Antimicrobial Resistance in Escherichia coli Isolated from Marine Sediment Samples from Kuwait Bay
Abstract
:1. Introduction
2. Materials and Methods
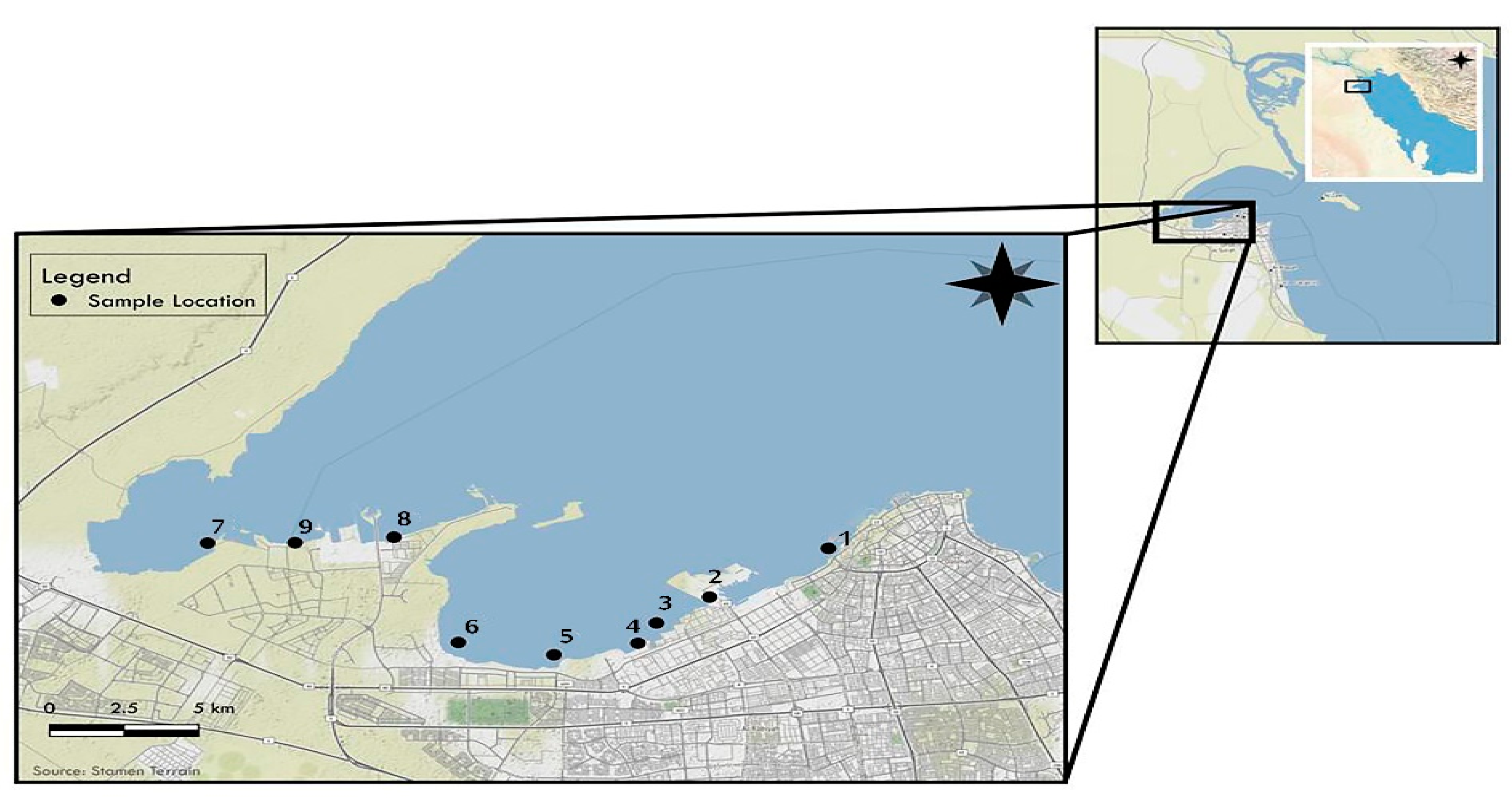
2.1. Sampling Sites
2.1.1. Sample Collection
2.1.2. Enumeration of E. coli
