Evolutionary Participatory Selection for Organic Heterogeneous Material: A Case Study with Ox-Heart Tomato in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Trial Locations
2.3. Participatory Selection
2.4. Multi-Location Trial
2.5. Statistical Analysis
3. Results
3.1. Climatic Data
3.2. Participatory Selection and Evaluation
3.3. Multi-Location Trial
3.3.1. Genotype Comparison
3.3.2. Comparing Farmers’ Selection and Natural Selection
3.3.3. Direction of Selection in Terms of Yield Components
3.4. Correlation Analysis
3.5. Principal Component Analysis
4. Discussion
4.1. Efficacy of Farmers’ Selection
4.2. Evidence of Local Adaptation
4.3. Participant’s Preferences
4.4. Limiting Factors and Lessons Learned
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A Safe Operating Space for Humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture Production as a Major Driver of the Earth System Exceeding Planetary Boundaries. Ecol. Soc. 2017, 22, 8. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for Feeding the World More Sustainably with Organic Agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef]
- van Bueren, E.L.; Jones, S.S.; Tamm, L.; Murphy, K.M.; Myers, J.R.; Leifert, C.; Messmer, M.M. The Need to Breed Crop Varieties Suitable for Organic Farming, Using Wheat, Tomato and Broccoli as Examples: A Review. NJAS-Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Bergougnoux, V. The History of Tomato: From Domestication to Biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Statistical Database; FAO: Rome, Italy, 2019. [Google Scholar]
- Mazzucato, A.; Papa, R.; Bitocchi, E.; Mosconi, P.; Nanni, L.; Negri, V.; Picarella, M.E.; Siligato, F.; Soressi, G.P.; Tiranti, B.; et al. Genetic Diversity, Structure and Marker-Trait Associations in a Collection of Italian Tomato (Solanum lycopersicum L.) Landraces. Theor. Appl. Genet. 2008, 116, 657–669. [Google Scholar] [CrossRef]
- Campanelli, G.; Acciarri, N.; Campion, B.; Delvecchio, S.; Leteo, F.; Fusari, F.; Angelini, P.; Ceccarelli, S. Participatory Tomato Breeding for Organic Conditions in Italy. Euphytica 2015, 204, 179–197. [Google Scholar] [CrossRef]
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What Have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [Green Version]
- European Commission. EU Plant Variety Database. Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchForm&ctl_type=H (accessed on 28 June 2022).
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; et al. Plant Breeding and Climate Changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Murphy, K.M.; Carter, A.H.; Jones, S.S. Evolutionary Breeding and Climate Change. In Genomics and Breeding for Climate-Resilient Crops; Springer: Berlin/Heidelberg, Germany, 2013; pp. 377–389. [Google Scholar]
- Mazzucato, A.; Ficcadenti, N.; Caioni, M.; Mosconi, P.; Piccinini, E.; Reddy Sanampudi, V.R.; Sestili, S.; Ferrari, V. Genetic Diversity and Distinctiveness in Tomato (Solanum lycopersicum L.) Landraces: The Italian Case Study of ‘A Pera Abruzzese’. Sci. Hortic. 2010, 125, 55–62. [Google Scholar] [CrossRef]
- Rete Semi Rurali. Alla ricerca delle varietà da conservazione. Notiziario #21 della Rete Seme Rurali. May 2019. Available online: https://rsr.bio/wp-content/uploads/2021/03/Notiziario-21.pdf (accessed on 1 June 2022).
- Boziné-Pullai, K.; Csambalik, L.; Drexler, D.; Reiter, D.; Tóth, F.; Tóthné Bogdányi, F.; Ladányi, M. Tomato Landraces Are Competitive with Commercial Varieties in Terms of Tolerance to Plant Pathogens—A Case Study of Hungarian Gene Bank Accessions on Organic Farms. Diversity 2021, 13, 195. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Goldman, I.; Ortiz, R. Pursuing the Potential of Heirloom Cultivars to Improve Adaptation, Nutritional, and Culinary Features of Food Crops. Agronomy 2019, 9, 441. [Google Scholar] [CrossRef]
- Hoagland, L.; Navazio, J.; Zystro, J.; Kaplan, I.; Vargas, J.G.; Gibson, K. Key Traits and Promising Germplasm for an Organic Participatory Tomato Breeding Program in the U.S. Midwest. HortScience 2015, 50, 1301–1308. [Google Scholar] [CrossRef]
- Fruit Logistica. European Statistics Handbook; Fruit Logistica: Berlin, Germany, 2020. [Google Scholar]
- Crespo-Herrera, L.A.; Ortiz, R. Plant Breeding for Organic Agriculture: Something New? Agric. Food Secur. 2015, 4, 25. [Google Scholar] [CrossRef]
- Del Bravo, F.; Raeli, M. Bio in Cifre 2020; SINAB—Sistema d’Informazione Nazionale sull’Agricoltura Biologica: Rome, Italy, 2020. [Google Scholar]
- CREA-DC. Italian Organic Seed Derogation Report. Available online: https://www.crea.gov.it/web/difesa-e-certificazione/pubblicazioni-istituzionali-e-schede-tecniche (accessed on 1 June 2022).
