Application Progress of O3/PMS Advanced Oxidation Technology in the Treatment of Organic Pollutants in Drinking Water
Abstract
:1. Introduction
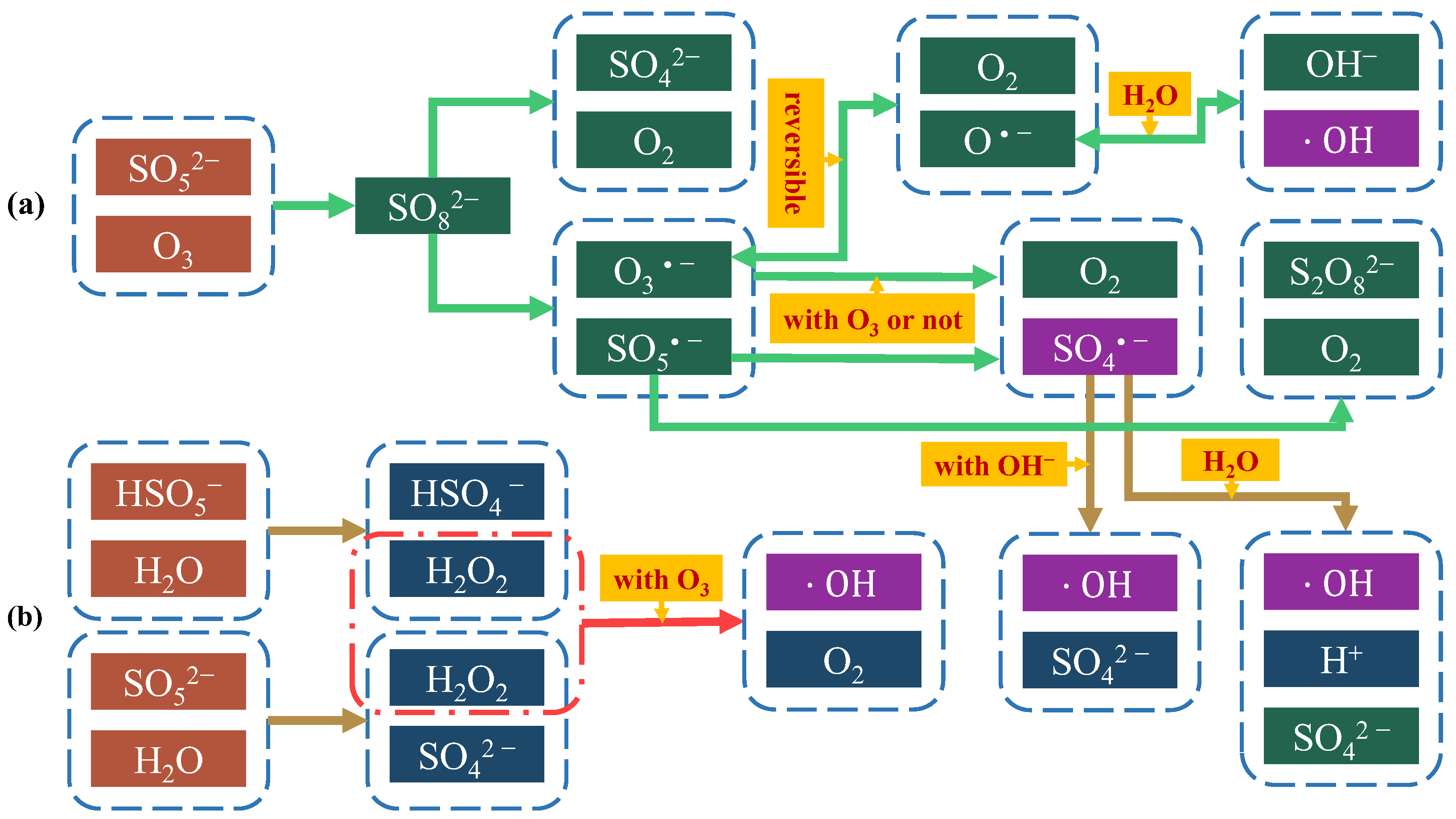
2. Reaction Mechanism of O3/PMS Process
- (1)
- O3 molecule directly oxidises and degrades pollutants;
- (2)
- Persulfate (S2O82−/SO52−) directly oxidises pollutants;
- (3)
- O3 reacts with H2O to produce •OH, •OH indirectly oxidises pollutants;
- (4)
- O3 guides PMS to generate SO4•−, and SO4•− and •OH work together to oxidise pollutants indirectly. At the same time, SO4•− can promote •OH generation in turn.