2.1.3. Antimicrobial Susceptibility Testing
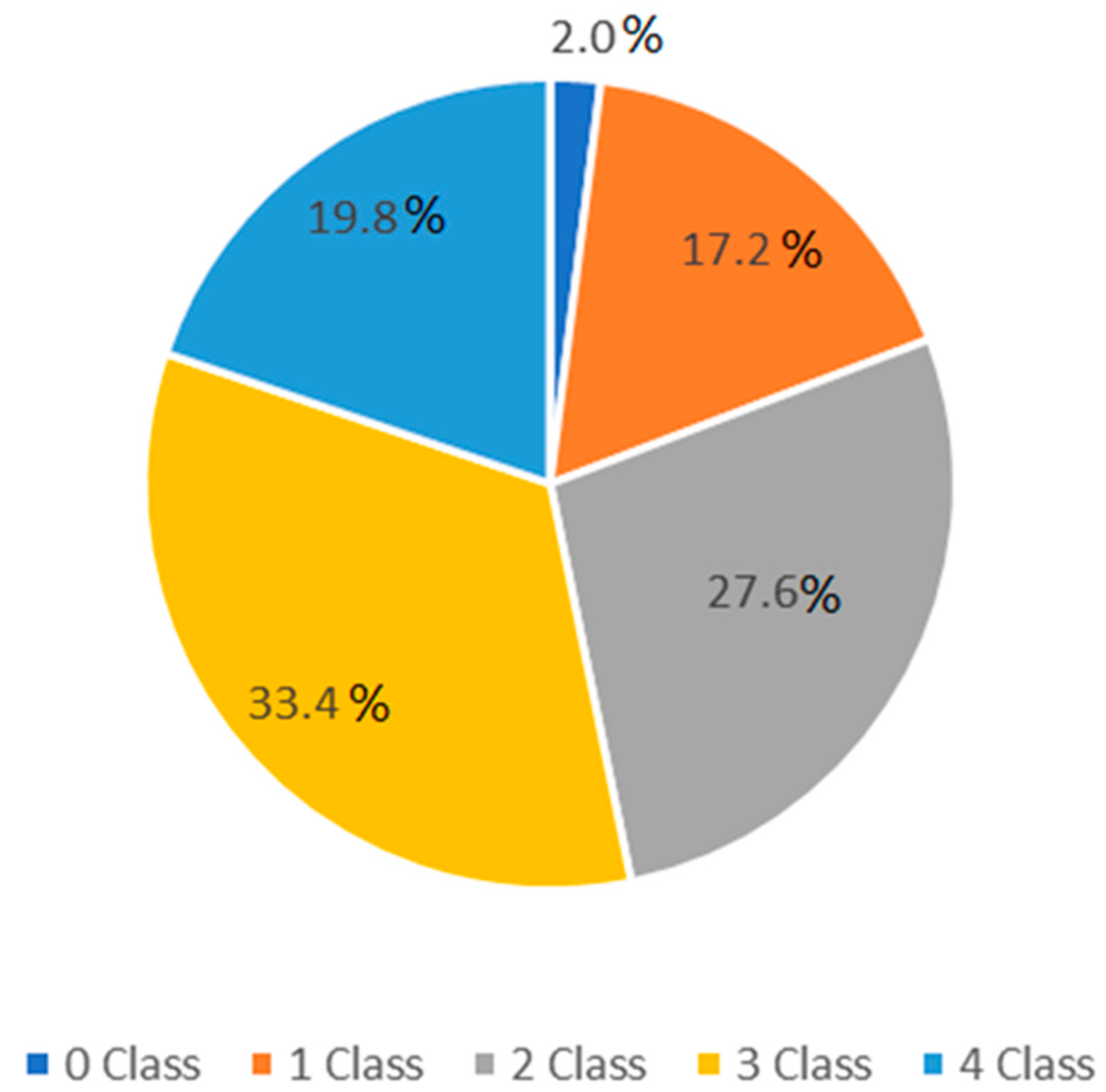
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. 2022. Available online: https://www.who.int/news-room/factsheets/detail/antimicrobial-resistance (accessed on 29 April 2022).
