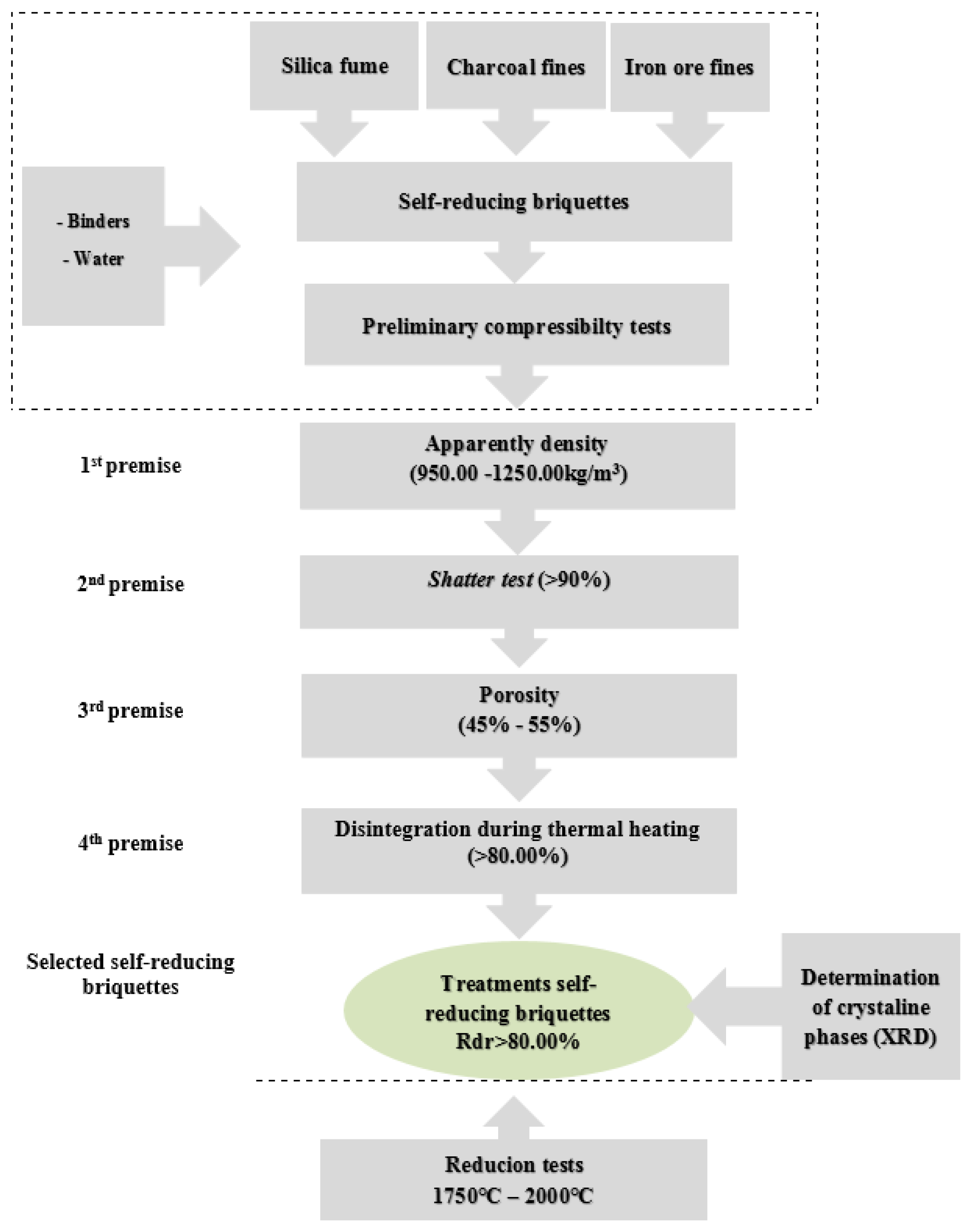
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Sample Characterization of Wastes and Composition of Self-Reducing Briquettes
2.3. Physical Characterization of Self-Reducing Briquettes
2.3.1. Apparent Density
2.3.2. Shatter Test (Adapted Methodology)
2.3.3. Porosity
2.3.4. Disintegration during Thermal Heating
2.3.5. Determination of Crystalline Phases by X-ray Diffraction (XRD)
2.4. Metallurgical Characterization of Self-Reducing Briquettes
2.4.1. Reduction-Fusion Tests
2.4.2. Scanning Electron Microscopy (SEM) and Semi-Quantitative Analyses by Energy Dispersive X-ray Spectroscopy (EDS)
3. Results and Discussion
3.1. Sample Characterization of Wastes
3.2. Apparent Density and Shatter Test in Briquettes
3.3. Porosity in Self-Reducing Briquettes
3.4. Disintegration during Thermal Heating
3.5. Analysis of the Crystalline Phases
3.6. Reduction Tests
Phase Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society of Testing and Materials |
| JIS | Japanese Industrial Standards |
| NBR | Norma Brasileira Regulamentadora |
| ISO | International Organization for Standardization |
| NTNU | Norwegian University of Science and Technology |
| SAF | Submerged Arc Furnace |
References
- Willms, T.; Echterhof, T.; Steinlechner, S.; Aula, M.; Abdelrahim, A.; Fabritius, T.; Mombelli, D.; Mapelli, C.; Preiss, S. Investigation on the Chemical and Thermal Behavior of Recycling Agglomerates from EAF by-Products. Appl. Sci. 2020, 10, 8309. [Google Scholar] [CrossRef]
- Yap, Z.S.; Khalid, N.H.A.; Haron, Z.; Mohamed, A.; Tahir, M.M.; Hasyim, S.; Saggaff, A. Waste Mineral Wool and Its Opportunities—A Review. Materials 2021, 14, 5777. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.; Nayak, D.; Dash, N.; Rath, S.S. Utilization of low-grade banded hematite jasper ores: Recovery of iron values and production of ferrosilicon. Clean Technol. Environ. Policy 2018, 20, 1761–1771. [Google Scholar] [CrossRef]
- Nemchinova, N.V.; Mineev, G.G.; Tyutrin, A.A.; Yakovleva, A.A. Utilization of Dust from Silicon Production. Steel Transl. 2017, 47, 948–957. [Google Scholar] [CrossRef]
- Lemos, L.R.; Rocha, S.H.F.S.; Castro, L.F.A.; Assunção, G.B.M.; Silva, G.L.R. Mechanical strength of briquettes for use in blast furnaces. REM Int. Eng. J. 2019, 72, 63–69. [Google Scholar] [CrossRef]