3. Status of O3/PMS Process Research
3.1. Using the O3/PMS Process for the Pretreatment of Drinking Water
3.2. Using the O3/PMS Process for the Advanced Treatment of Drinking Water
4. Evaluation of Bromate in the O3/PMS Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Acesulfame |
| AOPs | Advanced oxidation process |
| ATZ | Atrazine |
| BOD | Biochemical oxygen demand |
| CAP | Chloramphen-icol |
| COD | Chemical oxygen demand |
| DBPs | Disinfection by-products |
| DCAcAm | Dichloroacetamide |
| DOC | Dissolved organic carbon |
| DOM | Dissolved organic matter |
| EPA | Environmental Protection Agency |
| HA | Humic acid |
| IARC | International Agency for Research on Cancer |
| IBP | Ibuprofen |
| I-DBPs | Iodinated by-products |
| IPM | Iopamidol |
| I-THMS | Iodinated tri-halomethanes |
| MEMBRO3X | Membrane pore aeration |
| NDMA | N-Dimethylnitrosamine |
| NOM | Natural organic matter |
| O3/PMS | Ozone/peroxymonosulfate |
| O3/PMS/(O/C) | Ozone/persulfate/coagulation |
| PMS | Peroxymonosulfate |
| PMT | Prometon |
| pNBA | P-nitrobenzoic acid |
| PPCPs | Pharmaceutical and personal care products |
| rGO | Reduced graphene oxide |
| SMX | Sulfamethoxazole |
| SMZ | Sulfadimidine |
| SR-AOPs | Sulfate radical-based advanced oxidation processes |
| TBPs | Transformation by-products |
| TN | Total nitrogen |
| TP | Total phosphorus |
| TOC | Total organic carbon |
| TOX | Total organic halogens |
| TrOCs | Refractory trace organic compounds |
| UDMH | Unsymmetrical dimethylhydrazine |
| WHO | World Health Organization |
References
- Kallenborn, R.; Brorström-Lundén, E.; Reiersen, L.; Wilson, S. Pharmaceuticals and personal care products (PPCSs) in Arctic environments: Indicator contaminants for assessing local and remote anthropogenic sources in a pristine ecosystem in change. Environ. Sci. Pollut. Res. 2017, 25, 33001–33013. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.R.; Reemtsma, H.; Grimm, D.A.; Mitrac, S. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci. Total Environ. 2003, 311, 135–149. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.T.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J.V. Landfill leachate treatment methods: A review. Environ. Chem. Lett. 2006, 4, 51–61. [Google Scholar] [CrossRef]
- Luo, Y.L.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Gunten, U.V.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Helbling, D.E.; Johnson, D.R.; Honti, M.; Fenner, K. Micropollutant biotransformation kinetics associate with wwtp process parameters and microbial community characteristics. Environ. Sci. Technol. 2012, 46, 10579–10588. [Google Scholar] [CrossRef]
- Collado, N.; Rodriguez-Mozaz, S.; Gros, M.; Rubirola, A.; Barcelo, D.; Comas, J.; Rodriguez-Roda, I.; Buttiglieri, G. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014, 185, 202–212. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef]