- WHO. Containing Antimicrobial Resistance HO Policy Perspectives on Medicines; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the antibiotic resistome. Science 2006, 311, 374. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for Health and Wealth of Nations; HM Government Wellcome Trust Publication: London, UK, 2014; 20p. [Google Scholar]
- Baquero, F.; Martinez, J.L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review-Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Uddin, S.; Bottein, M.-Y.D.; Faizuddin, M. Ciguatera in the Indian Ocean with Special Insights on the Arabian Sea and Adjacent Gulf and Seas: A Review. Toxins 2021, 13, 525. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial metabolites in nutrition, healthcare and agriculture. Boitechnology 2017, 7, 15. [Google Scholar] [CrossRef]
- Annabi-Trabelsi, N.; Guermazi, W.; Karam, Q.; Ali, M.; Uddin, S.; Leignel, V.; Ayadi, H. Concentrations of trace metals in phytoplankton and zooplankton in the Gulf of Gabès, Tunisia. Mar. Pollut. Bull. 2021, 168, 112392. [Google Scholar] [CrossRef]
- Williams, M.R.; Stedtfeld, R.D.; Guo, X.; Hashsham, S.A. Antimicrobial Resistance in the Environment. Water Environ. Res. 2016, 88, 1951–1967. [Google Scholar] [CrossRef]
- Taylor, N.G.H.; Verner-Jeffreys, D.W.; Baker-Austin, C. Aquatic systems: Maintaining, mixing and mobilising antimicrobial resistance? Trends Ecol. Evol. 2011, 26, 278–284. [Google Scholar] [CrossRef]
- Le Quesne, W.J.F.; Baker-Austin, C.; Verner-Jeffreys, D.W.; Al-Sarawi, H.A.; Balkhy, H.H.; Lyons, B.P. Antimicrobial resistance in the Gulf Cooperation Council region: A proposed framework to assess threats, impacts and mitigation measures associated with AMR in the marine and aquatic environment. Environ. Int. 2018, 121, 1003–1010. [Google Scholar] [CrossRef]
- Kim, D.W.; Cha, C.J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Moller, L.; Torndal, U.B.; Eriksson, L.C.; Gustafsson, J.A. The air pollutant 2-nitrofluorene as initiator and promoter in a liver model for chemical carcinogenesis. Carcinogenesis 1989, 10, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Uddin, S.; Krishnan, D.; Rajagopalan, S.; Habibi, N. Antibiotics in Wastewater: Baseline of the Influent and Effluent Streams in Kuwait. Toxics 2022, 10, 174. [Google Scholar] [CrossRef]
- Lyons, B.P.; Devlin, M.J.; Abdul Hamid, S.A.; Al-Otiabi, A.F.; Al-Enezi, M.; Massoud, M.S.; Al-Zaidan, A.S.; Smith, A.J.; Morris, S.; Bersuder, P.; et al. Microbial water quality and sedimentary faecal sterols as markers of sewage contamination in Kuwait. Mar. Pollut. Bull. 2015, 100, 689–698. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Shimmari, F.; Al-Mutairi, A.; Abdullah, H. Spatial assessment of the sewage contamination of Kuwait’s marine areas. Mar. Pollut. Bull. 2015, 94, 307–317. [Google Scholar] [CrossRef]
- Al-Sarawi, H.A.; Jha, A.N.; Al-Sarawi, M.A.; Lyons, B.P. Historic and contemporary contamination in the marine environment of Kuwait: An overview. Mar. Pollut. Bull. 2015, 100, 621–628. [Google Scholar] [CrossRef]