- Zhdanov, A.V.; Zhuchkov, V.I.; Dashevskii, V.Y.; Leont’Ev, L.I. Problems with waste generation and recycling in the ferroalloys industry. Metallurgist 2015, 58, 1064–1070. [Google Scholar] [CrossRef]
- Chashin, G.A.; Kashlev, I.M.; Efimov, G.P.; Bondarev, A.A. Mastering a technology for making a new commercial product—Densified Microsilica. Metallurgist 2009, 53, 233–235. [Google Scholar] [CrossRef]
- Kero, I.; Gradahl, S.; Tranell, G. Airborne Emissions from Si/FeSi Production. JOM 2017, 69, 365–380. [Google Scholar] [CrossRef]
- Schei, A.; Tuset, J.K.; Tveit, H. Production of High Silicon Alloys; Tapir Forlag: Trondheim, Norway, 1998. [Google Scholar]
- Gasic, M. Handbook of Ferro Alloys: Theory and Technology; Elsevier: Cambridge, MA, USA, 2013. [Google Scholar]
- Vorob’ev, V.P. Carborundum-Bearing Reducing Agents in High-Silicon Alloy Production. Steel Transl. 2017, 47, 688–690. [Google Scholar] [CrossRef]
- Polyakh, O.A.; Rudneva, V.V.; Yakushevich, N.F.; Galevskii, G.V.; Anikin, A.E. Silicon Carbide Production from Steel Plant Wastes. Steel Transl. 2014, 44, 565–572. [Google Scholar] [CrossRef]
- Silveira, R.C.; Almeida, A.M.M. Tecnologia da Fabricação das Ligas à Base de Sílicio; Consultoria e Participações LTDA: Belo Horizonte, Brazil, 1988. [Google Scholar]
- Aasly, K. Properties and Behavior of Quartz for the Silicon Process. Ph.D. Thesis, Faculty of Engineering Science and Technology Department of Geology and Mineral Resources Engineering, Norwegian University of Science and Technology, Trondheim, Norway, 2008; 236p. [Google Scholar]
- Kadkhodabeigi, M.; Tveit, H.; Johansen, S.T. Modelling the Tapping Process in Submerged Arc Furnaces Used in High Silicon Alloys Production. ISIJ Int. 2011, 51, 193–202. [Google Scholar] [CrossRef]
- Hustad, H.M. Tapping of FeSi Furnaces. Ph.D. Thesis, Department of Materials Science and Engineering, Norwegian University of Science and Technology, Trondheim, Norway, 2018; 80p. [Google Scholar]
- Jia, F. A Kind of Briquette Binder. China Patent CN 101747970A, 23 June 2010. [Google Scholar]
- Buzin, P.J.W.K. Desenvolvimento de Briquetes Autorredutores a Partir de Carepas de Processamento Siderúrgico para Utilização em Forno Elétrico a Arco. Master’s Thesis, Programa de Pós-Graduação em Engenharia de Minas, Metalúrgica e de Materiais, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2009; 138p. [Google Scholar]