- Wang, R.M.; Ji, M.; Zhai, H.Y.; Guo, Y.J.; Liu, Y. Occurrence of antibiotics and antibiotic resistance genes in WWTP effluent-receiving water bodies and reclaimed wastewater treatment plants. Sci. Total Environ. 2021, 796, 148919. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, J.; Lu, X.L.; Ma, J.; Liu, Y.Z. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: A novel advanced oxidation process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.M.; Zeng, Z.Q.; Arowo, M.; Zou, H.K.; Chen, J.F.; Shao, L. Degradation of methyl orange by ozone in the presence of ferrous and persulfate ions in a rotating packed bed. Chemosphere 2016, 146, 413–418. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Zhang, B.T.; Zhang, Y.; Teng, Y.G.; Fan, M.H. Sulfate radical and its application in decontamination technologies. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1756–1800. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Dionysiou, D.D.; Kumar, V. Oxidative removal of bisphenol a by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Shabiimam, M.A.; Dikshit, A.K. Treatment of municipal landfill leachate by oxidants. Am. J. Environ. Eng. 2012, 2, 1–5. [Google Scholar]
- Yang, S.Y.; Yang, X.; Shao, X.T.; Niu, R.; Wang, L.L. Activated carbon catalysed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Cong, J.; Wen, G.; Huang, T.L.; Deng, L.Y.; Ma, J. Study on enhanced ozonation degradation of para-chlorobenzoic acid by peroxymonosulfate in aqueous solution. Chem. Eng. J. 2015, 264, 399–403. [Google Scholar] [CrossRef]
- Peyton, G.R. The free-radical chemistry of persulfate-based total organic carbon analysers. Mar. Chem. 1993, 41, 91–103. [Google Scholar] [CrossRef]
- Fischbacher, A.; Sonntag, J.V.; Sonntag, C.V.; Schmidt, T.C. The •OH radical yield in the H2O2 + O3 (peroxone) reaction. Environ. Sci. Technol. 2013, 47, 9959–9964. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.X.; Dong, H.Y.; Liu, S.G.; Zhang, L.P.; Qiang, Z.M. Accelerated oxidation of iopamidol by ozone/peroxymonosulfate (O3/PMS) process: Kinetics, mechanism, and simultaneous reduction of iodinated disinfection by-product formation potential. Water Res. 2020, 173, 115615. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.; Merényi, G.; Johansson, E.; Brinck, T. Reaction of peroxyl radicals with ozone in water. J. Phys. Chem. A 2003, 107, 676–681. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Yuan, Z.; Sui, M.H.; Yuan, B.J.; Li, P.; Wang, J.Y.; Qin, J.; Xu, G.Y. Degradation of ibuprofen using ozone combined with peroxymonosulfate. Environ. Sci. Water Res. Technol. 2017, 3, 960–969. [Google Scholar] [CrossRef]