- Solfanelli, F.; Ozturk, E.; Zanoli, R.; Orsini, S.; Schäfer, F. The State of Organic Seed in Europe 2021. Available online: https://www.liveseed.eu/tools-for-practitioners/booklets/ (accessed on 1 June 2022).
- Orsini, S.; Costanzo, A.; Solfanelli, F.; Zanoli, R.; Padel, S.; Messmer, M.M.; Winter, E.; Schaefer, F. Factors Affecting the Use of Organic Seed by Organic Farmers in Europe. Sustainability 2020, 12, 8540. [Google Scholar] [CrossRef]
- Ceccarelli, S. Plant Breeding with Farmers; ICARDA: Aleppo, Syria, 2012. [Google Scholar]
- Dawson, J.C.; Murphy, K.M.; Jones, S.S. Decentralized Selection and Participatory Approaches in Plant Breeding for Low-Input Systems. Euphytica 2008, 160, 143–154. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Organic Agriculture and Evolutionary Populations to Merge Mitigation and Adaptation Strategies to Fight Climate Change. South Sustain. 2020, 1, e013. [Google Scholar] [CrossRef]
- Harlan, H.V.; Martini, M.L. A Composite Hybrid Mixture. Agron. J. 1929, 21, 487–490. [Google Scholar] [CrossRef]
- Suneson, C.A. An Evolutionary Plant Breeding Method. Agron. J. 1956, 48, 188–191. [Google Scholar] [CrossRef]
- Campanelli, G.; Sestili, S.; Acciarri, N.; Montemurro, F.; Palma, D.; Leteo, F.; Beretta, M. Multi-Parental Advances Generation Inter-Cross Population, to Develop Organic Tomato Genotypes by Participatory Plant Breeding. Agronomy 2019, 9, 119. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Decentralized-Participatory Plant Breeding: An Example of Demand Driven Research. Euphytica 2007, 155, 349–360. [Google Scholar] [CrossRef]
- Ceccarelli, S. Efficiency of Plant Breeding. Crop Sci. 2015, 55, 87–97. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Return to Agrobiodiversity: Participatory Plant Breeding. Diversity 2022, 14, 126. [Google Scholar] [CrossRef]
- Colley, M.R.; Dawson, J.C.; McCluskey, C.; Myers, J.R.; Tracy, W.F.; van Bueren, E.L. Exploring the Emergence of Participatory Plant Breeding in Countries of the Global North–a Review. J. Agric. Sci. 2021, 159, 320–338. [Google Scholar] [CrossRef]
- Bocci, R.; Bussi, B.; Petitti, M.; Franciolini, R.; Altavilla, V.; Galluzzi, G.; Di Luzio, P.; Migliorini, P.; Spagnolo, S.; Floriddia, R.; et al. Yield, Yield Stability and Farmers’ Preferences of Evolutionary Populations of Bread Wheat: A Dynamic Solution to Climate Change. Eur. J. Agron. 2020, 121, 126–156. [Google Scholar] [CrossRef]
- Chable, V. Final Report Summary-SOLIBAM (Strategies for Organic and Low-Input Integrated Breeding and Management). 2015. Available online: www.solibam.eu (accessed on 1 October 2020).
- Petitti, M. Dieci anni di sperimentazione con la popolazione di pomodoro SOLIBAM Cuor di Bue. Notiziario #24 della Rete Seme Rurali. October 2020, pp. 5–7. Available online: https://rsr.bio/dieci-anni-di-sperimentazione-con-la-popolazione-di-pomodoro-solibam-cuor-di-bue/ (accessed on 1 June 2022).
