Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
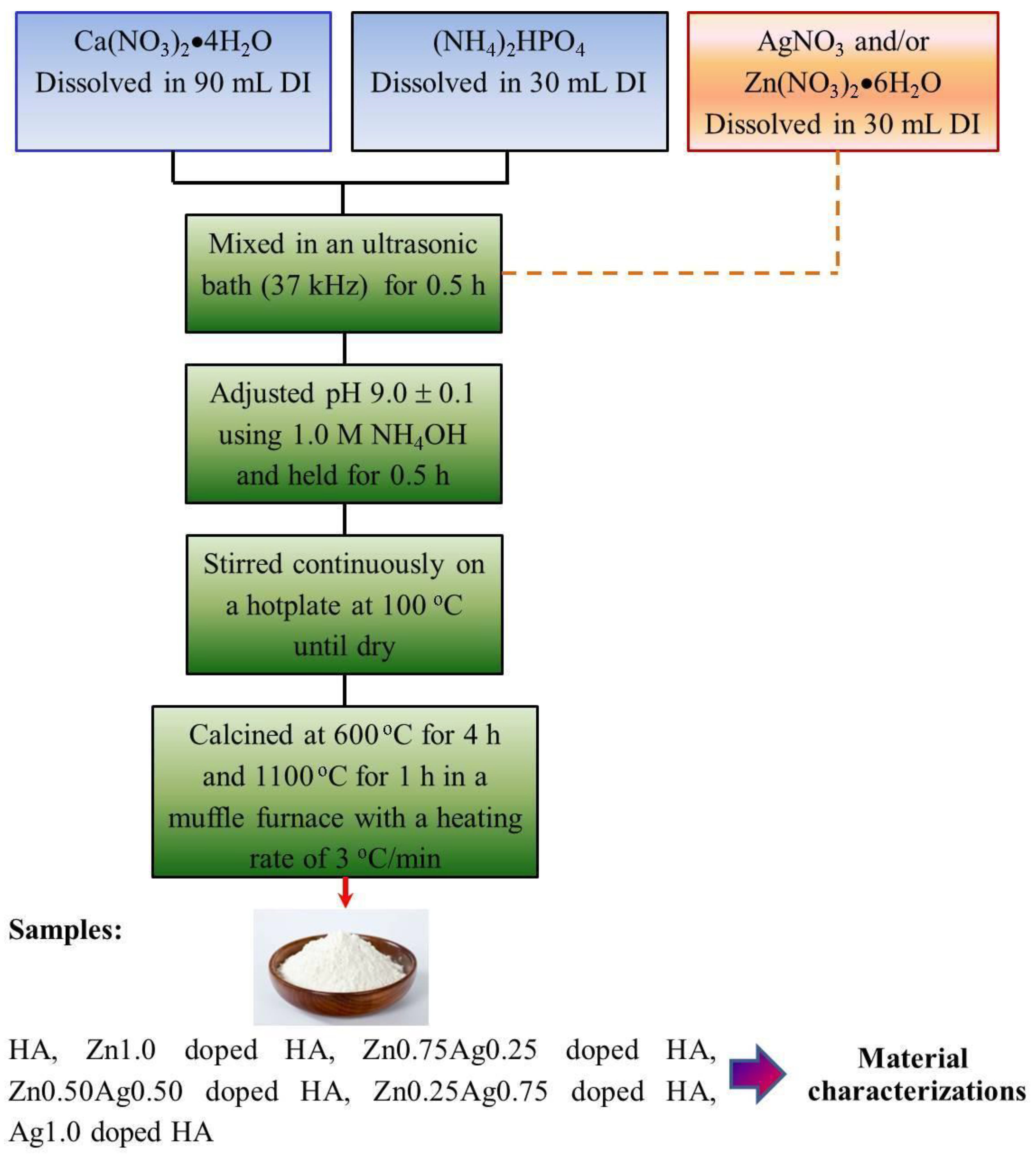
2.2. Synthesis of Zn-Ag Doped HA Samples
2.3. Characterization Analysis
2.4. Antibacterial and Antimicrobial Activity of HA and ZnAg Doped HA
2.5. Examination of Minium Bactericidal Concentration (MBC) and Minimum Inhibitory Concentration (MIC)
3. Results and Discussion
3.1. XRD Analysis
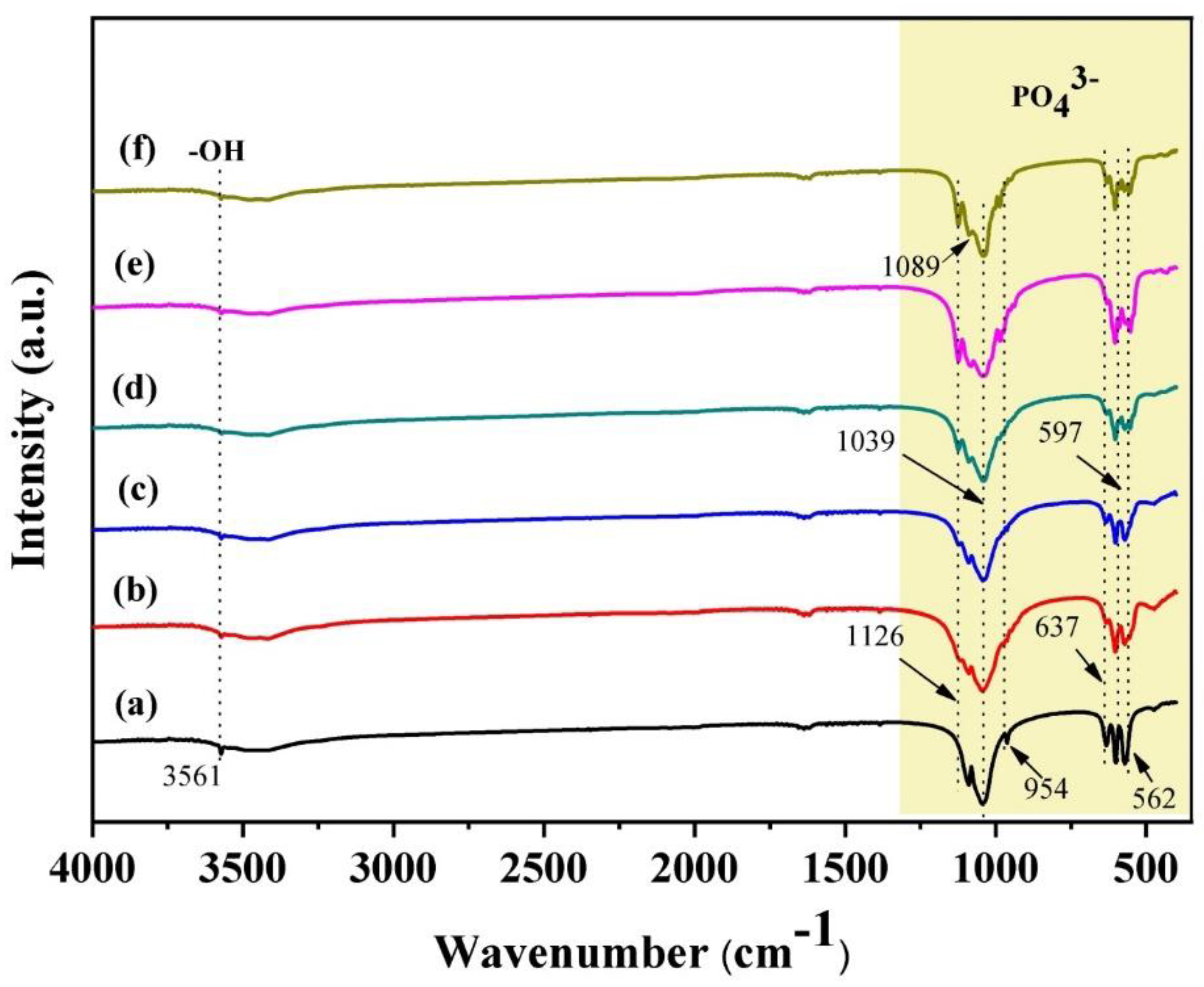
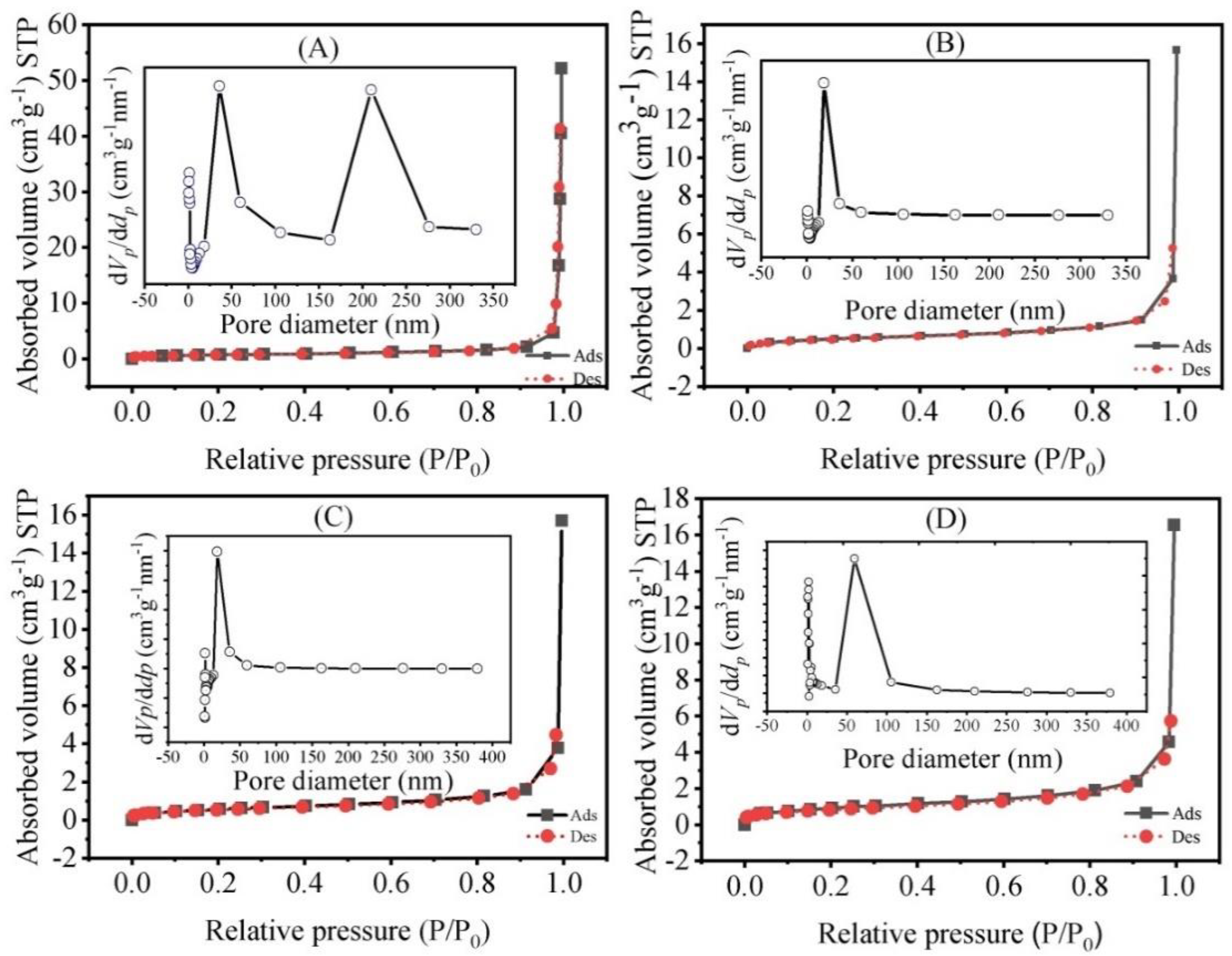
3.2. FT-IR Analysis and N2 Adsorption-Desorption
3.3. TEM Analysis
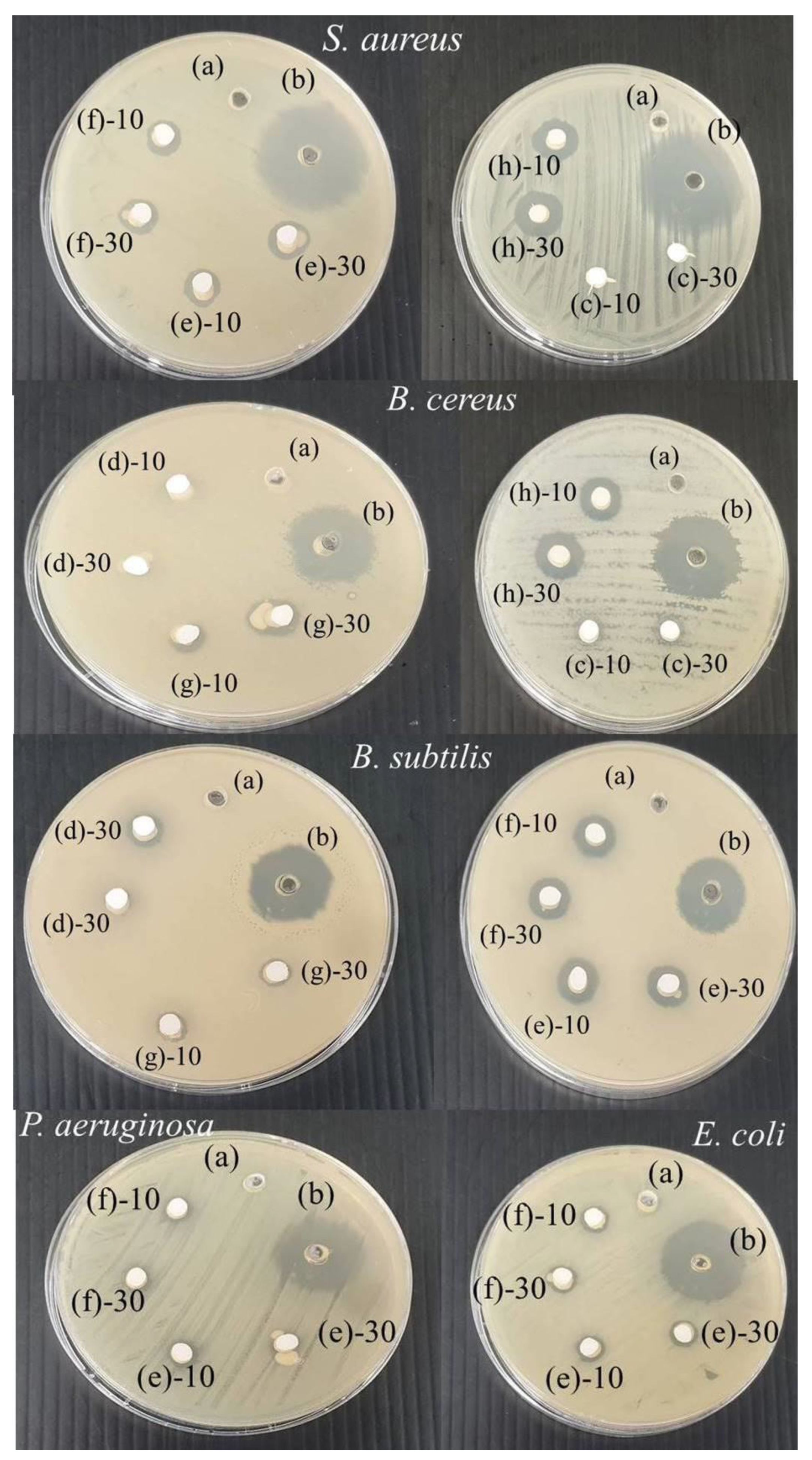
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone Mineral: Update on Chemical Composition and Structure. Osteoporos Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Nawang, R.; Hussein, M.Z.; Matori, K.A.; Che Abdullah, C.A.; Hashim, M. Physicochemical Properties of Hydroxyapatite/Montmorillonite Nanocomposite Prepared by Powder Sintering. Results Phys. 2019, 15, 102540. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhardwaj, A.; Bhatt, H.A. Nanocrystalline Calcium Phosphate Ceramics in Biomedical Engineering. Mater. Sci. Eng. C 2007, 27, 441–449. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The Future of Biologic Coatings for Orthopaedic Implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- Currey, J. Sacrificial Bonds Heal Bone. Nature 2001, 414, 699. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 18, Available online: https://www.elsevier.com/books/structure-and-chemistry-of-the-apatites-and-other-calcium-orthophosphates/elliott/978-0-444-81582-8 (accessed on 30 June 2022).
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite Nano Bioceramics Optimized 3D Printed Poly Lactic Acid Scaffold for Bone Tissue Engineering Application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Bhuvaneshwari, N.; Indira, J.; Kavitha, L. Novel Banana Peel Pectin Mediated Green Route for the Synthesis of Hydroxyapatite Nanoparticles and Their Spectral Characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 589–597. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Saraf, R. Ag-Doped Hydroxyapatite as Efficient Adsorbent for Removal of Congo Red Dye from Aqueous Solution: Synthesis, Kinetic and Equilibrium Adsorption Isotherm Analysis. Microporous Mesoporous Mater. 2016, 219, 134–144. [Google Scholar] [CrossRef]
