Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review
Abstract
:1. Introduction
2. Chemical and Physical Characteristics of K-Struvite
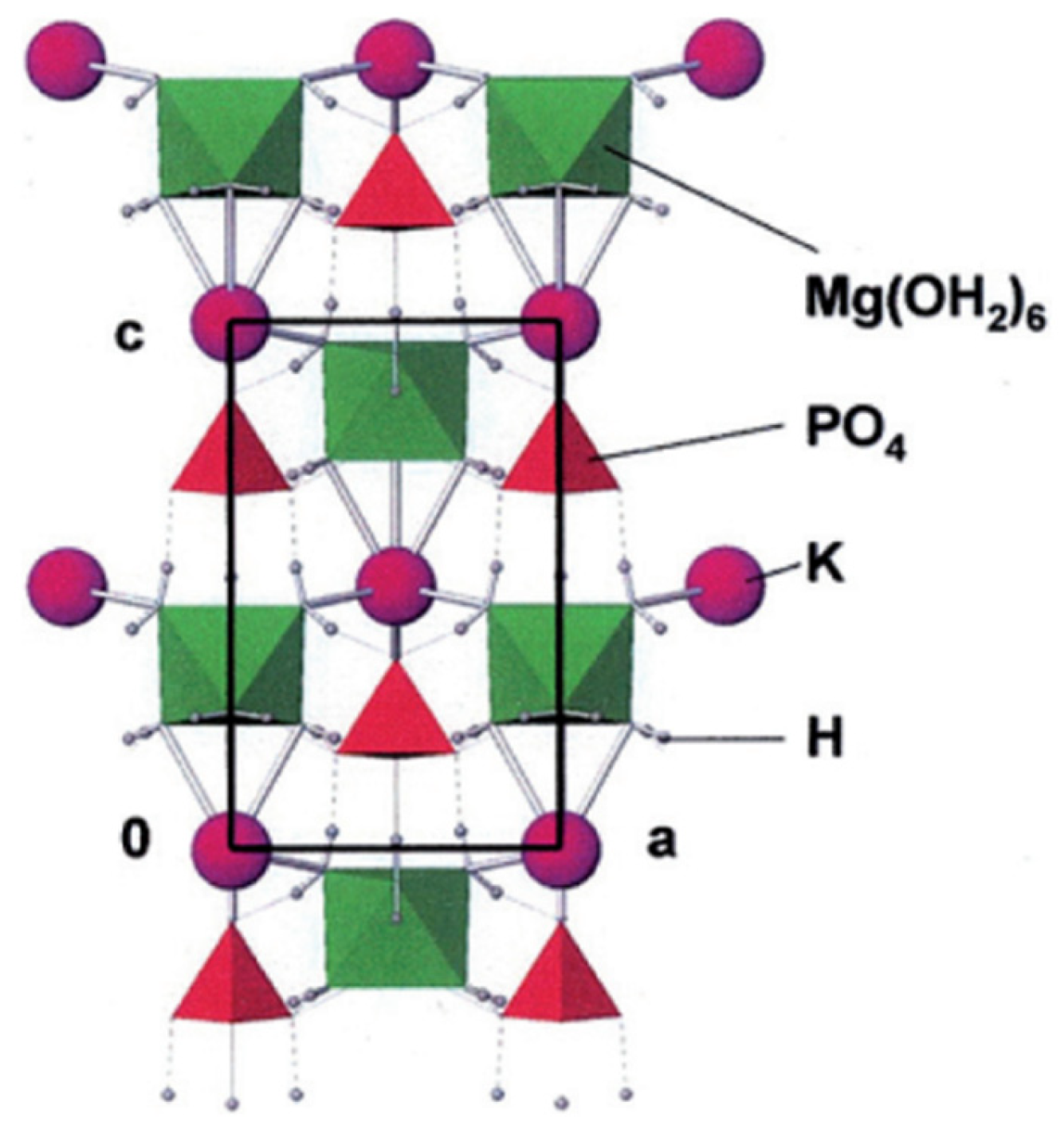
2.1. Crystallographic Properties
2.2. Thermodynamic Properties of K-Struvite
2.3. Solubility Product and Thermodynamic Modeling
3. Parameters Affecting K-Struvite Precipitation Performance
3.1. Operation pH
3.2. Magnesium and Phosphate Sources
3.3. Presence of Competitive Ions
3.3.1. Ammonia
3.3.2. Calcium
3.3.3. Sodium
3.4. Reactor Type and Operational Conditions
4. Nutrient Recovery by K-Struvite Precipitation
4.1. Nutrient Recovery by K-Struvite Precipitation from Human Urine
4.2. Other Sources
4.2.1. Livestock Wastewater
4.2.2. Agro-Industrial Waste
4.2.3. Industrial Wastewater
5. Challenges and Perspectives Ahead
5.1. Use of K-Struvite as a Fertilizer
5.2. Economic Aspects of K-Struvite Precipitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAP | Magnesium ammonium phosphate (struvite) |
| MPP | Magnesium potassium phosphate (K-struvite) |
| MSP | Magnesium sodium phosphate (S-struvite) |
| MP | Magnesium phosphate |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| EDTA | Ethylenediaminetetraacetic acid |
| SCWG | Supercritical water gasification |
| FT-IR | Fourier-transformed Infrared spectroscopy |
| SPU | Synthetically prepared urine |
| SPS | Synthetically prepared sample |
| SSHU | Source-separated human urine |
| SA | Stabilizing agent |
| LG-MO | Low-grade magnesium oxide |
| HAP | Hydroxyapatite |
| TCP | Tricalcium phosphate |
| OCP | Octacalcium phosphate |
| DCP | Dicalcium phosphate |
| DCPD | Dicalcium phosphate dehydrate |
| ACP | Amorphous calcium phosphate |
| STRs | Stirred-tank reactors |
| BSTR | Batch stirred-tank reactor |
| CSTR | Continuous stirred-tank reactor |
| FBRs | Fluidized bed reactors |
| FBHC | Fluidized bed homogeneous crystallization |
| DTBR | Draft tube and baffle reactor |
| BCRDT | Bubble column reactor with draft tube |
| CRT | Crystal retention time |
| HRT | Hydraulic retention time |
| Q | Hydraulic flowrate |
| V | Reactor volume |
| EC | Electrochemical |
| TAN | Total ammonia nitrogen |
| NF | Nanofiltration |
| CEME | Cation exchange membrane electrolysis |
| ED | Electrodialysis |
| FO | Forward osmosis |
| EU | European Union |
References
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in Struvite Precipitation Technologies for Nutrients Removal and Recovery from Aqueous Waste and Wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Tünay, O.; Kabdaşlı, I.; Orhon, D.; Kolçak, S. Ammonia Removal by Magnesium Ammonium Phosphate Precipitation in Industrial Wastewaters. Water Sci. Technol. 1997, 36, 225–228. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Tünay, O. Nutrient Recovery by Struvite Precipitation, Ion Exchange and Adsorption from Source-Separated Human Urine—A Review. Environ. Technol. Rev. 2018, 7, 106–138. [Google Scholar] [CrossRef]
- Tünay, O.; Kabdaşlı, I.; Mehmet, B. Nitrogen Removal and Recovery from Human Urine by Struvite Precipitation. Int. J. Environ. Waste Manag. 2009, 3, 382–392. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Tünay, O.; Işlek, Ç.; Erdinç, E.; Hüskalar, S.; Tatli, M.B. Nitrogen Recovery by Urea Hydrolysis and Struvite Precipitation from Anthropogenic Urine. Water Sci. Technol. 2006, 53, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Tünay, O.; Özcan, P. Application of Struvite Precipitation Coupled with Biological Treatment to Slaughterhouse Wastewaters. Environ. Technol. 2009, 30, 1095–1101. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Şafak, A.; Tünay, O. Bench-Scale Evaluation of Treatment Schemes Incorporating Struvite Precipitation for Young Landfill Leachate. Waste Manag. 2008, 28, 2386–2392. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Atalay, Z.; Tünay, O. Effect of Solution Composition on Struvite Crystallization. J. Chem. Technol. Biotechnol. 2017, 92, 2921–2928. [Google Scholar] [CrossRef]