- Li, S.Y.; Huang, J.; Li, X.K.; Li, L.S. The relation of interface electron transfer and PMS activation by the H-bonding interaction between composite metal and MCM-48 during sulfamethazine ozonation. Chem. Eng. J. 2020, 398, 125529. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone-Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Gunten, U.V. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Zaidan, L.E.M.C.; Sales, R.V.D.L.; Salgado, J.B.D.A.; Silva, A.M.R.B.D.; Napoleão, D.C.; Rodríguez-Díaz, J.M.; Marques, O.M.; Benachour, M.; Silva, V.L.D. Photodegradation applied to the treatment of phenol and derived substances catalysed by TiO2/BiPO4 and biological toxicity analysis. Environ. Sci. Pollut. Res. 2017, 24, 6002–6012. [Google Scholar] [CrossRef]
- Miklos, D.B.; Wang, W.L.; Linden, K.G.; Drewes, J.E.; Hübner, U. Comparison of UV-AOPs (UV/H2O2, UV/PDS and UV/Chlorine) for TOrC removal from municipal wastewater effluent and optical surrogate model evaluation. Chem. Eng. J. 2019, 362, 537–547. [Google Scholar] [CrossRef]
- Tian, F.X.; Ye, W.K.; Xu, B.; Hua, X.J.; Ma, S.X.; Lai, F.; Gao, Y.Q.; Xing, H.B.; Xia, W.H.; Wang, B. Comparison of UV-induced AOPs (UV/Cl2, UV/NH2Cl, UV/ClO2 and UV/H2O2) in the degradation of iopamidol: Kinetics, energy requirements and DBPs-related toxicity in sequential disinfection processes. Chem. Eng. J. 2020, 398, 125570. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Fan, T.Y.; Wang, L.P.; Cheng, T.W.; Chen, S.S.; Yuan, M.H.; Cheng, S.K. Application of Fenton Method for the Removal of Organic Matter in Sewage Sludge at Room Temperature. Sustainability 2020, 12, 1518. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Arslan, I.; Balcioglu, I.A.; Tuhkanen, T. Advanced Oxidation of Synthetic Dyehouse Effluent by O3, H2O2/O3 and H2O2/UV Processes. Environ. Technol. 2010, 20, 921–931. [Google Scholar] [CrossRef]
- Rosenfeldta, E.J.; Linden, K.G.; Canonica, S.; Gunten, U.V. Comparison of the efficiency of •OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res. 2006, 40, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Q.P.; Feng, W.H. Application progress of O3/UV advanced oxidation technology in the treatment of organic pollutants in water. Sustainability 2022, 14, 1556. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate constants and mechanism of reaction of SO4•− with aromatic compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Cao, Y.; Qiu, W.; Zhao, Y.M.; Li, J.; Jiang, J.; Yang, Y.; Pang, S.Y.; Liu, G.Q. The degradation of chloramphenicol by O3/PMS and the impact of O3-based AOPs pre-oxidation on dichloroacetamide generation in post-chlorination. Chem. Eng. J. 2020, 401, 126146. [Google Scholar] [CrossRef]
- Du, X.; Yang, W.P.; Liu, Y.; Zhang, W.X.; Wang, Z.H.; Nie, J.X.; Li, G.B.; Liang, H. Removal of manganese, ferrous and antibiotics from groundwater simultaneously using peroxymonosulfate-assisted in-situ oxidation/coagulation integrated with ceramic membrane process. Sep. Purif. Technol. 2020, 252, 117492. [Google Scholar] [CrossRef]
