Pluralistic Valuation of Codling Moth Regulation by Brown Long-Eared Bats in English Apple Orchards
Abstract
:1. Introduction
2. Background
2.1. Ecosystem Services and Economics
2.2. Apples and Arthropods
2.3. Bat Ecology
2.4. Bat Pest Regulation Services
3. Method
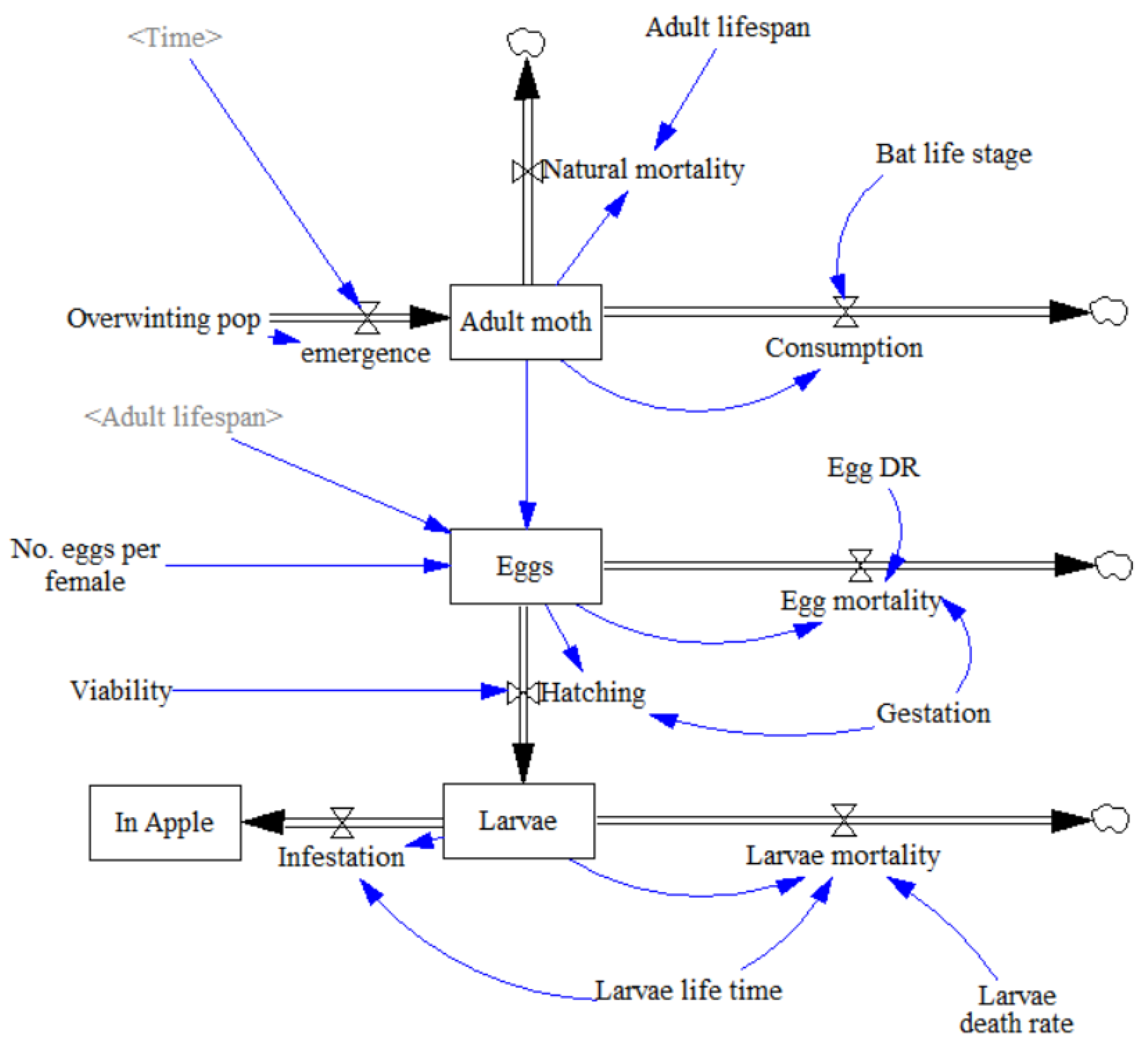
3.1. Establishing the Model
- 1
- Spring emergence of adult moths from their overwintering pupating stage, with a peak between May and June, was first established using a differential equation:where P = Overwintering population; te = Time at end of emergence period (day); t = Current time (day).