- Bizhanov, A.M.; Kurunovb, I.F.; Dashevskii, V.Y.A. Mechanical Strength of Extrusion Briquettes (Brex) for Blast-Furnace and Ferroalloy Production: I. Dependence of the Strength Properties of Extrusion Briquettes on the Binder. Russ. Metall. 2015, 2015, 185–190. [Google Scholar] [CrossRef]
- Eremin, A.Y.; Babanin, V.I.; Kozlova, S.Y. Establishing the Requirements for Indices Characterizing the Mechanical Strength of Briquets with Binders. Metallurgist 2003, 47, 437–446. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.; Xu, Y. Review of briquette binders and briquetting mechanism. Renew. Sustain. Energy Rev. 2018, 82, 477–487. [Google Scholar] [CrossRef]
- Rejdak, M.; Robak, J.; Czardybon, A.; Ignasiak, K.; Fudala, P. Research on the production of composite fuel on the basis of fine-grained coal fractions and biomass—The impact of process parameters and the type of binder on the quality of briquetes produced. Minerals 2020, 10, 31. [Google Scholar]
- NBR 10004; Resíduos sólidos—Classificação. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brasil, 2004; p. 21.
- Han, H.; Duan, D.; Yuan, P. Binders and Bonding Mechanism for RHF Briquette Made from Blast Furnace Dust. ISIJ Int. 2014, 54, 1781–1789. [Google Scholar] [CrossRef]
- Oliveira, S.J. Avaliação de Briquetes de Misturas de Finos de Minérios de Ferro e Rejeito de Mineração para Uso em altos-Fornos. Master’s Thesis, Programa de Pós-graduação em Engenharia de Materiais. Universidade Federal de Ouro Preto, Ouro Preto, Brazil, 2019; 115p. [Google Scholar]
- ASTM D440-86; Standard Test Method of Drop Shatter Test for Coal. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002; 4p.
- ISO 616; Coke—Determination of Shatter Indices. International Organization for Standardization: England, UK, 1995; 5p.
- JIS M 8711; Test Method for Determination of Shatter Strength of Iron Ore Sinter. Japanese Industrial Standard: Tokyo, Japan, 2011; 4p.
- Kumar, M.; Jena, S.; Patel, S.K. Characterization of properties andreduction behavior of iron ores for application in sponge ironmaking. Miner. Processing Extr. Metall. Rev. 2008, 29, 118–129. [Google Scholar] [CrossRef]
- ISO 8371; Iron Ores for Blast Furnace Feedstocks—Determination of the Decrepitation Index. International Organization for Standardization: Geneva, Switzerland, 2015; 7p.
- ISO 7215; Iron Ores for Blast Furnace Feedstocks—Determination of the Reducibility by the Final Degree of Reduction Index. International Organization for Standardization: Geneva, Switzerland, 2015; 11p.