- Liu, X.S.; Su, X.M.; Tian, S.J.; Li, Y.; Yuan, R.F. Mechanisms for simultaneous ozonation of sulfamethoxazole and natural organic matters in secondary effluent from sewage treatment plant. Front. Environ. Sci. Eng. 2021, 15, 75–86. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interfac. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Hong, Y.T.; Ding, S.K.; Wei, J.; Dong, S.K.; Xiao, R.; Chu, W.H. Transformation of antiviral ribavirin during ozone/pms intensified disinfection amid COVID-19 pandemic. Sci. Total Environ. 2021, 790, 148030. [Google Scholar] [CrossRef] [PubMed]
- Deniere, E.; Alagappan, R.P.; Langenhove, H.V.; Hulle, S.V.; Demeestere, K. The ozone-activated peroxymonosulfate process (O3/PMS) for removal of trace organic contaminants in natural and wastewater: Effect of the (in)organic matrix composition. Chem. Eng. J. 2022, 430, 133000. [Google Scholar] [CrossRef]
- Deniere, E.; Hulle, S.V.; Langenhove, H.V.; Demeestere, K. Advanced oxidation of pharmaceuticals by the ozone-activated peroxymonosulfate process: The role of different oxidative species. J. Hazard. Mater. 2018, 360, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Andrés, J.; Morillo-Ponce, J.; Ibáñez-López, M.E.; Acevedo-Merino, A.; García-Morales, J.L. Disinfection enhancement of single ozonation by combination with peroxymonosulfate salt. J. Environ. Chem. Eng. 2020, 8, 104335. [Google Scholar] [CrossRef]
- Gadgil, A. Drinking water in developing countries. Annu. Rev. Energy Environ. 1998, 23, 253–286. [Google Scholar] [CrossRef]
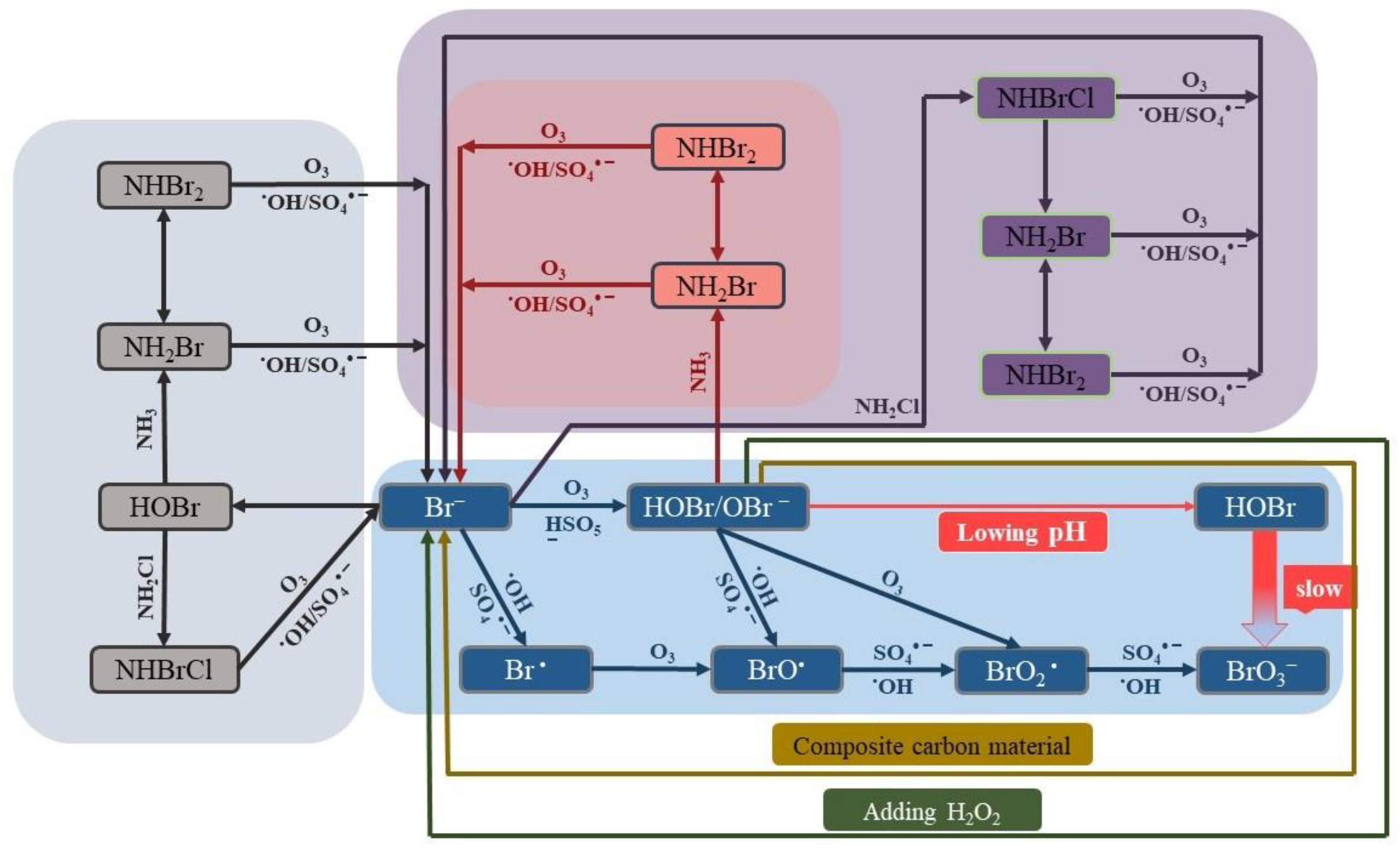
- Wen, G.; Wang, S.B.; Wang, T.; Feng, Y.B.; Chen, Z.H.; Lin, W.; Huang, T.L.; Ma, J. Inhibition of bromate formation in the O3/PMS process by adding low dosage of carbon materials: Efficiency and mechanism. Chem. Eng. J. 2020, 402, 126207. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.B.; Ma, W.X.; Wang, J.Y.; Xu, H.N.; Li, K.; Huang, T.L.; Ma, J.; Wen, G. Adding CuCo2O4-GO to inhibit bromate formation and enhance sulfamethoxazole degradation during the ozone/peroxymonosulfate process: Efficiency and mechanism. Chemosphere 2022, 286, 131829. [Google Scholar] [CrossRef]
- Shao, Y.; Pang, Z.C.; Wang, L.L.; Liu, X.W. Efficient degradation of acesulfame by ozone/peroxymonosulfate advanced oxidation process. Molecules 2019, 24, 2874. [Google Scholar] [CrossRef]
- Wu, G.Y.; Qin, W.L.; Sun, L.; Yuan, X.J.; Xia, D.S. Role of peroxymonosulfate on enhancing ozonation for micropollutant degradation: Performance evaluation, mechanism insight and kinetics study. Chem. Eng. J. 2019, 360, 115–123. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Mitch, W.A.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef] [PubMed]
- Lunn, G.; Sansone, E.B. Oxidation of 1,1-dimethylhydrazine (UDMH) in aqueous solution with air and hydrogen peroxide. Chemosphere 1994, 29, 1577–1590. [Google Scholar] [CrossRef]
- Huang, Y.J.; He, Z.X.; Liao, X.B.; Cheng, Y.S.; Qi, H. NDMA reduction mechanism of UDMH by O3/PMS technology. Sci. Total Environ. 2022, 805, 150418. [Google Scholar] [CrossRef]
- Merle, T.; Pronk, W.; Gunten, U.V. MEMBRO3X, a novel combination of a membrane contactor with advanced oxidation (O3/H2O2) for simultaneous micropollutant abatement and bromate minimisation. Environ. Sci. Technol. Lett. 2017, 4, 180–185. [Google Scholar] [CrossRef]