- 2
- Natural mortality rate of adults was calculated through current number of adults divided by average life expectancy assuming that all adult moths which are not consumed by the bat live out their full life expectancy and fecundity.where N = Current C. pomonella population; Ta = Adult C. pomonella lifespan.
- 3
- Consumption by the bat was also dependent upon the density of adult moths as follows:where C = P. auritus nightly consumption (g); η = Proportion of C. pomonella in P. auritus diet; γ = Mean C. pomonella body weight (mg); N = Current C. pomonella population; κ = Initial overwintering C. pomonella population.As well as being dependent on bat reproductive stage, nightly pest consumption rate is also dependent on the weight of prey and proportion of prey in the bat’s diet. Studies attempting to calculate the average proportion of Lepidoptera in P. auritus diet have attained widely different values. Although a West of Ireland study by Shiel et al. [64] is the geographically closest estimation, a more recent faecal analysis study in Switzerland by Andriollo et al. [65] utilised DNA metabarcoding, identifying a broader taxonomic diversity, Andriollo et al. [65] found that C. pomonella occurred in 7% of P. auritus guano from across 9 different roost sites. It was assumed C. pomonella constituted up to 7% of P. auritus diet. The current moth population expressed as a proportion of the peak population ensures that the bat was not able to consume more moths than were currently present. Where the current population of C. pomonella reached peak population size, codling moth was assumed to account for the full 7% in the P. auritus diet as it was deemed abundant.
- 4
- The egg-laying function was integrated into the egg life stage parameter. Number of eggs at any given time was calculated as “Eggs laid–Egg mortality–Hatching”. Assuming a sex ratio of 1:1 [66], half of the adult moth population were assumed to be female and lay a set number of eggs in their full lifetime, established with a rate of number of eggs divided by adult life expectancy. This can be visualised in the equation:where N = Current C. pomonella population; Ef = Number of eggs per female; Ta = Adult C. pomonella life expectancy.
- 5
- Egg mortality was calculated by number of eggs at given time multiplied by egg death rate divided by gestation time. Dividing egg death rate by gestation time ensured that the net proportion of eggs dying was equal to the egg death rate.where Et = Number of eggs at time t; De = Egg death rate; Tg = Gestation time.
- 6
- Hatching was calculated as a rate of viable eggs over gestation time:where Et = Number of eggs at time t; V = Proportion of viable eggs; Tg = Gestation time.
- 7
- Larvae mortality was calculated in a similar manner to egg mortality with number of larvae (i.e., Eggs hatched) multiplied by Larvae death rate over larvae life time.where Lt = Number of larvae at time t; Dl = Larvae death rate; Tl = Larvae life expectancy.
- 8
- The equation for infestation was number of larvae at time t divided by larvae life expectancy. It was assumed that one larva infests one apple and multiple larvae cannot infest the same apple [67].where Lt = Number of larvae at time t; Tl = Larvae life expectancy.