- Loewenthal, R.E.; Kornmuller, U.R.C.; van Heerden, E.P. Modelling Struvite Precipitation in Anaerobic Treatment Systems. Water Sci. Technol. 1994, 30, 107–116. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Mavinic, D.S.; Meikleham, A.; Ellis, N. Modeling Phosphorus Removal and Recovery from Anaerobic Digester Supernatant through Struvite Crystallization in a Fluidized Bed Reactor. Water Res. 2014, 51, 1–10. [Google Scholar] [CrossRef]
- González-Morales, C.; Fernández, B.; Molina, F.J.; Naranjo-Fernández, D.; Matamoros-Veloza, A.; Camargo-Valero, M.A. Influence of PH and Temperature on Struvite Purity and Recovery from Anaerobic Digestate. Sustainability 2021, 13, 10730. [Google Scholar] [CrossRef]
- Wang, L.; Gu, K.; Zhang, Y.; Sun, J.; Gu, Z.; Zhao, B.; Hu, C. Enhanced Struvite Generation and Separation by Magnesium Anode Electrolysis Coupled with Cathode Electrodeposition. Sci. Total Environ. 2022, 804, 150101. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Long, S.; Zhang, H.; Lichtfouse, E. Electrochemical Crystallization for Recovery of Phosphorus and Potassium from Urine as K-Struvite with a Sacrificial Magnesium Anode. Environ. Chem. Lett. 2022, 20, 27–33. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Liu, H.; Qian, Y. Simultaneous Removal of Phosphorus and Potassium from Synthetic Urine through the Precipitation of Magnesium Potassium Phosphate Hexahydrate. Chemosphere 2011, 84, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, E.; Harada, H. Simultaneously Recovery of Phosphorus and Potassium Using Bubble Column Reactor as Struvite-K and Implementation on Crop Growth. In Crystallizations and Applications; Ben Smida, Y., Marzouki, R., Eds.; IntechOpen Limited: London, UK, 2021. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Kuşçuoğlu, S.; Tünay, O.; Siciliano, A. Assessment of K-Struvite Precipitation as a Means of Nutrient Recovery from Source Separated Human Urine. Sustainability 2022, 14, 1082. [Google Scholar] [CrossRef]
- Wilsenach, J.A.; Schuurbiers, C.A.H.; van Loosdrecht, M.C.M. Phosphate and Potassium Recovery from Source Separated Urine through Struvite Precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Kuşcuoğlu, S. Determination of K-Struvite Application Bases; İstanbul Technical University: İstanbul, Turkey, 2008. [Google Scholar]
- Xu, K.; Li, J.; Zheng, M.; Zhang, C.; Xie, T.; Wang, C. The Precipitation of Magnesium Potassium Phosphate Hexahydrate for P and K Recovery from Synthetic Urine. Water Res. 2015, 80, 71–79. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, B.; Liu, R.; Zhang, T. A Thermodynamic Modeling Approach for Solubility Product from Struvite-k. Comput. Mater. Sci. 2019, 157, 51–59. [Google Scholar] [CrossRef]
- Bennett, A.M.; Lobanov, S.; Koch, F.A.; Mavinic, D.S. Improving Potassium Recovery with New Solubility Product Values for K-Struvite. J. Environ. Eng. Sci. 2017, 12, 93–103. [Google Scholar] [CrossRef]
- Lothenbach, B.; Xu, B.; Winnefeld, F. Thermodynamic Data for Magnesium (Potassium) Phosphates. Appl. Geochem. 2019, 111, 104450. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, B.; Chen, H.; Yin, P. An Experimental Study on the Recovery of Potassium (K) and Phosphorous (P) from Synthetic Urine by Crystallization of Magnesium Potassium Phosphate. Chem. Eng. J. 2018, 337, 19–29. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kumar, R.; Jeon, B.-H. Struvite Precipitation under Changing Ionic Conditions in Synthetic Wastewater: Experiment and Modeling. J. Colloid Interface Sci. 2016, 474, 93–102. [Google Scholar] [CrossRef]