- Vakili, S.N.; Rezayi, M.; Chahkandi, M.; Meshkat, Z.; Fani, M.; Moattari, A. A Novel Electrochemical DNA Biosensor Based on Hydroxyapatite Nanoparticles to Detect BK Polyomavirus in the Urine Samples of Transplant Patients. IEEE Sens. J. 2020, 20, 12088–12095. [Google Scholar] [CrossRef]
- Amjad, M.U.; Ahmed, B.A.; Ahmed, F.; Saeed, H.A. Development and Characterization of Silver-Doped Multi-Walled Carbon Nanotube Membranes for Water Purification Applications. Membranes 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Fanta, F.T.; Dubale, A.A.; Bebizuh, D.F.; Atlabachew, M. Copper Doped Zeolite Composite for Antimicrobial Activity and Heavy Metal Removal from Waste Water. BMC Chem. 2019, 13, 44. [Google Scholar] [CrossRef]
- Hong, X.; Wu, X.; Zhang, Q.; Xiao, M.; Yang, G.; Qiu, M.; Han, G. Hydroxyapatite Supported Ag3PO4 Nanoparticles with Higher Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2012, 258, 4801–4805. [Google Scholar] [CrossRef]
- Ghahremani, D.; Mobasherpour, I.; Salahi, E.; Ebrahimi, M.; Manafi, S.; Keramatpour, L. Potential of Nano Crystalline Calcium Hydroxyapatite for Tin(II) Removal from Aqueous Solutions: Equilibria and Kinetic Processes. Arab. J. Chem. 2017, 10, S461–S471. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Darmokoesoemo, H.; Kusuma, H.S. Hydroxyapatite-Based Adsorbents: Applications in Sequestering Heavy Metals and Dyes. J. Environ. Manag. 2022, 302, 113989. [Google Scholar] [CrossRef]
- Sangeetha, K.; Vidhya, G.; Vasugi, G.; Girija, E.K. Lead and Cadmium Removal from Single and Binary Metal Ion Solution by Novel Hydroxyapatite/Alginate/Gelatin Nanocomposites. J. Environ. Chem. Eng. 2018, 6, 1118–1126. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Wang, Y.; Song, X.; Fang, D.; Ge, S. The Sorption of Single- and Multi-Heavy Metals in Aqueous Solution Using Enhanced Nano-Hydroxyapatite Assisted with Ultrasonic. J. Environ. Chem. Eng. 2021, 9, 105240. [Google Scholar] [CrossRef]