3.2. Data Collection
3.3. Sensitivity Analysis
3.4. Avoided Costs
4. Results
4.1. Baseline Model Results
4.2. Sensitivity Analysis
4.3. Avoided Costs
5. Discussion
5.1. Analysis
5.2. Integrated Pest Management
5.3. Wider Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Sensitivity Analysis Results
| Parameter | Parameter Value | Cumulative Crop Loss (Apples Infested) |
|---|---|---|
| Adult lifespan (days) | 16.100 17.389 18.678 19.967 21.256 22.544 23.833 25.122 26.411 27.700 | 434.61 409.87 387.79 367.98 350.09 333.86 319.06 305.53 293.09 281.63 |
| Number of eggs per female | 161.8 170.7 179.6 188.5 197.4 206.3 215.2 224.1 233.0 241.9 | 296.20 312.49 328.78 345.07 361.37 377.66 393.95 410.24 426.54 442.83 |
| Egg death rate (proportion of eggs dying per day) | 0.12 0.20 0.28 0.37 0.45 0.53 0.61 0.70 0.78 0.86 | 481.74 441.59 407.60 378.45 353.19 331.08 311.58 294.24 278.73 264.77 |
| Gestation (days) | 7.00 7.33 7.67 8.00 8.33 8.67 9.00 9.33 9.67 10.00 | 342.00 341.94 341.89 341.85 341.80 341.76 341.71 341.66 341.60 341.55 |
| Larvae lifespan (days) | 13.00 16.79 20.58 24.37 28.16 31.95 35.74 39.53 43.32 47.11 | 343.12 342.90 342.62 342.20 341.59 340.72 339.54 338.02 336.17 333.98 |
| Egg viability (proportion of eggs hatching) | 0.7170 0.7306 0.7441 0.7577 0.7712 0.7848 0.7983 0.8119 0.8255 0.8390 | 330.84 333.37 335.84 338.25 340.62 342.93 345.20 347.41 349.59 351.72 |
| Larvae death rate | 0.241000 0.317111 0.393222 0.469333 0.545444 0.621556 0.697667 0.773778 0.849889 0.926000 | 521.14 492.71 467.05 443.80 422.66 403.37 385.70 369.48 354.54 340.73 |
References
- National Statistics: Latest Horticulture Statistics; Department for Environment, Food & Rural Affairs: London, UK, 2020.
- Trade Map—Trade Statistics for International Business Development; International Trade Centre: Geneva, Switzerland, 2021.
- Burrough, A.E.; Oines, C.M.; Oram, S.P.; Robertson, H.J. Traditional Orchard Project in England—The Creation of an Inventory to Support the UK Habitat Action Plan. In Natural England Commissioned Reports; Natural England: Worcester, UK, 2010. [Google Scholar]
- Gimber, M. Traditional Orchard Decline; People’s Trust for Endangered Species: London, UK, 2021. [Google Scholar]
- Kunz, T.H.; De Torrez, E.B.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Turner, K.; Zylstra, M.; Brouwer, R.; de Groot, R.; Farber, S.; Ferraro, P.; Green, R.; Hadley, D.; Harlow, J.; et al. Ecosystem services and economic theory: Integration for policy-relevant research. Ecol. Appl. 2008, 18, 2050–2067. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, R.B. The case for methodological pluralism. Ecol. Econ. 1989, 1, 37–57. [Google Scholar] [CrossRef]
- Spash, C.L.; Aslaksen, I. Re-establishing an ecological discourse in the policy debate over how to value ecosystems and biodiversity. J. Environ. Manag. 2015, 159, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Daily, G.C. (Ed.) Nature’s Services: Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997. [Google Scholar]
- Study of Critical Environmental Problems (SCEP). Man’s Impact on the Global Environment; MIT Press: Cambridge, MA, USA, 1970. [Google Scholar]
- Holdren, J.P.; Ehrlich, P.R. Human population and the global environment. Am. Sci. 1974, 62, 282–292. [Google Scholar] [PubMed]