- Swirkowsky, M. Avaliação do Aproveitamento do Resíduo Borra Metálica da Produção de Tubos de aço com Costura para Elaboração de Briquetes Autorredutores. Master’s Thesis, Universidade da Região de Joinville—UNIVILLE, Joinville, Brazil, 2018; 78p. [Google Scholar]
- Kulikov, B.P.; Istomin, S.P. Pererabotka Otkhodov Alyuminievogo Proizvodstva (Waste Recycling of Alumnum Industry); LLC “Klassik Tsentr”: Krasnoyarsk, Russia, 2004; 480p. [Google Scholar]
- Broggi, A.; Ringdalen, E.; Tangstad, M. Evolution of SiOx Shell Layers on SiC-SiOx Core-Shell Nanowires. In Materials Science Forum; Trans Tech. Publications Ltd.: Stafa-Zurich, Switzerland, 2020; Volume 1004, pp. 479–489. [Google Scholar]
- Folstad, M.B.; Ringdalen, E.; Tveit, H.; Tangstad, M. Effect of Different SiO2 Polymorphs on the Reaction Between SiO2 and SiC in Si production. Met. Mater. Trans. A 2021, 52, 792–803. [Google Scholar] [CrossRef]
- Aarnæs, T.S.; Tangstad, M.; Ringdalen, E. SiC formation and SiO reactivity of methane at high temperatures. Mater. Chem. Phys. 2021, 276, 125355. [Google Scholar] [CrossRef]
- Coetsee, T. MnO reduction in high carbon ferromanganese production: Practice and theory. Miner. Process. Extr. Metall. Rev. 2018, 39, 351–358. [Google Scholar] [CrossRef]
- Vining, K.R.; Khosa, J.; Sparrow, G.J. Briquetting conditions for australian hematite-goethite iron ore fines. ISIJ Int. 2017, 57, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Murray, J.L., Bennet, L.H., Baker, H., Eds.; ASMT International: West Conshohocken, PA, USA, 1990. [Google Scholar]
- Lucena, D.A.; Medeiros, R.D.; Fonseca, U.T.; Assis, P.S. Aglomeração de Moinha de Carvão Vegetal e sua Possível Aplicação em Alto-forno e Geração de Energia. Tecnol. Metal. Mater. 2008, 4, 1–6. [Google Scholar] [CrossRef]
- Sousa, F.O.; Araújo, G.M. Estudo da Influência da Hidratação Complementar nas Propriedades Mecânicas de Pelotas Autorredutoras. Tecnol. Metal. Mater. Min. 2015, 12, 134–139. [Google Scholar] [CrossRef]
- Castro, A.L.; Pandolfelli, V.C. Revisão: Conceitos de Dispersão e Empacotamento de Partículas para a Produção de Concretos Especiais Aplicados na Construção Civil. Rev. Cerâm. 2009, 55, 18–32. [Google Scholar] [CrossRef]
- Hermann, A.; Langaro, E.A.; Silva, S.H.L.; Klein, N.S. Particle packing of cement and silica fume in pastes using an analytical model. IBRACON Struct. Mater. J. 2016, 9, 48–65. [Google Scholar] [CrossRef]
- Li, N.; Ma, Z.; Zhu, Y. Experimental study on drying and agglomerating moulding of lignite. Adv. Mater. Res. 2011, 158, 64–70. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Factors affecting strength and durability of densified biomass products. Biomass Bioenergy 2009, 33, 337–359. [Google Scholar] [CrossRef]
- Singh, M. Studies on the Cement-Bonded Briquettes of Iron and Steel Plant Byproducts as Burden Material for Blast Furnaces. 2003. Ph.D. Thesis, Chemical and Metallurgical Engineering, Process Metallurgy, Luleå University of Technologia, Suécia, Sweden, 2003; 158p. [Google Scholar]
- Carvalho, E.A.; Brinck, V. Briquetagem—Parte I. In Tratamento de Minérios, 5th ed.; Luz, A.B.d., Sampaio, J.A., França, S.C.A., Eds.; CETEM/MCT: Rio de Janeiro, Brasil, 2010; pp. 683–702. [Google Scholar]