- Coombes, N. DiGGeR, a Spatial Design Program. In Biometric Bulletin; NSW Department of Primary Industries: Orange, Australia, 2009. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R. Rstudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Rodríguez-Álvarez, M.X.; Boer, M.P.; van Eeuwijk, F.A.; Eilers, P.H.C. Correcting for Spatial Heterogeneity in Plant Breeding Experiments with P-Splines. Spat. Stat. 2018, 23, 52–71. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-4200-4037-1. [Google Scholar]
- Olivoto, T.; Lúcio, A.D. Metan: An R Package for Multi-Environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M(CA), FAMD, MFA, HCPC, Factoextra; STHDA: Exeter, UK, 2017; ISBN 978-1-975721-13-8. [Google Scholar]
- Casals, J.; Rull, A.; Segarra, J.; Schober, P.; Simó, J. Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park. Agronomy 2019, 9, 486. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Pereira-Dias, L.; Casanova, C.; García-Martínez, M.D.; Rosa, E.; Soler, E.; Plazas, M.; Soler, S. Insights into the Adaptation to Greenhouse Cultivation of the Traditional Mediterranean Long Shelf-Life Tomato Carrying the Alc Mutation: A Multi-Trait Comparison of Landraces, Selections, and Hybrids in Open Field and Greenhouse. Front. Plant Sci. 2018, 4, 1774. [Google Scholar] [CrossRef] [PubMed]
- Panthee, D.R.; Labate, J.A.; McGrath, M.T.; Breksa, A.P.; Robertson, L.D. Genotype and Environmental Interaction for Fruit Quality Traits in Vintage Tomato Varieties. Euphytica 2013, 193, 169–182. [Google Scholar] [CrossRef]
- Maeder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Mangano, A. Seconda Condanna per Trapianto di Pomodoro. Otto Mesi a Due Agricoltori Siciliani. Available online: https://www.terrelibere.org/seconda-condanna-semi-pomodoro-otto-mesi-agricoltori-siciliani/ (accessed on 28 June 2022).
- Trouche, G.; Aguirre Acuña, S.; Castro Briones, B.; Gutiérrez Palacios, N.; Lançon, J. Comparing Decentralized Participatory Breeding with On-Station Conventional Sorghum Breeding in Nicaragua: I. Agronomic Performance. Field Crops Res. 2011, 121, 19–28. [Google Scholar] [CrossRef]
- Van Ploeg, D.; Heuvelink, E. Influence of Sub-Optimal Temperature on Tomato Growth and Yield: A Review. J. Hortic. Sci. Biotechnol. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Bertin, N. Competition for Assimilates and Fruit Position Affect Fruit Set in Indeterminate Greenhouse Tomato. Ann. Bot. 1995, 75, 55–65. [Google Scholar] [CrossRef]
- Heuvelink, E. Effect of Fruit Load on Dry Matter Partitioning in Tomato. Sci. Hortic. 1997, 69, 51–59. [Google Scholar] [CrossRef]
- Heuvelink, E.; Buiskool, R.P.M. Influence of Sink-Source Interaction on Dry Matter Production in Tomato. Ann. Bot. 1995, 75, 381–389. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Hoekstra, H.E.; Hoekstra, J.M.; Berrigan, D.; Vignieri, S.N.; Hill, C.E.; Hoang, A.; Gibert, P.; Beerli, P. The Strength of Phenotypic Selection in Natural Populations. Am. Nat. 2001, 157, 245–261. [Google Scholar] [CrossRef]
- Wade, M.J.; Kalisz, S. The Causes of Natural Selection. Evolution 1990, 44, 1947–1955. [Google Scholar] [CrossRef]
- Weltzien, E.; Smith, M.; Meitzner, L.; Sperling, L. Technical and Institutional Issues in Participatory Plant Breeding—From the Perspective of Formal Plant Breeding: A Global Analysis of Issues, Results, and Current Experience; CGIAR: Cali, Colombia, 2003; ISBN 958-694-051-9. [Google Scholar]
- Sperling, L.; Ashby, J.A.; Smith, M.E.; Weltzien, E.; McGuire, S. A Framework for Analyzing Participatory Plant Breeding Approaches and Results. Euphytica 2001, 122, 439–450. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Russi, L.; Romani, M.; Pecetti, L.; Nazzicari, N. Farmer-Participatory vs. Conventional Market-Oriented Breeding of Inbred Crops Using Phenotypic and Genome-Enabled Approaches: A Pea Case Study. Field Crops Res. 2019, 232, 30–39. [Google Scholar] [CrossRef]
- Tufan, H.A.; Grando, S.; Meola, C. State of the Knowledge for Gender in Breeding: Case Studies for Practitioners. Available online: https://cgspace.cgiar.org/handle/10568/92819 (accessed on 13 May 2021).