- Gibert, O.; Valderrama, C.; Martínez, M.M.; Darbra, R.M.; Moncunill, J.O.; Martí, V. Hydroxyapatite Coatings on Calcite Powder for the Removal of Heavy Metals from Contaminated Water. Water 2021, 13, 1493. [Google Scholar] [CrossRef]
- Rastgordani, M.; Zolgharnein, J. Simultaneous Determination and Optimization of Titan Yellow and Reactive Blue 4 Dyes Removal Using Chitosan@hydroxyapatite Nanocomposites. J. Polym. Environ. 2021, 29, 1789–1807. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction Plasma Sprayed Sr and Mg Doped Nano Hydroxyapatite Coatings on Ti for Bone Implant. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Milán, Z.; de Las Pozas, C.; Cruz, M.; Borja, R.; Sánchez, E.; Ilangovan, K.; Espinosa, Y.; Luna, B. The Removal of Bacteria by Modified Natural Zeolites. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2001, 36, 1073–1087. [Google Scholar] [CrossRef]
- DeAlba-Montero, I.; Guajardo-Pacheco, J.; Morales-Sánchez, E.; Araujo-Martínez, R.; Loredo-Becerra, G.M.; Martínez-Castañón, G.-A.; Ruiz, F.; Compeán Jasso, M.E. Antimicrobial Properties of Copper Nanoparticles and Amino Acid Chelated Copper Nanoparticles Produced by Using a Soya Extract. Bioinorg. Chem. Appl. 2017, 2017, 1064918. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic Substitutions in Calcium Phosphates Synthesized at Low Temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Jankauskaitė, V.; Vitkauskienė, A.; Lazauskas, A.; Baltrusaitis, J.; Prosyčevas, I.; Andrulevičius, M. Bactericidal Effect of Graphene Oxide/Cu/Ag Nanoderivatives against Escherichia Coli, Pseudomonas Aeruginosa, Klebsiella Pneumoniae, Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Aureus. Int. J. Pharm. 2016, 511, 90–97. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-Substituted Calcium Phosphate Coatings Deposited by Plasma-Assisted Techniques: A Review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Singh, B.; Dubey, A.K.