- Song, Y.; Feng, S.; Qin, W.; Li, J.; Guan, C.T.; Zhou, Y.; Gao, Y.; Zhang, Z.; Jiang, J. Formation mechanism and control strategies of N-nitrosodimethylamine (NDMA) formation during ozonation. Sci. Total Environ. 2022, 823, 153679. [Google Scholar] [CrossRef]
- Wen, G.; Wang, S.J.; Ma, J.; Huang, T.L.; Liu, Z.Q.; Zhao, L.; Su, J.F. Enhanced ozonation degradation of di-n-butyl phthalate by zero-valent zinc in aqueous solution: Performance and mechanism. J. Hazard. Mater. 2014, 265, 69–78. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Z.T.; Li, K.; Huang, T.L.; Ma, J.; Wen, G. Degradation of micropollutants and formation of oxidation by-products during the ozone/peroxymonosulfate system: A critical review. Water 2021, 13, 3126. [Google Scholar] [CrossRef]
- Wen, G.; Qiang, C.; Feng, Y.B.; Huang, T.L.; Ma, J. Bromate formation during the oxidation of bromide-containing water by ozone/peroxymonosulfate process: Influencing factors and mechanisms. Chem. Eng. J. 2018, 352, 316–324. [Google Scholar] [CrossRef]
- Soyluoglu, M.; Ersan, M.S.; Ateia, M.; Karanfil, T. Removal of bromide from natural waters: Bromide-selective vs. Conventional ion exchange resins. Chemosphere 2020, 238, 124583. [Google Scholar] [CrossRef]
- Ye, B.; Chen, Z.; Li, X.Z.; Liu, J.N.; Wu, Q.Y.; Yang, C.; Hu, H.Y.; Wang, R.H. Inhibition of bromate formation by reduced graphene oxide supported cerium dioxide during ozonation of bromide-containing water. Front. Environ. Sci. Eng. 2019, 13, 86–93. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.J.; Zhou, J.Z.; Huang, Z.F.; Liu, S.; Qian, G.R.; Gao, N.Y. Bromate inhibition by reduced graphene oxide in thermal/PMS process. Water Res. 2017, 122, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wen, G.; Ni, Y.L.; Wang, S.B.; Wang, S.; Yu, Y.; Huang, T.L.; Ma, J. Inhibition of bromate formation in the ozone/peroxymonosulfate process by ammonia, ammonia-chlorine and chlorine-ammonia pretreatment: Comparisons with ozone alone. Sep. Purif. Technol. 2022, 278, 119600. [Google Scholar] [CrossRef]
- Ling, L.; Deng, Z.; Fang, J.Y.; Shang, C. Bromate control during ozonation by ammonia-chlorine and chlorine-ammonia pretreatment: Roles of bromine-containing haloamines. Chem. Eng. J. 2020, 389, 123447. [Google Scholar] [CrossRef]
- Yang, J.X.; Li, J.; Dong, W.Y.; Ma, J.; Yang, Y.; Li, J.Y.; Yang, Z.C.; Zhang, X.L.; Gu, J.; Xie, W.Y.; et al. Enhancement of bromate formation by pH depression during ozonation of bromide-containing water in the presence of hydroxylamine. Water Res. 2017, 109, 135–143. [Google Scholar] [CrossRef]
- Wang, L.; Jing, K.; Hu, B.W.; Lu, J.H. Hydrogen peroxide suppresses the formation of brominated oxidation by-products in heat-activated peroxydisulfate oxidation process. Chem. Eng. J. 2021, 417, 129138. [Google Scholar] [CrossRef]



| No. | Reaction | Reaction Rate Constant (L·M−1·s−1) | References |