- Zengin, G.; Ölmez, T.; Doğruel, S.; Kabdaşlı, I.; Tünay, O. Assessment of source-based nitrogen removal alternatives in leather tanning industry wastewater. Water Sci. Technol. 2002, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.B.M.; Brasil, Y.L.; Silva, A.F.R.; Andrade, L.H.; Amaral, M.C.S. Potassium Recovery from Vinasse by Integrated Electrodialysis—Precipitation Process: Effect of the Electrolyte Solutions. J. Environ. Chem. Eng. 2020, 8, 104238. [Google Scholar] [CrossRef]
- Liu, J.C. Recovery of Phosphate and Ammonium as Struvite from Semiconductor Wastewater. Sep. Purif. Technol. 2009, 64, 368–373. [Google Scholar] [CrossRef]
- Shashvatt, U.; Benoit, J.; Aris, H.; Blaney, L. CO2-Assisted Phosphorus Extraction from Poultry Litter and Selective Recovery of Struvite and Potassium Struvite. Water Res. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Wang, X.; Qian, Y. Laboratory Experiments on Simultaneous Removal of K and P from Synthetic and Real Urine for Nutrient Recycle by Crystallization of Magnesium–Potassium–Phosphate–Hexahydrate in a Draft Tube and Baffle Reactor. Chemosphere 2012, 88, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Le, V.-G.; Vu, C.-T.; Shih, Y.-J.; Bui, X.-T.; Liao, C.-H.; Huang, Y.-H. Phosphorus and Potassium Recovery from Human Urine Using a Fluidized Bed Homogeneous Crystallization (FBHC) Process. Chem. Eng. J. 2020, 384, 123282. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel Production and Phosphorus Recovery through an Integrated Treatment of Agro-Industrial Waste. Sustainability 2019, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhang, D.; Wang, W.; Li, B.; Zhao, N.; Li, J.; Dai, J. Alleviating Na+ Effect on Phosphate and Potassium Recovery from Synthetic Urine by K-Struvite Crystallization Using Different Magnesium Sources. Sci. Total Environ. 2019, 655, 211–219. [Google Scholar] [CrossRef]
- Banks, E.; Chianelli, R.; Korenstein, R. Crystal Chemistry of Struvite Analogs of the Type MgMPO4·6H2O (M+ = K+, Rb+, Cs+, Ti+, NH4+). Inorg. Chem. 1975, 14, 1634–1639. [Google Scholar] [CrossRef]
- Mathew, M.; Schroeder, L.W. Crystal Structure of a Struvite Analogue, MgKPO4.6H2O. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 11–13. [Google Scholar] [CrossRef]
- Mathew, M.; Kingsbury, P.; Takagi, S.; Brown, W.E. A New Struvite-Type Compound, Magnesium Sodium Phosphate Heptahydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 40–44. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.B.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO46H2O, the Potassium Equivalent of Struvite a New Mineral. Eur. J. Mineral. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Ohlinger, K.N.; Young, T.M.; Schroeder, E.D. Predicting Struvite Formation in Digestion. Water Res. 1998, 32, 3607–3614. [Google Scholar] [CrossRef]
- Chauhan, C.K.; Vyas, P.M.; Joshi, M.J. Growth and characterization of Struvite-K crystals. Cryst. Res. Technol. 2011, 46, 187–194. [Google Scholar] [CrossRef]
- Shih, K.; Yan, H. The Crystallization of Struvite and Its Analog (K-Struvite) From Waste Streams for Nutrient Recycling. Environ. Mater. Waste: Resour. Recovery Pollut. Prev. 2016, 665–686. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Hausherr, R.; Gujer, W. Struvite Precipitation from Urine—Influencing Factors on Particle Size. Water Res. 2010, 44, 2038–2046. [Google Scholar] [CrossRef]
- Frost, R.L.; Weier, M.L.; Martens, W.N.; Henry, D.A.; Mills, S.J. Raman Spectroscopy of Newberyite, Hannayite and Struvite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shi, H.-S.; Huang, S.-W.; Zhang, P. Dehydration Characteristics of Struvite-K Pertaining to Magnesium Potassium Phosphate Cement System in Non-Isothermal Condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Gardner, L.J.; Walling, S.A.; Lawson, S.M.; Sun, S.; Bernal, S.A.; Corkhill, C.L.; Provis, J.L.; Apperley, D.C.; Iuga, D.; Hanna, J.V.; et al. Characterization of and Structural Insight into Struvite-K, MgKPO4·6H2O, an Analogue of Struvite. Inorg. Chem. 2021, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Stillitano, M.A.; Limonti, C.; Marchio, F. Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition. Appl. Sci. 2016, 6, 375. [Google Scholar] [CrossRef]