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In Vitro Biocompatibility and Antimicrobial Activity of Wet Chemically Prepared Ca10−xAgx(PO4)6(OH)2 (0.0 ≤ x ≤ 0.5) Hydroxyapatites. Mater. Sci. Eng. C 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Iqbal, N.; Abdul Kadir, M.R.; Nik Malek, N.A.N.; Humaimi Mahmood, N.; Raman Murali, M.; Kamarul, T. Rapid Microwave Assisted Synthesis and Characterization of Nanosized Silver-Doped Hydroxyapatite with Antibacterial Properties. Mater. Lett. 2012, 89, 118–122. [Google Scholar] [CrossRef]
- Lim, P.N.; Chang, L.; Thian, E.S. Development of Nanosized Silver-Substituted Apatite for Biomedical Applications: A Review. Nanomedicine 2015, 11, 1331–1344. [Google Scholar] [CrossRef]
- Guo, C.; Xue, J.; Dong, Y. Fabrication and Characterization of Hydroxyapatite Nanomaterial Dual Deposited with Nano Silver and Zinc Oxide. Mater. Lett. 2018, 219, 182–185. [Google Scholar] [CrossRef]
- Arumugam, M.; Pandi, P.; Balasubramanian, N.; Palanichamy, R.; Neyvasagam, K. Structural, Optical and Antimicrobial Activity of Copper and Zinc Doped Hydroxyapatite Nanopowders Using Sol-Gel Method. Mech. Mater. Sci. Eng. 2017, 9, 1. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chen, C.-C.; Wang, J.-B.; Wang, Y.-C.; Lin, F.-H. Flame Sprayed Zinc Doped Hydroxyapatite Coating with Antibacterial and Biocompatible Properties. Ceram. Int. 2017, 43, S829–S835. [Google Scholar] [CrossRef]
- Sery, A.A.; El-Boraey, H.A.; Abo-Elenein, S.A.; ElKorashey, R.M. CuFe2O4@ Hydroxyapatite Composite for the Environmental Remediation of Some Heavy Metal Ions: Synthesis and Characterization. Water Sci. 2021, 35, 154–164. [Google Scholar] [CrossRef]
- Panneerselvam, K.; Arul, K.T.; Warrier, A.R.; Asokan, K.; Dong, C.-L. Rapid Adsorption of Industrial Pollutants Using Metal Ion Doped Hydroxyapatite. AIP Conf. Proc. 2019, 2117, 020004. [Google Scholar] [CrossRef]