- D’abreu, J.C.; Filho, R.N.R. Contribuição ao estudo da aglomeração de finos utilizando cimento ARI, cimento AL—61 e cal hidratada. Tecnol. Metal. Mater. 2004, 1, 5–9. [Google Scholar] [CrossRef]
- Zambrano, A.P.; Takano, C.; Mourao, M.B.; Tagusagawa, S.Y. Binder behavior on Cromite-Carbon composite pellets. Mater. Res. 2016, 19, 1344–1350. [Google Scholar] [CrossRef]
- Borowski, G.; Stępniewski, W.; Wójcik-Oliveira, K. Effect of starch binder on charcoal briquette properties. Int. Agrophys Lub. 2017, 31, 571–574. [Google Scholar] [CrossRef]
- Sindland, C.; Tangstad, M. Production Rate of SiO Gas from Industrial Quartz and Silicon. Metall. Mater. Trans. B 2021, 52, 1755–1771. [Google Scholar] [CrossRef]
- Muwanguzi, A.J.B.; Karasev, A.V.; Byaruhanga, J.K.; Jönsson, P.G. Characterisation of the Physical and Metallurgical Properties of Natural Iron Ore for Iron production. ISRN Mater. Sci. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Bao, S.; Tangstad, M.; Tang, K.; Ringdalen, E. Production of SiO Gas in the Silicon Process. In Proceedings of the Thirteenth International Ferroalloys Congress—Efficient technologies in ferroalloy industry, Almaty, Kazakhstan, 9–13 June 2013; pp. 273–282. [Google Scholar]
- Li, X.; Zhang, G.; Ostrovski, O.; Tronstad, R. Effect of gas atmosphere on the formation of silicon by reaction of SiC and SiO2. J. Mater. Sci. 2016, 51, 876–884. [Google Scholar] [CrossRef]
- Andersen, V. Reaction Mechanism and Kinetics of the High Temperature Reactions in the Silicon Process. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2010; 99p. [Google Scholar]
- Khajavi, L.T.; Barati, M. Thermodynamics of Phosphorus Removal from Silicon in Solvent Refining of Silicon. High Temp. Mater. Process. 2012, 31, 627–631. [Google Scholar] [CrossRef]
- Tangstad, M.; Ksiazek, M.; Andersen, V.; Ringdalen, E. Small scale laboratory experiments simulating an industrial silicon furnace. In Proceedings of the Twelfth International Ferroalloys Congress: ‘Sustainable Future’, Helsinki, Finland, 6–9 June 2010; pp. 661–669. [Google Scholar]
- Yefimets, A.M.; Tesleva, E.P.; Solovyan, A.V. Influence of boric anhydrideupon physical and chemical properties of ferrosilicon slag. IOP Conf. Ser. Mater. Sci. Eng. 2015, 91, 012049. [Google Scholar] [CrossRef]
- Jayakumari, S. Formation and Characterizationof β- and α-Silicon CarbideProduced During Silicon/Ferrosilicon Process. Ph.D. Thesis, Faculty of Natural Sciences, Department of Materials Science and Engineering, Norwegian University of Science and Technology, Trondheim, Norway, 2020; 252p. [Google Scholar]
- Ciftja, A. Solar Silicon Refining; Inclusions, Settling, Filtration, Wetting. Ph.D. Thesis, Faculty of Natural Sciences, Department of Materials Science and Engineering, Norwegian University of Science and Technology, Trondheim, Norway, 2009; 192p. [Google Scholar]
- Tangstad, M.; Safarian, J.; Bao, S.; Ringdalen, E.; Valderhaug, A. Reaction rates of 2SiO2+SiC=3SiO+CO in pellets at elevated temperatures. Asp. Min. Miner. Sci. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Ananina, S.A.; Verushkin, V.V.; Iskhakov, F.M.; Budennyi, O.V.; Shesterin, V.P. Production of Ferrosilicon with a low impurity contents. Russ. Met. 2009, 2009, 748–751. [Google Scholar] [CrossRef]






