- Lelet, M.I.; Yakun’kova, M.L.; Mikhailov, D.A.; Lelet, J.N. Experimental Calorimetric Study of Thermodynamic Properties of Two Magnesium Phosphates, MgHPO4·3H2O and MgKPO4·6H2O. J. Chem. Eng. Data 2021, 66, 2723–2732. [Google Scholar] [CrossRef]
- Luff, B.B.; Reed, R.B. Thermodynamic Properties of Magnesium Potassium Orthophosphate Hexahydrate. J. Chem. Eng. Data 1980, 25, 310–312. [Google Scholar] [CrossRef]
- Snoeyink, V.L.; Jenkins, D. Water Chemistry; Wiley: New York, NY, USA, 1980; ISBN 9780471051961. [Google Scholar]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Barat, R.; Bouzas, A.; Martí, N.; Ferrer, J.; Seco, A. Precipitation Assessment in Wastewater Treatment Plants Operated for Biological Nutrient Removal: A Case Study in Murcia, Spain. J. Environ. Manag. 2009, 90, 850–857. [Google Scholar] [CrossRef]
- Satoshi, Y.; Seichiro, O.; Hiroyuki, H.; Kotaro, A.; Mitoma, Y.; Hidetaka, K.; Biswas, B.K. Simultaneous Crystallization of Phosphate and Potassium as Magnesium Potassium Phosphate Using Bubble Column Reactor with Draught Tube. J. Environ. Chem. Eng. 2013, 1, 1154–1158. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Potentiometric Study of the Formation of Magnesium Potassium Phosphate Hexahydrate. J. Mater. Civil. Eng. 2012, 24, 586–591. [Google Scholar] [CrossRef]
- Lahalle, H.; Coumes, C.C.D.; Mesbah, A.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Investigation of Magnesium Phosphate Cement Hydration in Diluted Suspension and Its Retardation by Boric Acid. Cem. Concr. Res. 2016, 87, 77–86. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of K-Struvite Formation in Magnesium Phosphate Cements. Cem. Concr. Res. 2017, 91, 117–122. [Google Scholar] [CrossRef]
- Lahalle, H.; Coumes, C.C.D.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c Ratio on the Hydration Process of a Magnesium Phosphate Cement and on Its Retardation by Boric Acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Harada, H.; Katayama, Y.; Afriliana, A.; Inoue, M.; Teranaka, R.; Mitoma, Y. Effects of Co-Existing Ions on the Phosphorus Potassium Ratio of the Precipitate Formed in the Potassium Phosphate Crystallization Process. J. Environ. Prot. 2017, 8, 1424–1434. [Google Scholar] [CrossRef]
- Tarragó, E.; Ruscalleda, M.; Colprim, J.; Balaguer, M.D.; Puig, S. Towards a Methodology for Recovering K-Struvite from Manure. J. Chem. Technol. Biotechnol. 2018, 93, 1558–1562. [Google Scholar] [CrossRef]
- Liu, J.C. Selective Precipitation of Phosphate from Semiconductor Wastewater. J. Environ. Eng. 2009, 135, 1063–1070. [Google Scholar] [CrossRef]
- Taylor, A.W.; Frazier, A.W.; Gurney, E.L. Solubility Products of Magnesium Ammonium and Magnesium Potassium Phosphates. Trans. Faraday Soc. 1963, 59, 1580–1584. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, D.; Li, J.; Guo, G.; Tang, S. Phosphate Recovery from Swine Wastewater Using Plant Ash in Chemical Crystallization. J. Clean. Prod. 2017, 168, 338–345. [Google Scholar] [CrossRef]
- Mamais, D.; Pitt, P.A.; Cheng, Y.W.; Loiacono, J.; Jenkins, D. Determination of Ferric Chloride Dose to Control Struvite Precipitation in Anaerobic Sludge Digesters. Water Environ. Res. 1994, 66, 912–918. [Google Scholar] [CrossRef]
- Çelen, I.; Buchanan, J.R.; Burns, R.T.; Robinson, R.B.; Raman, D.R. Using a Chemical Equilibrium Model to Predict Amendments Required to Precipitate Phosphorus as Struvite in Liquid Swine Manure. Water Res. 2007, 41, 1689–1696. [Google Scholar] [CrossRef]