- Sopyan, I.; Pusparini, E.; Ramesh, S.; Tan, C.Y.; Ching, Y.C.; Wong, Y.H.; Abidin, N.I.Z.; Chandran, H.; Ramesh, S.; Bang, L.T. Influence of Sodium on the Properties of Sol-Gel Derived Hydroxyapatite Powder and Porous Scaffolds. Ceram. Int. 2017, 43, 12263–12269. [Google Scholar] [CrossRef]
- Triyono, D.; Hanifah, U.; Laysandra, H. Structural and Optical Properties of Mg-Substituted LaFeO3 Nanoparticles Prepared by a Sol-Gel Method. Results Phys. 2020, 16, 102995. [Google Scholar] [CrossRef]
- Chahkandi, M. Mechanism of Congo Red Adsorption on New Sol-Gel-Derived Hydroxyapatite Nano-Particle. Mater. Chem. Phys. 2017, 202, 340–351. [Google Scholar] [CrossRef]
- Varadarajan, N.; Balu, R.; Rana, D.; Ramalingam, M.; Kumar, T.S.S. Accelerated Sonochemical Synthesis of Calcium Deficient Hydroxyapatite Nanoparticles: Structural and Morphological Evolution. J. Biomater. Tissue Eng. 2014, 4, 295–299. [Google Scholar] [CrossRef]
- Rouhani, P.; Taghavinia, N.; Rouhani, S. Rapid Growth of Hydroxyapatite Nanoparticles Using Ultrasonic Irradiation. Ultrason. Sonochem. 2010, 17, 853–856. [Google Scholar] [CrossRef]
- Kim, W.; Saito, F. Sonochemical Synthesis of Hydroxyapatite from H3PO4 Solution with Ca(OH)2. Ultrason. Sonochem. 2001, 8, 85–88. [Google Scholar] [CrossRef]
- Kamonwannasit, S.; Futalan, C.M.; Khemthong, P.; Butburee, T.; Karaphun, A.; Phatai, P. Synthesis of Copper-Silver Doped Hydroxyapatite via Ultrasonic Coupled Sol-Gel Techniques: Structural and Antibacterial Studies. J. Sol-Gel Sci. Technol. 2020, 96, 452–463. [Google Scholar] [CrossRef]
- Ni, Z.; Gu, X.; He, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Synthesis of Silver Nanoparticle-Decorated Hydroxyapatite (HA@Ag) Poriferous Nanocomposites and the Study of Their Antibacterial Activities. RSC Adv. 2018, 8, 41722–41730. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yan, H.; Zhao, S.; Li, Z.; Li, Y.; Liang, X. Effect of Calcining Temperature on Particle Size of Hydroxyapatite Synthesized by Solid-State Reaction at Room Temperature. Adv. Powder Technol. 2013, 24, 1034–1038. [Google Scholar] [CrossRef]