|---|---|---|---|
| 1 | 2.12 × 104 | [12,21] | |
| 2 | none | [12] | |
| 3 | none | [12] | |
| 4 | none | [22] | |
| 5 | none | [22] | |
| 6 | 1.6 × 105 | [22,23] | |
| 7 | 2.1 × 108 | [22,23] | |
| 8 | 2.2 × 108 | [22,23] | |
| 9 | <3 × 103 | [22,24] | |
| 10 | 7.3 × 107 | [22,24] |
| No. | Reaction | Reaction Rate Constant (L·M−1·s−1) | References |
|---|---|---|---|
| 11 | none | [12] | |
| 12 | none | [25] | |
| 13 | none | [25] | |
| 14 | (6.5 ± 1.0) × 107 M−1·s−1 | [26] | |
| 15 | <3 × 103 M−1·s−1 | [22] |
| Process | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| UV | (a) Green and environmentally friendly, with little impact on water quality; (b) Stable maturity and sustainable output of light. | (a) It will lead to the phenomenon of photoreactivation and dark repair. (b) The effect is average when used alone and is usually used in combination with other processes, such as UV/H2O2, UV/O3, UV/NH2Cl, etc. | [29,30,31] |
| Fenton method | (a) The equipment is simple, the reaction conditions are mild, and it can be operated under normal temperature and pressure; (b) Fast oxidation speed and high efficiency; (c) Green and environmentally friendly, low environmental pressure. | (a) H2O2 is unstable, and FeSO4 is added to generate Fe2+, which is difficult to operate; (b) It needs to operate under strong acid conditions and is highly corrosive; (c) High cost and a lot of sludge. | [32,33] |
| O3/H2O2 | (a) Fast oxidation speed and high efficiency; (b) Less secondary pollution; (c) It has excellent colour removal performance. | (a) H2O2 is unstable; (b) The effective reaction time is short; (c) Complex equipment, high energy consumption and high cost. | [34,35] |
| O3/PMS | (a) The equipment is simple, the reaction conditions are relatively mild, and it can be operated under normal temperature and pressure; (b) Fast oxidation speed and high efficiency; (c) Green and environmentally friendly, with less toxic by-products; (d) Two active free radicals with stronger applicability. | (a) The mineralisation ability is average; (b) It generally needs to operate in a weak alkaline environment. | [12,25] |
| Application Occasions | Process Types | Research Results | References |
|---|---|---|---|
| Pretreatment | O3/PMS | Under optimal conditions, the O3/PMS process can reduce the CAP to below the detection concentration within 5 min. Furthermore, compared with other AOPs processes, O3/PMS produced the least DCAcAm because SO4•− reduced the attack of oxidant on the DCAcAm side chain in CAP. | [38] |
| The O3/PMS process can degrade IBP by 72% within 20 min. Using HA to simulate the natural water matrix, any concentration of HA can inhibit the degradation of IBP, but there was no negative correlation. | [25] | ||
| The PMS in the system did not directly degrade ribavirin but indirectly acted on it by promoting the generation of •OH, and the effect was more evident when the pH value increased. | [42] | ||