- Perwitasari, D.S.; Muryanto, S.; Tauviqirrahman, M.; Jamari, J.; Bayuseno, A.P. Optimization of Key Parameters in Struvite (K) Production for Phosphorus and Potassium Recovery Using a Batch Crystallizer. Rasayan J. Chem. 2019, 12, 787–795. [Google Scholar] [CrossRef]
- Musvoto, E.V.; Wentzel, M.C.; Ekama, G.A. Integrated Chemical–Physical Processes Modelling—II. Simulating Aeration Treatment of Anaerobic Digester Supernatants. Water Res. 2000, 34, 1868–1880. [Google Scholar] [CrossRef]
- Musvoto, E.V.; Ekama, G.A.; Wentzel, M.C.; Loewenthal, R.E. Extension and Application of the Three-Phase Weak Acid/Base Kinetic Model to the Aeration Treatment of Anaerobic Digester Liquors. Water SA 2000, 26, 417–438. [Google Scholar]
- Wang, Y.; Mou, J.; Liu, X.; Chang, J. Phosphorus Recovery from Wastewater by Struvite in Response to Initial Nutrients Concentration and Nitrogen/Phosphorus Molar Ratio. Sci. Total Environ. 2021, 789, 147970. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Jefferson, B.; Parsons, S.A. Agglomeration of Struvite Crystals. Water Res. 2007, 41, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, K.N.; Li, J.Y.; Wang, C.W.; Zheng, M. Recovery of Phosphorus and Potassium from Source-Separated Urine Using a Fluidized Bed Reactor: Optimization Operation and Mechanism Modeling. Ind. Eng. Chem. Res. 2017, 56, 3033–3039. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.M.; Boiarkina, I.; Yu, W.; Huang, Y.F.; Wang, G.Q.; Young, B.R. Phosphorus Recovery through Struvite Crystallisation: Recent Developments in the Understanding of Operational Factors. J. Environ. Manag. 2019, 248, 109254. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Tünay, O.; Udert, K.M. Transfer into the solid phase. In Source Separation and Decentralization for Wastewater Management; Larsen, T.A., Udert, K.M., Lienert, J., Eds.; IWA Publishing: London, UK, 2013; pp. 351–365. [Google Scholar]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus Recovery as Struvite: Recent Concerns for Use of Seed, Alternative Mg Source, Nitrogen Conservation and Fertilizer Potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Li, B.; Zhang, D.; Zhao, N.; Tang, S. Comparison of Different K-Struvite Crystallization Processes for Simultaneous Potassium and Phosphate Recovery from Source-Separated Urine. Sci. Total Environ. 2019, 651, 787–795. [Google Scholar] [CrossRef]
- Siciliano, A.; de Rosa, S. Recovery of Ammonia in Digestates of Calf Manure through a Struvite Precipitation Process Using Unconventional Reagents. Environ. Technol. 2014, 35, 841–850. [Google Scholar] [CrossRef]
- Siciliano, A.; Ruggiero, C.; de Rosa, S. A New Integrated Treatment for the Reduction of Organic and Nitrogen Loads in Methanogenic Landfill Leachates. Process. Saf. Environ. Prot. 2013, 91, 311–320. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y. Simultaneous Nitrogen and Phosphorus Recovery from Sludge-Fermentation Liquid Mixture and Application of the Fermentation Liquid to Enhance Municipal Wastewater Biological Nutrient Removal. Environ. Sci. Technol. 2009, 43, 6164–6170. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, C.; Li, J.; Cheng, X.; Wang, C. Removal and Recovery of N, P and K from Urine via Ammonia Stripping and Precipitations of Struvite and Struvite-K. Water Sci. Technol. 2017, 75, 155–164. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, B.H.; Lim, S.J.; Kim, T.H.; Kim, S.K.; Kim, J.Y. Development and Validation of an Equilibrium Model for Struvite Formation with Calcium Co-Precipitation. J. Cryst. Growth 2013, 372, 129–137. [Google Scholar] [CrossRef]