- Sompech, S.; Dasri, T.; Thaomola, S. Preparation and Characterization of Amorphous Silica and Calcium Oxide from Agricultural Wastes. Orient. J. Chem. 2016, 32, 1923–1928. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Dan Batalu, N.; Cătălin Ciocoiu, R.; Voicu, Ş.I.; Stan, G.E.; Thakur, V.K. Synthesis and Characterization of Jellified Composites from Bovine Bone-Derived Hydroxyapatite and Starch as Precursors for Robocasting. ACS Omega 2018, 3, 1338–1349. [Google Scholar] [CrossRef]
- Nayak, B.; Samant, A.; Patel, R.; Misra, P.K. Comprehensive Understanding of the Kinetics and Mechanism of Fluoride Removal over a Potent Nanocrystalline Hydroxyapatite Surface. ACS Omega 2017, 2, 8118–8128. [Google Scholar] [CrossRef]
- Huang, A.; Dai, H.; Wu, X.; Zhao, Z.; Wu, Y. Synthesis and Characterization of Mesoporous Hydroxyapatite Powder by Microemulsion Technique. J. Mater. Res. Technol. 2019, 8, 3158–3166. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In Vitro Evaluation of Ag-Containing Calcium Phosphates: Effectiveness of Ag-Incorporated β-Tricalcium Phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef]
- Nath, S.; Kalmodia, S.; Basu, B. Densification, Phase Stability and in Vitro Biocompatibility Property of Hydroxyapatite-10 wt% Silver Composites. J. Mater. Sci. Mater. Med. 2010, 21, 1273–1287. [Google Scholar] [CrossRef]
- Rajendran, A.; Barik, R.C.; Natarajan, D.; Kiran, M.S.; Pattanayak, D.K. Synthesis, Phase Stability of Hydroxyapatite–Silver Composite with Antimicrobial Activity and Cytocompatability. Ceram. Int. 2014, 40, 10831–10838. [Google Scholar] [CrossRef]


| Samples | Symbols | Weight (g) | |||
|---|---|---|---|---|---|
| Ca(NO3)2 4H2O | Zn(NO3)2 6H2O | (NH4)2HPO4 | AgNO3 | ||
| Ca10(PO4)6(OH)2 | HA | 35.3469 | - | 11.8307 | - |
| Ca9.0Zn1.0(PO4)6(OH)2 | Zn1.0 doped HA | 30.9530 | 4.7391 | 11.5398 | - |
| Ca9.0Zn0.75Ag0.25(PO4)6(OH)2 | Zn0.75Ag0.25 doped HA | 30.6357 | 2.5455 | 10.6462 | 0.6133 |
| Ca9.0Zn0.50Ag0.50(PO4)6(OH)2 | Zn0.50Ag0.50 doped HA | 30.3182 | 1.6793 | 11.3066 | 1.2119 |