- Westman, W.E. How much are nature’s services worth? Science 1977, 197, 960–964. [Google Scholar] [CrossRef]
- Spash, C.L. The New Environmental Pragmatists, Pluralism and Sustainability. Environ. Values 2009, 18, 253–256. [Google Scholar] [CrossRef]
- Hanley, N.; Shogren, J.F.; White, B. Introduction to Environmental Economics; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Mols, C.M.M.; Visser, M.E. Great tits can reduce caterpillar damage in apple orchards. J. Appl. Ecol. 2002, 39, 888–899. [Google Scholar] [CrossRef]
- Glen, D.M. The effects of predators on the eggs of codling moth Cydia pomonella, in a cider-apple orchard in south-west England. Ann. Appl. Biol. 1975, 80, 115–119. [Google Scholar] [CrossRef]
- Cross, J.; Fountain, M.; Markó, V.; Nagy, C. Arthropod ecosystem services in apple orchards and their economic benefits. Ecol. Entomol. 2015, 40, 82–96. [Google Scholar] [CrossRef]
- Carreck, N.; Williams, I. The economic value of bees in the UK. Bee World 1998, 79, 115–123. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Li, X.; Yang, X.; Zhao, L.; Liu, L.; Zhu, C.; Li, R. Linking plant ecological stoichiometry with soil nutrient and bacterial communities in apple orchards. Appl. Soil Ecol. 2017, 126, 1–10. [Google Scholar] [CrossRef]
- Helm, D. Natural Capital: Valuing the Planet; Yale University Press: New Haven, CT, USA; London, UK, 2016. [Google Scholar]
- Gómez-Baggethun, E.; Ruiz-Pérez, M. Economic valuation and the commodification of ecosystem services. Prog. Phys. Geogr. Earth Environ. 2011, 35, 613–628. [Google Scholar] [CrossRef]
- de Groot, R. Investing in Natural Capital: The Ecological Economics Approach To Sustainability; Jansson, A., Hammer, M., Folke, C., Costanza, R., Eds.; Island Press: Washington, DC, USA, 1994; ISBN 978-1-55963-316-1. [Google Scholar]
- Gowdy, J.M. Terms and concepts in ecological economics. Wildl. Soc. Bull. 2000, 28, 26–33. [Google Scholar]
- Gomez-Baggethun, E.; Martin-Lopez, B. Ecological economics perspectives on ecosystem services valuation. In Handbook of ecological economics; Edward Elgar Publishing: Cheltenham, UK, 2015; pp. 260–282. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic Value of Biological Control in Integrated Pest Management of Managed Plant Systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Truslove, L.; Coston, D.; Evans, R.; Moss, E.; Dodson, C.; Jenner, N.; Biesmeijer, J.; Potts, S. Pollination deficits in UK apple orchards. J. Pollinat. Ecol. 2013, 12, 9–14. [Google Scholar] [CrossRef]
- EUR Exchange Rates. Bank of England. Available online: https://www.bankofengland.co.uk/boeapps/database/Rates.asp?TD=13&TM=Sep&TY=2021&into=EUR&rateview=D (accessed on 13 September 2021).
- Mace, A.; Ridley, L.; Parrish, G.; MacArthur, R.; Rainford, J.; Garthwaite, D. Pesticide Usage Survey Report 286: Orchards in the United Kingdom 2018; Land Use and Sustainability Team, Fera Science Ltd.: York, UK, 2018. [Google Scholar]
- Jackson, D.M. Codling Moth1 Egg Distribution on Unmanaged Apple Trees2. Ann. Entomol. Soc. Am. 1979, 72, 361–368. [Google Scholar] [CrossRef]
- AHDB and Department for Environment, Food and Rural Affairs. Apple Best Practice Guide: Codling Moth (Cydia pomonella L.). 2021. Available online: https://apples.ahdb.org.uk/codling-moth/ (accessed on 10 July 2021).