- Yan, H.; Shih, K. Effects of Calcium and Ferric Ions on Struvite Precipitation: A New Assessment Based on Quantitative X-Ray Diffraction Analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Abbona, F.; Madsen, H.E.L.; Boistelle, R. The Initial Phases of Calcium and Magnesium Phosphates Precipitated from Solutions of High to Medium Concentrations. J. Cryst. Growth 1986, 74, 581–590. [Google Scholar] [CrossRef]
- Abbona, F.; Lundager Madsen, H.E.; Boistelle, R. The Final Phases of Calcium and Magnesium Phosphates Precipitated from Solutions of High to Medium Concentration. J. Cryst. Growth 1988, 89, 592–602. [Google Scholar] [CrossRef]
- Abbona, F.; Franchini-Angela, M.; Boistelle, R. Crystallization of Calcium and Magnesium Phosphates from Solutions of Medium and Low Concentrations. Cryst. Res. Technol. 1992, 27, 41–48. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Young, B. A New Thermodynamic Approach for Struvite Product Quality Prediction. Environ. Sci. Pollut. Res. 2019, 26, 3954–3964. [Google Scholar] [CrossRef]
- Capdevielle, A.; Sýkorová, E.; Béline, F.; Daumer, M.L. Kinetics of Struvite Precipitation in Synthetic Biologically Treated Swine Wastewaters. Environ. Technol. 2014, 35, 1250–1262. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J. Impact of Calcium on Struvite Crystallization in the Wastewater and Its Competition with Magnesium. Chem. Eng. J. 2019, 378, 122121. [Google Scholar] [CrossRef]
- Enyemadze, I.; Momade, F.W.Y.; Oduro-Kwarteng, S.; Essandoh, H. Phosphorus Recovery by Struvite Precipitation: A Review of the Impact of Calcium on Struvite Quality. J. Water Sanit. Hyg. Dev. 2021, 11, 706–718. [Google Scholar] [CrossRef]
- Yang, H.; Sun, H.J. Crystal Structure of a New Phosphate Compound, Mg2KNa(PO4)2·14H2O. J. Solid State Chem. 2004, 177, 2991–2997. [Google Scholar] [CrossRef]
- Tansel, B.; Lunn, G.; Monje, O. Struvite Formation and Decomposition Characteristics for Ammonia and Phosphorus Recovery: A Review of Magnesium-Ammonia-Phosphate Interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lobanov, S.; Lo, V.K. An Overview of Technologies to Recover Phosphorus as Struvite from Wastewater: Advantages and Shortcomings. Environ. Sci. Pollut. Res. 2019, 26, 19063–19077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, K.; Zheng, M.; Li, J.; Wang, C. Factors Affecting the Crystal Size of Struvite-K Formed in Synthetic Urine Using a Stirred Reactor. Ind. Eng. Chem. Res. 2018, 57, 17301–17309. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Demirer, G.N. Removal and Recovery of Nutrients as Struvite from Anaerobic Digestion Residues of Poultry Manure. Environ. Technol. 2011, 32, 783–794. [Google Scholar] [CrossRef]
- Rech, I.; Kamogawa, M.Y.; Jones, D.L.; Pavinato, P.S. Synthesis and Characterization of Struvite Derived from Poultry Manure as a Mineral Fertilizer. J. Environ. Manag. 2020, 272, 111072. [Google Scholar] [CrossRef]
- Atalay, S.; Sargin, I.; Arslan, G. Crystallization of Struvite-K from Pumpkin Wastes. J. Sci. Food Agric. 2022, 102, 523–530. [Google Scholar] [CrossRef]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient Removal and Recovery from Digestate: A Review of the Technology. Biofuels 2018, 9, 247–262. [Google Scholar] [CrossRef]
- Tao, W.; Fattah, K.P.; Huchzermeier, M.P. Struvite Recovery from Anaerobically Digested Dairy Manure: A Review of Application Potential and Hindrances. J. Environ. Manag. 2016, 169, 46–57. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.J.; Lee, K.; Yu, M.S.; Park, K.Y. Effects of PH, Molar Ratios and Pre-Treatment on Phosphorus Recovery through Struvite Crystallization from Effluent of Anaerobically Digested Swine Wastewater. Environ. Eng. Res. 2017, 22, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Li, X. Nutrient Removal from Anaerobically Digested Cattle Manure by Struvite Precipitation. J. Environ. Eng. Sci. 2015, 5, 285–294. [Google Scholar] [CrossRef]