| Ca9.0Zn0.25Ag0.75(PO4)6(OH)2 | Zn0.25Ag0.75 doped HA | 30.0244 | 0.8313 | 11.1935 | 1.7997 |
| Ca9.0Ag1.0(PO4)6(OH)2 | Ag1.0 doped HA | 29.7268 | - | 11.0825 | 2.3758 |
| Samples | Lattice Parameters (nm) | Crystallite Size (nm) | |
|---|---|---|---|
| a | c | ||
| HA1100 | 0.9302 ± 0.0181 | 0.6865 ± 0.0008 | 71.24 |
| Zn1.0 doped HA1100 | 0.9323 ± 0.0146 | 0.6880 ± 0.0007 | 57.56 |
| Zn0.75Ag0.25 doped HA1100 | 0.9332 ± 0.0141 | 0.6886 ± 0.0001 | 67.62 |
| Zn0.50Ag0.50 doped HA1100 | 0.9316 ± 0.0139 | 0.6876 ± 0.0008 | 62.96 |
| Zn0.25Ag0.75 doped HA1100 | 0.9341 ± 0.0103 | 0.6876 ± 0.0007 | 70.78 |
| Ag1.0 doped HA1100 | 0.9279 ± 0.0114 | 0.6888 ± 0.0009 | 83.20 |
| Samples | SBET (m2/g) a | VTotal (cm3/g) b | DAV (nm) c |
|---|---|---|---|
| HA1100 | 2.675 | 0.030 | 45.027 |
| Zn1.0 doped HA1100 | 1.820 | 0.014 | 28.041 |
| Zn0.75Ag0.25 doped HA1100 | 2.117 | 0.012 | 23.252 |
| Zn0.50Ag0.50 doped HA1100 | 2.255 | 0.016 | 27.579 |
| Zn0.25Ag0.75 doped HA1100 | 3.139 | 0.018 | 23.354 |
| Ag1.0 doped HA1100 | 3.351 | 0.017 | 20.640 |
| Types of Bacteria | Inhibition Diameter (mm) (10 mg/mL) | Inhibition Diameter (mm) (30 mg/mL) | Inhibition Diameter (mm) for Tetracycline (30 μg) |
|---|---|---|---|
| HA1100 | |||
| S. aureus | ND | ND | - |
| B. cereus | ND | ND | - |
| B. subtilis | ND | ND | - |
| P. aeruginosa | ND | ND | - |
| E. coli | ND | ND | - |
| Zn1.0 doped HA1100 | |||
| S. aureus | ND | ND | - |
| B. cereus | ND | ND | - |
| B. subtilis | ND | 7.60 ± 0.01 | - |
| P. aeruginosa | ND | ND | - |
| E. coli | ND | ND | - |
| Zn0.75Ag0.25 doped HA1100 | |||
| S. aureus | 8.68 ± 0.47 | 9.60 ± 0.30 | - |
| B. cereus | 9.60 ± 0.60 | 10.02 ± 0.00 | - |
| B. subtilis | 10.33 ± 0.47 | 10.90 ± 0.16 | - |
| P. aeruginosa | ND | ND | - |
| E. coli | 7.81 ± 0.03 | 7.86 ± 0.09 | - |
| Zn0.5Ag0.5 doped HA1100 | |||
| S. aureus | 8.55 ± 0.40 | 8.88 ± 0.24 | - |
| B. cereus | 9.63 ± 0.00 | 9.76 ± 0.01 | - |
| B. subtilis | 9.98 ± 0.65 | 10.27 ± 0.08 | - |
| P. aeruginosa | ND | ND | - |
| E. coli | 7.97 ± 0.07 | 8.05 ± 0.04 | - |