- Selck, H.; Handy, R.D.; Fernandes, T.F.; Klaine, S.J.; Petersen, E.J. Nanomaterials in the aquatic environment: A European Union-United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead. Environ. Toxicol. Chem. 2016, 35, 1055–1067. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Blotevogel, J.; Stewart, P.S.; Borch, T. Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility, degradation, and toxicity. Environ. Sci. Technol. 2015, 49, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.E.; McNamara, P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Front. Microbiol. 2014, 5, 780. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.A.; Whitehead, R.N.; Mount, M.; Loman, N.J.; Pallen, M.J.; Piddock, L.J. Parallel evolutionary pathways to antibiotic resistance selected by biocide exposure. J. Antimicrob. Chemother. 2015, 70, 2241–2248. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Uddin, S.; Dupont, S. Baseline concentrations of pharmaceuticals in Kuwait’s coastal marine environment. Mar. Pollut. Bull. 2021, 173, 113040. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Liang, X.; Yu, K.; Zhang, T.; Li, X. Metagenomic profiles of antibiotic resistance genes (ARGs) between human impacted estuary and deep ocean sediments. Environ. Sci. Technol. 2013, 47, 12753–12760. [Google Scholar] [CrossRef]
- Chen, J.; McIlroy, S.E.; Archana, A.; Baker, D.M.; Panagiotou, G. A pollution gradient contributes to the taxonomic, functional, and resistome diversity of microbial communities in marine sediments. Microbiome 2019, 7, 104. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Guo, X.-P.; Zhao, S.; Chen, Y.-R.; Yang, J.; Hou, L.-J.; Liu, M.; Yang, Y. Antibiotic resistance genes in sediments of the Yangtze Estuary: From 2007 to 2019. Sci. Total Environ. 2020, 744, 140713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.; Wu, J.; Wang, J.; Lin, Y. Occurrence and distribution of antibiotic resistance genes in sediments in a semi-enclosed continental shelf sea. Sci. Total Environ. 2020, 720, 137712. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Le Quesne, W.J.F.; Lyons, B.P. The Marine Environment of Kuwait—Emerging issues in a rapidly changing environment. Mar. Pollut. Bull. 2015, 100, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.P.; Barber, J.L.; Rumney, H.S.; Bolam, T.P.C.; Bersuder, P.; Law, R.J.; Mason, C.; Smith, A.J.; Morris, S.; Devlin, M.J.; et al. Baseline survey of marine sediments collected from the State of Kuwait: PAHs, PCBs, brominated flame retardants and metal contamination. Mar. Pollut. Bull. 2015, 100, 629–636. [Google Scholar] [CrossRef]
- Uddin, S.; Al-Ghadban, A.N.; Al Khabbaz, A. Localized Hyper Saline Waters in Arabian Gulf from Desalination Activity—An Example from South Kuwait. Environ. Monit. Assess. 2010, 181, 587–594. [Google Scholar] [CrossRef]
- Uddin, S.; Al Ghadban, A.N.; Aba, A.; Behbehani, M. Concentration of selected radionuclides in seawater from Kuwait. Mar. Pollut. Bull. 2012, 64, 1261–1264. [Google Scholar] [CrossRef]
- Uddin, S.; Al Ghadban, A.N.; Behbahani, M. Baseline concentrations of strontium and Sr-90 in seawater from the northern Gulf. Mar. Pollut. Bull. 2013, 75, 301–304. [Google Scholar] [CrossRef]
- Uddin, S.; Aba, A.; Behbehani, M.; Al-Ghadban, A.N.; Al-Zekri, W.; Al-Shammari, H. Plutonium and cesium baseline concentrations in seawater from northern Arabian Gulf. Mar. Pollut. Bull. 2017, 120, 396–400. [Google Scholar] [CrossRef]
- Uddin, S.; Behbehani, M.; Aba, A.; Al Ghadban, A.N. Naturally Occurring Radioactive Material (NORM) in seawater of the northern Arabian Gulf—Baseline measurements. Mar. Pollut. Bull. 2017, 123, 365–372. [Google Scholar] [CrossRef]