- Gorazda, K.; Tarko, B.; Werle, S.; Wzorek, Z. Sewage Sludge as a Fuel and Raw Material for Phosphorus Recovery: Combined Process of Gasification and P Extraction. Waste Manag. 2018, 73, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Acelas, N.Y.; López, D.P.; Wim Brilman, D.W.F.; Kersten, S.R.A.; Kootstra, A.M.J. Supercritical Water Gasification of Sewage Sludge: Gas Production and Phosphorus Recovery. Bioresour. Technol. 2014, 174, 167–175. [Google Scholar] [CrossRef]
- Atoufi, H.D.; Lampert, D.J. Impacts of Oil and Gas Production on Contaminant Levels in Sediments. Curr. Pollut. Rep. 2020, 6, 43–53. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous Recovery of Ammonium, Potassium and Magnesium from Produced Water by Struvite Precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Wu, Y.; Wang, C.; Zheng, J.; Xu, K.; Li, J. Recovery of Potassium from Landfill Leachate Concentrates Using a Combination of Cation-Exchange Membrane Electrolysis and Magnesium Potassium Phosphate Crystallization. Sep. Purif. Technol. 2015, 144, 1–7. [Google Scholar] [CrossRef]
- Wu, S.; Zou, S.; Liang, G.; Qian, G.; He, Z. Enhancing Recovery of Magnesium as Struvite from Landfill Leachate by Pretreatment of Calcium with Simultaneous Reduction of Liquid Volume via Forward Osmosis. Sci. Total Environ. 2018, 610, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Gaterell, M.R.; Gay, R.; Wilson, R.; Gochin, R.J.; Lester, J.N. An Economic and Environmental Evaluation of the Opportunities for Substituting Phosphorus Recovered from Wastewater Treatment Works in Existing UK Fertiliser Markets. Environ. Technol. 2011, 21, 1067–1084. [Google Scholar] [CrossRef]
- Dunseth, M.G.; Salutsky, M.L.; Ries, K.M.; Shapiro, J.J. Ultimate Disposal of Phosphates from Wastewater by Recovery as Fertilizer; Water Pollution Control Research Series, 17070ESJ 01/70; US Department of the Interior; Federal Water Quality Administration: Washington, DC, USA, 1970.
- El Diwani, G.; El Rafie, S.; El Ibiari, N.N.; El-Aila, H.I. Recovery of Ammonia Nitrogen from Industrial Wastewater Treatment as Struvite Slow Releasing Fertilizer. Desalination 2007, 214, 200–214. [Google Scholar] [CrossRef]
- Ryu, H.D.; Lim, C.S.; Kang, M.K.; Lee, S.I. Evaluation of Struvite Obtained from Semiconductor Wastewater as a Fertilizer in Cultivating Chinese Cabbage. J. Hazard. Mater. 2012, 221–222, 248–255. [Google Scholar] [CrossRef]
- Uysal, A.; Yilmazel, Y.D.; Demirer, G.N. The Determination of Fertilizer Quality of the Formed Struvite from Effluent of a Sewage Sludge Anaerobic Digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Demir, S.; Sayilgan, E.; Eraslan, F.; Kucukyumuk, Z. Optimization of Struvite Fertilizer Formation from Baker’s Yeast Wastewater: Growth and Nutrition of Maize and Tomato Plants. Environ. Sci. Pollut. Res. 2014, 21, 3264–3274. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A. Assessment of Fertilizer Potential of the Struvite Produced from the Treatment of Methanogenic Landfill Leachate Using Low-Cost Reagents. Environ. Sci. Pollut. Res. 2016, 23, 5949–5959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Rahman, M.M.; Kwag, J.-H.; Kim, J.-H.; Ra, C.S. Eco-Friendly Production of Maize Using Struvite Recovered from Swine Wastewater as a Sustainable Fertilizer Source. Asian-Australas. J. Anim. Sci. 2011, 24, 1699–1705. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Geelen, D.; de Paepe, J.; Clauwaert, P.; de Pascale, S.; Rouphael, Y. An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability 2021, 13, 4218. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No. 1069/2009 and (EC) No. 1107/2009 and Repealing Regulation (EC) No. 2003/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 22 July 2022).