| Zn0.25Ag0.75 doped HA1100 | |||
| S. aureus | 8.25 ± 0.99 | 9.00 ± 0.22 | - |
| B. cereus | 7.71 ± 0.32 | 9.13 ± 0.03 | - |
| B. subtilis | ND | 5.46 ± 0.06 | - |
| P. aeruginosa | ND | ND | - |
| E. coli | ND | ND | - |
| Ag1.0 doped HA1100 | |||
| S. aureus | 13.31 ± 0.21 | 13.58 ± 0.40 | - |
| B. cereus | 11.68 ± 0.00 | 12.65 ± 0.24 | - |
| B. subtilis | 8.44 ± 0.26 | 10.35 ± 0.08 | - |
| P. aeruginosa | ND | ND | - |
| E. coli | ND | ND | - |
| Tetracycline | |||
| S. aureus | - | - | 25.60 ± 0.15 |
| B. cereus | - | - | 22.32 ± 1.93 |
| B. subtilis | - | - | 17.49 ± 1.01 |
| P. aeruginosa | - | - | 18.27 ± 0.55 |
| E. coli | - | - | 21.32 ± 2.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phatai, P.; Prachumrak, N.; Kamonwannasit, S.; Kamcharoen, A.; Roschat, W.; Phewphong, S.; Futalan, C.M.; Khemthong, P.; Butburee, T.; Youngjan, S.; et al. Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity. Sustainability 2022, 14, 11756. https://doi.org/10.3390/su141811756
Phatai P, Prachumrak N, Kamonwannasit S, Kamcharoen A, Roschat W, Phewphong S, Futalan CM, Khemthong P, Butburee T, Youngjan S, et al. Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity. Sustainability. 2022; 14(18):11756. https://doi.org/10.3390/su141811756
Chicago/Turabian StylePhatai, Piaw, Narid Prachumrak, Sirilak Kamonwannasit, Agarat Kamcharoen, Wuttichai Roschat, Sunti Phewphong, Cybelle Morales Futalan, Pongtanawat Khemthong, Teera Butburee, Saran Youngjan, and et al. 2022. "Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity" Sustainability 14, no. 18: 11756. https://doi.org/10.3390/su141811756
APA StylePhatai, P., Prachumrak, N., Kamonwannasit, S., Kamcharoen, A., Roschat, W., Phewphong, S., Futalan, C. M., Khemthong, P., Butburee, T., Youngjan, S., Millare, J. C., & Prasitnok, O. (2022). Zinc-Silver Doped Mesoporous Hydroxyapatite Synthesized via Ultrasonic in Combination with Sol-Gel Method for Increased Antibacterial Activity. Sustainability, 14(18), 11756. https://doi.org/10.3390/su141811756