| Material | Quantitative Composition of Burden (kg) | Proportion in the Briquettes (%) | Binders (%) |
|---|---|---|---|
| Silica fume | 178.57 | 43.72 | 0.00; 2.50; 5.00; 7.50; 10.00 |
| Ire ore fines | 131.36 | 32.16 | |
| Charcoal fines | 57.65 | 14.12 |
| Mix (M) | Percentage Composition of Blends | |||||
|---|---|---|---|---|---|---|
| Silica Fume (%) | Charcoal Fines (%) | Iron Ore Fines (%) | Binders (%) | Water (%) | ||
| M1 | 54.0 | 32.0 | 14.0 | - | 0.0 | |
| 5.0 | ||||||
| 10.0 | ||||||
| 15.0 | ||||||
| M2 | 51.5 | 32.0 | 14.0 | Portland cement | 2.5 | 0.0 |
| M3 | 49.0 | 32.0 | 14.0 | 5.0 | 5.0 | |
| M4 | 46.5 | 32.0 | 14.0 | 7.5 | 10.0 | |
| M5 | 44.0 | 32.0 | 14.0 | 10.0 | 15.0 | |
| M6 | 51.5 | 32.0 | 14.0 | Hydrated lime | 2.5 | 0.0 |
| M7 | 49.0 | 32.0 | 14.0 | 5.0 | 5.0 | |
| M8 | 46.5 | 32.0 | 14.0 | 7.5 | 10.0 | |
| M9 | 44.0 | 32.0 | 14.0 | 10.0 | 15.0 | |
| M10 | 51.5 | 32.0 | 14.0 | Sodium silicate | 2.5 | 0.0 |
| M11 | 49.0 | 32.0 | 14.0 | 5.0 | 5.0 | |
| M12 | 46.5 | 32.0 | 14.0 | 7.5 | 10.0 | |
| M13 | 44.0 | 32.0 | 14.0 | 10.0 | 15.0 | |
| Reduction Tests | Temperature (°C) | Time Interval (min.) | Time at Target Temperature (min.) | He (g) (L/min.) |
|---|---|---|---|---|
| 1 | 1500 | 00:25:00 | 30 | 0.1 |
| 1800 | 00:15:00 | |||
| 1800 | 00:30:00 | |||
| 25 | 00:30:00 | |||
| 2 | 1500 | 00:25:00 | 30 | 0.1 |
| 1850 | 00:15:00 | |||
| 1850 * | 00:30:00 | |||
| 25 | 00:30:00 | |||
| 3 | 1500 | 00:25:00 | 30 | 0.1 |
| 1750 | 00:15:00 | |||
| 1750 * | 00:30:00 | |||
| 25 | 00:30:00 | |||
| 4 | 1700 | 00:25:00 | 30 | 0.1 |
| 2000 | 00:15:00 | |||
| 2000 * | 00:30:00 | |||
| 25 | 00:30:00 |
| % Passing | Diameter (μm) |
|---|---|
| 10 | 4.19 |
| 50 | 17.90 |
| 90 | 48.10 |
| Medium diameter | 23.30 |
| Oxides (%) | Waste | ||
|---|---|---|---|
| Silica Fume | Charcoal Ashes | Iron Ore Fines | |
| SiO2 | 90.00 | 65.86 | 38.46 |
| Al2O3 | 0.16 | 10.10 | 0.32 |
| P2O5 | 0.14 | 0.46 | 0.05 |
| CaO | 0.38 | 6.99 | 0.07 |
| TiO2 | 0.007 | 0.34 | 0.012 |
| MnO | 0.08 | 0.25 | 0.02 |
| Fe2O3 | 0.96 | 7.26 | 60.68 |
| MgO | 0.85 | 1.12 | - |
| Na2O | 0.50 | - | - |
| K2O | 2.83 | 2.45 | - |
| PPC | 4.78 | - | 0.39 |
| Moisture (%) | 1.86 | 2.13 | 1.29 |
| Components | % |
|---|---|
| Volatile materials | 20.98 |
| Moisture | 2.13 |
| Ash | 26.88 |
| Fixed Carbon | 50.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoal, A.d.L.; Rossoni, H.A.V.; Kaffash, H.; Tangstad, M.; Henriques, A.B. Study of the Physical Behaviour and the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues. Sustainability 2022, 14, 10963. https://doi.org/10.3390/su141710963
Pascoal AdL, Rossoni HAV, Kaffash H, Tangstad M, Henriques AB. Study of the Physical Behaviour and the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues. Sustainability. 2022; 14(17):10963. https://doi.org/10.3390/su141710963
Chicago/Turabian StylePascoal, Aline da Luz, Hygor Aristides Victor Rossoni, Hamideh Kaffash, Merete Tangstad, and Andréia Bicalho Henriques. 2022. "Study of the Physical Behaviour and the Carbothermal Reduction of Self-Reducing Briquettes Developed with Iron Ore Fines, Charcoal and Silica Fume Residues" Sustainability 14, no. 17: 10963. https://doi.org/10.3390/su141710963