| Space Group | a (Å) | b (Å) | c (Å) | V (Å) | Z | |
|---|---|---|---|---|---|---|
| K-struvite (Lengenbach): from structure determination | Pmn21 | 6.892 (2) | 6.166 (2) | 11.139 (4) | 473.4 (3) | 2 |
| K-struvite (Lengenbach): refined from XRD data | Pmn21 | 6.903 (3) | 6.174 (2) | 11.146 (3) | 475.0 (2) | 2 |
| K-struvite (Rossblei). From XRD data | Pmn21 | 6.878 (1) | 6.161 (1) | 11.100 (1) | 470.41 (9) | 2 |
| K-struvite (PDF#35-0812) | Pmn21 | 6.873 | 6.160 | 11.087 | 469.40 | 2 |
| N-struvite (PDF#71-2089) | Pmn21 | 6.95 | 6.14 | 11.22 | 478.8 | 2 |
| Assignments | Reported Infrared (IR) Frequency Wavenumbers (cm−1) | Observed IR Frequency Wavenumbers (cm−1) | |
|---|---|---|---|
| Absorption peaks due to water of crystallization | H–O–H stretching vibrations of water crystallization | 3280 to 3550 | 3276.7, 3389.9,3521.6 |
| H–O–H stretching vibrations of cluster of water molecules of crystallization | 2060 to 2460 | 2375, 2480.5 | |
| H–O–H bending modes of vibrations | 1590 to 1650 | 1655.7, 1704.5 | |
| Wagging modes of vibration of coordinated water | 808 | 894 | |
| Absorption peaks due to PO4 units | ν1 symmetric stretching vibration of PO4 units | 930 to 995 | 1023.5 |
| ν2 symmetric bending vibration of PO4 units | 404 to 470 | 421.8 | |
| ν3 asymmetric stretching vibration of PO4 units | 1017 to 1163 | 1066.8, 1168.6, 1239.4 | |
| ν4 asymmetric bending modes | 509 to 554 | 507.8 | |
| Metal–oxygen bonds | Metal–oxygen bonds | 400–650 | 687.6 |
| Deformation of OH linked to Mg | 847 | 894 |
| Reagent | References | |||
|---|---|---|---|---|
| [14,19,29,30,75] a | [67] a | [13] b | [32] a | |
| CaCl2·2H2O | 4.4 | 0.7 | 0.09 c | 0.5 c |
| MgCl2·6H2O | 3.2 | 0.04 | 1.5 | |
| NaCl | 78.7 | 78.7 | 2.5 | 209 |
| Na2SO4 | 16.2 | 16.2 | 0.98 | 16.5 |
| Na3-citrate·2H2O | 2.6 | |||
| Na2-(COO)2 | 0.15 | 0.25 | ||
| KH2PO4 | 30.9 | 30.9 | 0.179 + 1.155 d | 25 |
| KCl | 21.5 | 21 | 0.955 | 24 |
| NH4Cl | 3.71 | 2.86 | 0.021 | |
| C4H7N3O (ceratinine) | 9.7 | 9.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabdaşlı, I.; Siciliano, A.; Limonti, C.; Tünay, O. Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review. Sustainability 2022, 14, 11680. https://doi.org/10.3390/su141811680
Kabdaşlı I, Siciliano A, Limonti C, Tünay O. Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review. Sustainability. 2022; 14(18):11680. https://doi.org/10.3390/su141811680
Chicago/Turabian StyleKabdaşlı, Işık, Alessio Siciliano, Carlo Limonti, and Olcay Tünay. 2022. "Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review" Sustainability 14, no. 18: 11680. https://doi.org/10.3390/su141811680
APA StyleKabdaşlı, I., Siciliano, A., Limonti, C., & Tünay, O. (2022). Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review. Sustainability, 14(18), 11680. https://doi.org/10.3390/su141811680