| In natural water, although SO4•− has a great contribution to the removal of TrOCs, •OH is still the main oxidant for the removal of TrOCs. SO4•− also promotes the generation of •OH. | [43,44] | ||
| O3/PMS/(O/C) | Fe2+ and Mn2+ in groundwater can activate PMS in situ and promote the generation of •OH and SO4•−. However, NOM promotes the formation of by-products such as ferric hydroxide crystallisation or manganese precipitation, which reduces the filtration efficiency of the ceramic membrane, and the precipitation needs to be removed regularly. | [39] | |
| Advanced treatment | O3/PMS | SO4•− is the primary oxidative radical in the system, and the degradation contribution rate is 47.5%. Components such as chloride and NOM greatly affect the degradation of ACE in the system. Furthermore, the degradation performance of ACE in the system was the best at neutral pH. | [49] |
| Under the condition of pH 6.5, 7.5 mg·min−1 O3 and 100 mg·L−1 PMS was introduced, and after 7 min of reaction, the removal rate of PMT was up to 97.34%; this process produced 17 TBPs, which were finally mineralised into inorganic ions, CO2 and H2O. | [50] | ||
| •OH and SO4•− rapidly oxidise I− in the system to iodate, which rapidly reduces COD and BOD concentrations in the system, accelerates the degradation of IPM and effectively controls the formation of I-DBPs. | [22] | ||
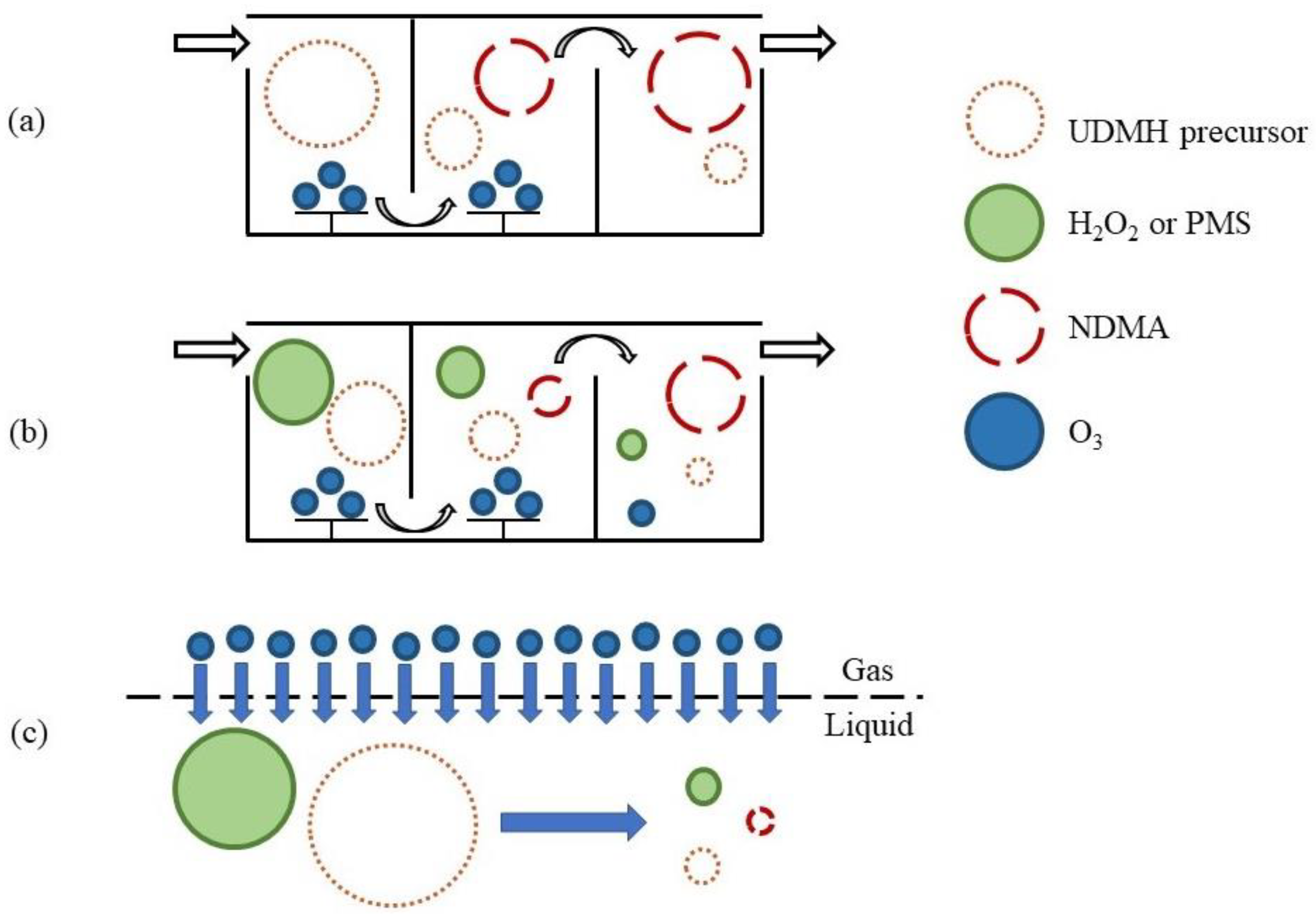
| O3 oxidises UDMH to NDMA, a strong carcinogen, before reacting with PMS to generate free radicals. Excessive PMS inhibits the formation of NDMA. | [54] | ||
| O3/PMS/CuCo2O4-GO | After the O3/PMS/CuCo2O4-GO process was reacted for 10 min, the removal rate of SMX was almost 100%. Compared with CuCo2O4, CuCo2O4-GO sacrifices a small part of the catalytic performance but brings the control ability of the composite carbon material to bromate. Furthermore, the GO material has a negative effect on the oxidation properties of O3. | [48] | |
| O3/PMS/MEMBRO3X | Using MEMBRO3X equipment, O3 was quickly oxidised to form •OH or SO4•−, which can quickly reduce UDMH below the detection concentration and effectively control the generation of disinfection by-product NDMA. | [55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Li, Q.; Feng, W.; Zhang, X. Application Progress of O3/PMS Advanced Oxidation Technology in the Treatment of Organic Pollutants in Drinking Water. Sustainability 2022, 14, 11718. https://doi.org/10.3390/su141811718
Lu H, Li Q, Feng W, Zhang X. Application Progress of O3/PMS Advanced Oxidation Technology in the Treatment of Organic Pollutants in Drinking Water. Sustainability. 2022; 14(18):11718. https://doi.org/10.3390/su141811718
Chicago/Turabian StyleLu, Hai, Qingpo Li, Weihao Feng, and Xiaoyu Zhang. 2022. "Application Progress of O3/PMS Advanced Oxidation Technology in the Treatment of Organic Pollutants in Drinking Water" Sustainability 14, no. 18: 11718. https://doi.org/10.3390/su141811718