- Uddin, S.; Behbehani, M. Concentrations of selected radionuclides and their spatial distribution in marine sediments from the northwestern Gulf, Kuwait. Mar. Pollut. Bull. 2018, 127, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Saeed, T. Microplastic particles in the Persian/Arabian Gulf—A review on sampling and identification. Mar. Pollut. Bull. 2020, 154, 111100. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Uddin, S.; Fowler, S.W.; Behbehani, M.; Aba, A. Depositional time trends of phosphorous accumulation in a dated sediment core from the northwestern Arabian Gulf: Can phosphorous be used to support 210Pb chronologies in coastal aquatic sediments? Mar. Pollut. Bull. 2021, 166, 112213. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Jupp, B.; Faizuddin, M. Petroleum hydrocarbon pollution in sediments from the Gulf and Omani waters: Status and review. Mar. Pollut. Bull. 2021, 173, 112913. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.A.; Shelton, D.R. Escherichia coli and Fecal Coliforms in Freshwater and Estuarine Sediments. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1067–1110. [Google Scholar] [CrossRef]
- Elkadiri, R.; Manche, C.; Sultan, M.; Al-Dousari, A.; Uddin, S.; Chouinard, K.; Abotalib, A.Z. Development of a Coupled Spatiotemporal Algal Bloom Model for Coastal Areas: A Remote Sensing and Data Mining-Based Approach. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 5159–5171. [Google Scholar] [CrossRef]
- Uddin, S.; Al-Ghadban, A.N.; Gevao, B.; Al-Shamroukh, D.; Al-Khabbaz, A. Estimation of Suspended Particulate Matter in Gulf Using MODIS Data. Aquat. Ecosyst. Health Manag. 2011, 15, 41–44. [Google Scholar] [CrossRef]
- Balkhy, H.H.; Assiri, A.M.; Al-Mousa, H.; Al-Abri, S.S.; Al-Katheeri, H.; Alansari, H.; Abdulrazzaq, N.M.; Aidara-Kane, A.; Pittet, D. The strategic plan for combating antimicrobial resistance in Gulf Cooperation Council States. J. Infect. Public Health 2016, 9, 375–385. [Google Scholar] [CrossRef]
- Light, E.; Baker-Austin, C.; Card, R.M.; Ryder, D.; Alves, M.T.; Al-Sarawi, H.A.; Abdulla, K.H.; Stahl, H.; AL-Ghabshi, A.; Ghoribi, M.A.; et al. Establishing a marine monitoring programme to assess antibiotic resistance: A case study from the Gulf Cooperation Council (GCC) region. Environ. Adv. 2022, 9, 100268. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Lyons, B.; Al-Sarawi, H.A.; Behbehani, M.; Shajan, A.; Razzack, N.A.; Zakir, F.; Alam, F. Antibiotic Resistance Genes Associated with Marine Surface Sediments: A Baseline from the Shores of Kuwait. Sustainability 2022, 14, 8029. [Google Scholar] [CrossRef]
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al-Belushi, K.I.A.; Elshafie, A.E.; Al-Harthy, A.; Bakheit, C.K. Coastal sewage discharge and its impact on fish with reference to antibiotic resistant enteric bacteria and enteric pathogens as bio-indicators of pollution. Chemosphere 2009, 77, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al-Zadjali, M.; Elshafie, A.; Al-Harthy, A.; Al-Alawi, W. Antibiotic resistant bacteria as bio-indicator of polluted effluent in the green turtles, Chelonia mydas in Oman. Mar. Environ. Res. 2011, 71, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarawi, H.A.; Jha, A.N.; Baker-Austin, C.; Al-Sarawi, M.A.; Lyons, B.P. Baseline screening for the presence of antimicrobial resistance in E. coli isolated from Kuwait’s marine environment. Mar. Pollut. Bull. 2018, 129, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.; Rotimi, V.O.; Albert, M.J.; Khodakhast, F.; Nordmann, P.; Poirel, L. High prevalence of VIM-4 and NDM-1 metallo-beta-lactamase among carbapenem-resistant Enterobacteriaceae. J. Med. Microbiol. 2013, 62, 1239–1244. [Google Scholar] [CrossRef]