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate Variation Explains a Third of Global Crop Yield Variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef] [Green Version]
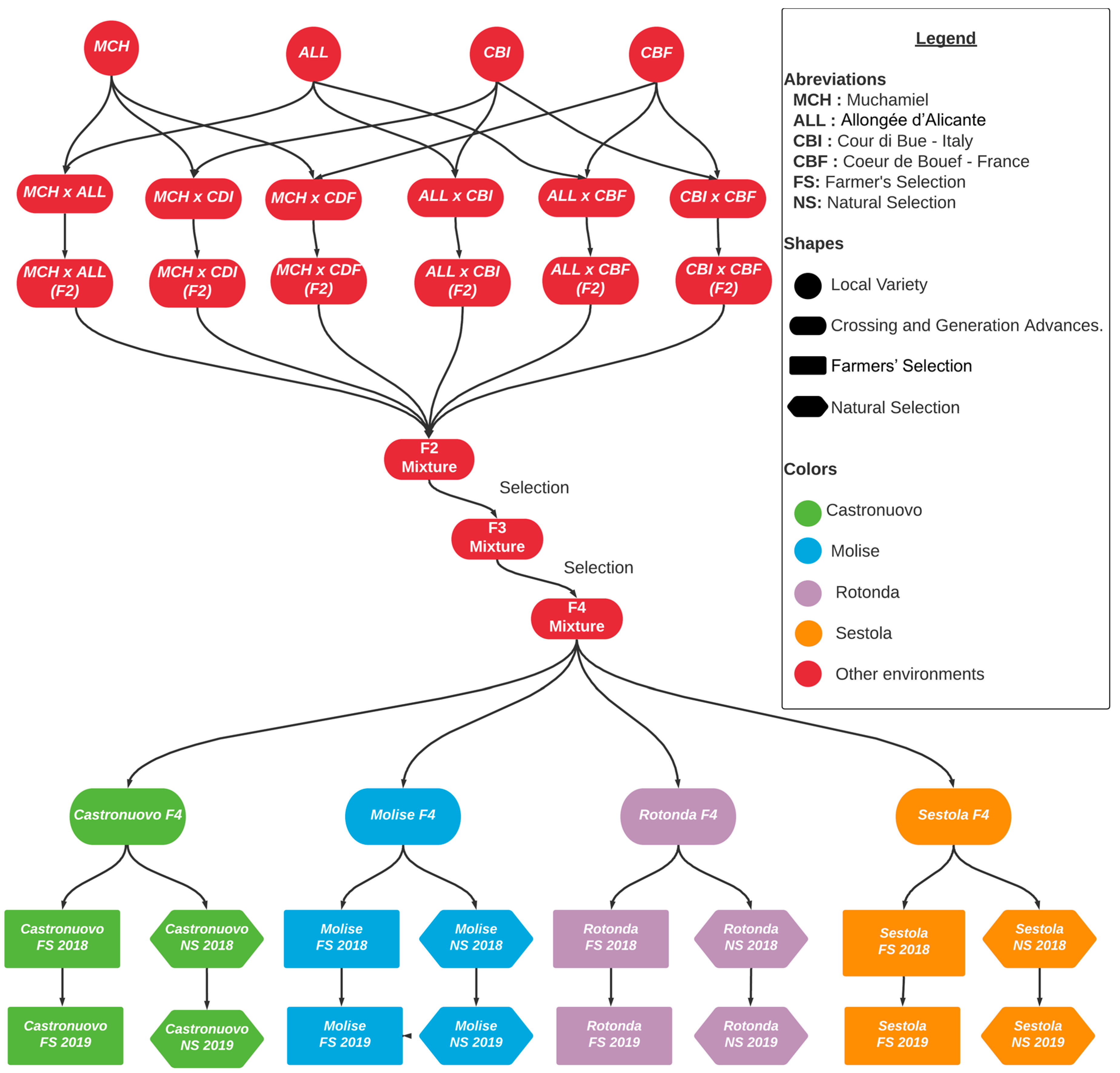











| Name | Allongée d’Alicante | Muchamiel | Cuor di Bue | Cœur de Bœuf |
|---|---|---|---|---|
| Origin | Spain | Spain | Italy | France |
| Shape | Rectangular | Marmande | Ox-Heart | Ox-Heart |
| Maturity | Late | Medium | Early | Medium |
| Growth Habit | Indeterminate | Determinate | Indeterminate | Indeterminate |
| Fruit weight (g) | 150–190 | 190–250 | 190–250 | 190–250 |
| Trait of interest | Homogeneity | Firmness | Shape | Shape |
| Research Question | Comparison of Interest |
|---|---|
| Was farmers’ selection effective for the evaluated traits? | Farmers’ selections vs. natural selections from the same location |
| Is there evidence of local adaptation in the populations to the location where selection occurred? | For each location, local vs. non-local natural and farmers’ selection populations |
| Genotype | Location | Genotype × Location | Replications/ Location | Residuals | |
|---|---|---|---|---|---|
| Degrees of freedom | 13 | 3 | 39 | 4 | 52 |
| Overall evaluation | 12.75 ** | 35.07 *** | 32.56 ** | 0.53 | 19.08 |
| Yield at first harvest | 15.59 ** | 45.75 *** | 23.19 ** | 0.79 | 14.69 |
| Total yield | 1.46 * | 88.40 *** | 7.47 *** | 0.13 | 2.54 |
| Mean fruit weight | 66.30 *** | 0.91 | 18.37 * | 0.74 | 13.66 |
| Total fruit number | 10.21 *** | 80.52 *** | 5.19 * | 0.10 | 3.96 |
| % of marketable yield | 2.54 *** | 87.06 *** | 9.12 *** | 0.09 | 1.18 |