- Zhang, R.; Eggleston, K.; Rotimi, V.; Zeckhauser, R.J. Antibiotic resistance as a global threat: Evidence from China, Kuwait and the United States. Glob. Health 2006, 2. [Google Scholar] [CrossRef]
- Walsh, T.R. A one-health approach to antimicrobial resistance. Nat. Microbiol. 2018, 3, 854–855. [Google Scholar] [CrossRef]
- Anjum, M.F.; Schmitt, H.; Börjesson, S.; Berendonk, T.U.; Donner, E.; Stehling, E.G.; Boerlin, P.; Topp, E.; Jardine, C.; Li, X.; et al. The potential of using E. coli as an indicator for the surveillance of antimicrobial resistance (AMR) in the environment. Curr. Opin. Microbiol. 2021, 64, 152–158. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Imre, K.; Ban-Cucerzan, A.; Herman, V.; Sallam, K.I.; Cristina, R.T.; Abd-Elghany, S.M.; Morar, D.; Popa, S.A.; Imre, M.; Morar, A. Occurrence, Pathogenic Potential and Antimicrobial Resistance of Escherichia coli Isolated from Raw Milk Cheese Commercialized in Banat Region, Romania. Antibiotics 2022, 11, 721. [Google Scholar] [CrossRef]
- Bagel, A.; Sergentet, D. Shiga Toxin-Producing Escherichia coli and Milk Fat Globules. Microorganisms 2022, 10, 496. [Google Scholar] [CrossRef]
- Kimera, Z.; Mgaya, F.; Mshana, S.; Karimuribo, E.; Matee, M. Occurrence of Extended Spectrum Beta Lactamase (ESBL) Producers, Quinolone and Carbapenem Resistant Enterobacteriaceae Isolated from Environmental Samples along Msimbazi River Basin Ecosystem in Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 8264. [Google Scholar] [CrossRef] [PubMed]
- Regional Organization for the Protection of the Marine Environment. The Manual of Oceanographic Observations and Pollutant Analysis Methods, 3rd ed.; Regional Organization for the Protection of the Marine Environment: Kuwait City, Kuwait, 2010. [Google Scholar]
- Ergine, P.; Salerno, C.; Barca, E.; Berardi, G.; Pollice, A. Identification of the faecal indicator Escherichia coli in wastewater through the β-D-glucuronidase activity: Comparison between two enumeration methods, membrane filtration with TBX agar, and Colilert®-18. J. Water Health 2017, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. European Society of Clinical Microbiology and Infectious Diseases; Version 7.0; EUCAST Development Laboratory for Antimicrobial Susceptibility Testing of Bacteria: Växjö, Sweden, 2021. [Google Scholar]
- Alkhalidi, M.A.; Al-Nasser, Z.H.; Al-Sarawi, H.A. Environmental Impact of Sewage Discharge on Shallow Embayment and Mapping of Microbial Indicators. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Devlin, M.J.; Massoud, M.S.; Hamid, S.A.; Al-Zaidan, A.; Al-Sarawi, H.; Al-Enezi, M.; Al-Ghofran, L.; Smith, A.J.; Barry, J.; Stentiford, G.D.; et al. Changes in the water quality conditions of Kuwait’s marine waters: Long term impacts of nutrient enrichment. Mar. Pollut. Bull. 2015, 100, 607–620. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Woolhouse, M.E.J. Using sewage for surveillance of antimicrobial resistance. Science 2020, 367, 630–632. [Google Scholar] [CrossRef]
- Matheu, J.; Aidara-Kane, A.; Andremont, A. he ESBL tricycle 731 AMR sueillance project: A simple, one health approach to global surveillance. AMR Control 2017. Available online: http://resistancecontrol.info/2017/the-esbl-tricycle-amr-surveillance-project-a-simple-one-health-approach-to-global-surveillance/ (accessed on 2 August 2022).
- Smith, A.J.; McGowan, T.; Devlin, M.J.; Massoud, M.S.; Al-Enezi, M.; Al-Zaidan, A.S.; Al-Sarawi, H.A.; Lyons, B.P. Screening for contaminant hotspots in the marine environment of Kuwait using ecotoxicological and chemical screening techniques. Mar. Pollut. Bull. 2015, 100, 681–688. [Google Scholar] [CrossRef]
- Aleisa, E.; Alshayji, K. Analysis on reclamation and reuse of wastewater in Kuwait. J. Eng. Res. 2019, 7, 1–13. [Google Scholar]
- Anastasi, E.M.; Matthews, B.; Stratton, H.M.; Katouli, M. Pathogenic Escherichia coli found in sewage treatment plants and environmental waters. Appl. Environ. Microbiol. 2012, 78, 5536–5541. [Google Scholar] [CrossRef]
- Redha, M.A.; Al Sweih, N.; Albert, M.J. Virulence and phylogenetic groups of Escherichia coli cultured from raw sewage in Kuwait. Gut Pathog. 2022, 14. [Google Scholar] [CrossRef]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef] [PubMed]
- Ajewole, O.A.; Ikhimiukor, O.O.; Adelowo, O.O. Heavy metals (Cu and Zn) contamination of pond sediment and co-occurrence of metal and antibiotic resistance in Escherichia coli from Nigerian aquaculture. Int. J. Environ. Stud. 2021, 87, 773–784. [Google Scholar] [CrossRef]
- Wainwright, M. The Mystery of the Plate: Fleming’s Discovery and Contribution to the Early Development of Penicillin. J. Med. Biogr. 1993, 1, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Kuper, K.; Schulz, L.T.; Bhowmick, T.; Postelnick, M.; Lee, F.; Walensky, R.P. Association Between Penicillin Allergy Documentation and Antibiotic Use. JAMA Intern. Med. 2020, 180, 1120–1122. [Google Scholar] [CrossRef]
- Kot, B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef] [Green Version]

| Number of Location | Name of Location | No. of Colonies CFU/100 mL Mean ± SD |
|---|---|---|
| 1 | KPC | 9 × 103 ± 0.4 × 103 |
| 2 | Al-Ghazali | 7.5 × 104 ± 1.5 × 104 |
| 3 | Maternity Hospital | 1.06 × 105 ± 0.2 × 105 |
| 4 | Chest Hospital | 9.8 × 104 ± 0.21 × 104 |
| 5 | Sulaibkhat Bay | 54 × 104 ± 0.2 × 104 |
| 6 | Sulaibkhat Sport Club | 367 ± 2 |
| 7 | Jaber City | 967 ± 2.1 |
| 8 | Doha East | 8.4 × 103 ± 1.4 × 103 |
| 9 | Doha West | 167 ± 1.5 |
| Percent of Resistant E. coli | ||||||
|---|---|---|---|---|---|---|
| Site No. | Antibiotics Sites | Ceftriaxone | Cefepime | Ampicillin | Ciprofloxacin | Cefpodoxime |
| 1 | KPC (n = 9) | 0 | 0 | 100 | 33.0 | 0 |
| 2 | Al-Ghazali (n = 75) | 36 | 28 | 96 | 51 | 79 |
| 3 | Chest Hospital (n = 106) | 44 | 42.5 | 99 | 43 | 70.75 |
| 4 | Maternity Hospital (n = 98) | 53 | 54 | 99 | 58 | 63.2 |
| 5 | Sulaibkhat Bay (n = 54) | 44.4 | 20.4 | 96.3 | 70.4 | 63 |
| 6 | Sulaibkhat Sport Club (n = 10) | 30 | 40 | 100 | 50 | 70 |
| 7 | Jabber City (n = 29) | 27.5 | 13.7 | 65.5 | 24 | 55 |
| 8 | Doha East (n = 9) | 11.1 | 0 | 100 | 33.3 | 100 |
| 9 | Doha West (n = 5) | 20 | 0 | 100 | 60 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sarawi, H.A.; Najem, A.B.; Lyons, B.P.; Uddin, S.; Al-Sarawi, M.A. Antimicrobial Resistance in Escherichia coli Isolated from Marine Sediment Samples from Kuwait Bay. Sustainability 2022, 14, 11325. https://doi.org/10.3390/su141811325
Al-Sarawi HA, Najem AB, Lyons BP, Uddin S, Al-Sarawi MA. Antimicrobial Resistance in Escherichia coli Isolated from Marine Sediment Samples from Kuwait Bay. Sustainability. 2022; 14(18):11325. https://doi.org/10.3390/su141811325
Chicago/Turabian StyleAl-Sarawi, Hanan A., Afrah B. Najem, Brett P. Lyons, Saif Uddin, and Mohammad A. Al-Sarawi. 2022. "Antimicrobial Resistance in Escherichia coli Isolated from Marine Sediment Samples from Kuwait Bay" Sustainability 14, no. 18: 11325. https://doi.org/10.3390/su141811325