| Trait | Castronuovo | Molise | Rotonda | Sestola |
|---|---|---|---|---|
| Overall evaluation | 2.3 ± 0.1 c | 3 ± 0.1 a | 3 ± 0.1 a | 2.6 ± 0.1 b |
| Yield at first harvest (g/plant) | 148.5 ± 16.6 b | 19.4 ± 3.8 c | 123.5 ± 17.4 b | 216.6 ± 17 a |
| Total yield (g/plant) | 441.4 ± 39.8 c | 872.6 ± 30.9 b | 3010.1 ± 117.1 a | 993.2 ± 53.6 b |
| Number of fruits per plant | 2.8 ± 0.4 c | 5.5 ± 0.5 b | 17.1 ± 0.7 a | 5.7 ± 0.4 b |
| Mean fruit weight (g) | 174.3 ± 11.7 a | 175.6 ± 8.6 a | 183.7 ± 9 a | 185.7 ± 10 a |
| % of marketable yield | 0 ± 0 d | 64.3 ± 1.3 b | 80.1 ± 1.6 a | 39.1 ± 4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petitti, M.; Castro-Pacheco, S.; Lo Fiego, A.; Cerbino, D.; Di Luzio, P.; De Santis, G.; Bocci, R.; Ceccarelli, S. Evolutionary Participatory Selection for Organic Heterogeneous Material: A Case Study with Ox-Heart Tomato in Italy. Sustainability 2022, 14, 11030. https://doi.org/10.3390/su141711030
Petitti M, Castro-Pacheco S, Lo Fiego A, Cerbino D, Di Luzio P, De Santis G, Bocci R, Ceccarelli S. Evolutionary Participatory Selection for Organic Heterogeneous Material: A Case Study with Ox-Heart Tomato in Italy. Sustainability. 2022; 14(17):11030. https://doi.org/10.3390/su141711030
Chicago/Turabian StylePetitti, Matteo, Sergio Castro-Pacheco, Antonio Lo Fiego, Domenico Cerbino, Paolo Di Luzio, Giuseppe De Santis, Riccardo Bocci, and Salvatore Ceccarelli. 2022. "Evolutionary Participatory Selection for Organic Heterogeneous Material: A Case Study with Ox-Heart Tomato in Italy" Sustainability 14, no. 17: 11030. https://doi.org/10.3390/su141711030