- Solomon, M.E.; Glen, D.M.; Kendall, D.A.; Milsom, N.F. Predation of Overwintering Larvae of Codling Moth (Cydia pomonella (L.)) by Birds. J. Appl. Ecol. 1976, 13, 341. [Google Scholar] [CrossRef]
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling Moth Management and Chemical Ecology. Annu. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef]
- Judd, G.J.R.; Gardiner, M.G.T.; Thomson, D.R. Control of codling moth in organically-managed apple orchards by combining pheromone-mediated mating disruption, post-harvest fruit removal and tree banding. Entomol. Exp. Appl. 1997, 83, 137–146. [Google Scholar] [CrossRef]
- Dewar, A.; Foster, S. Overuse of Pyrethroids may be implicated in the Recent BYDV Epidemics in Cereals. Outlooks Pest Manag. 2017, 28, 7–12. [Google Scholar] [CrossRef]
- Voudouris, C.C.; Sauphanor, B.; Franck, P.; Reyes, M.; Mamuris, Z.; Tsitsipis, J.A.; Vontas, J.; Margaritopoulos, J.T. Insecticide resistance status of the codling moth Cydia pomonella (Lepidoptera: Tortricidae) from Greece. Pestic. Biochem. Physiol. 2011, 100, 229–238. [Google Scholar] [CrossRef]
- Franck, P.; Reyes, M.; Olivares, J.; Sauphanor, B. Genetic architecture in codling moth populations: Comparison between microsatellite and insecticide resistance markers. Mol. Ecol. 2007, 16, 3554–3564. [Google Scholar] [CrossRef] [PubMed]
- Relyea, R.A. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol. Appl. 2005, 15, 618–627. [Google Scholar] [CrossRef]
- Szöcs, E.; Brinke, M.; Karaoglan, B.; Schäfer, R.B. Large Scale Risks from Agricultural Pesticides in Small Streams. Environ. Sci. Technol. 2017, 51, 7378–7385. [Google Scholar] [CrossRef]
- Flexner, J.; Lighthart, B.; Croft, B. The effects of microbial pesticides on non-target, beneficial arthropods. Agric. Ecosyst. Environ. 1986, 16, 203–254. [Google Scholar] [CrossRef]
- Koureas, M.; Tsakalof, A.; Tsatsakis, A.; Hadjichristodoulou, C. Systemic Review of Biomonitoring Studies to Determine the Association between Exposure to Organophosphorus and Pyrethroid Insecticides and Human Health Outcomes. Toxicol. Lett. 2012, 210, 155–168. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.W.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.A.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- McKerchar, M.; Potts, S.; Fountain, M.; Garratt, M.; Westbury, D. The potential for wildflower interventions to enhance natural enemies and pollinators in commercial apple orchards is limited by other management practices. Agric. Ecosyst. Environ. 2020, 301, 107034. [Google Scholar] [CrossRef]
- Altringham, J.D. British Bats; Harper Collins Publishers: New York, NY, USA, 2003. [Google Scholar]
- Verboom, B.; Huitema, H. The importance of linear landscape elements for the pipistrelle Pipistrellus pipistrellus and the serotine bat Eptesicus serotinus. Landsc. Ecol. 1997, 12, 117–125. [Google Scholar] [CrossRef]
- Mikula, P.; Čmoková, A. Lepidopterans in the summer diet of Eptesicus serotinus in Central Bohemia. Vespertilio 2012, 16, 197–201. [Google Scholar]
- Jay, M.; Roincé, C.B.; Ricard, J.M.; Garcin, A.; Mandrin, J.F.; Lavigne, C.; Bouvier, J.C.; Tupinier, Y.; Puechmaille, S. Biodiversité fonctionelle en verger de pommier: Les chauves-souris consomment-elles des ravageurs? Infos.-Ctifl. 2012, 286, 28–34. [Google Scholar]
- Andriollo, T.; Michaux, J.R.; Ruedi, M. Food for everyone: Differential feeding habits of cryptic bat species inferred from DNA metabarcoding. Mol. Ecol. 2021, 30, 4584–4600. [Google Scholar] [CrossRef]
- Baroja, U.; Garin, I.; Aihartza, J.; Arrizabalaga-Escudero, A.; Vallejo, N.; Aldasoro, M.; Goiti, U. Pest consumption in a vineyard system by the lesser horseshoe bat (Rhinolophus hipposideros). PLoS ONE 2019, 14, e0219265. [Google Scholar] [CrossRef] [PubMed]
- Boyd, I.L.; Stebbings, R.E. Population Changes of Brown Long-Eared Bats (Plecotus auritus) in Bat Boxes at Thetford Forest. J. Appl. Ecol. 1989, 26, 101–112. [Google Scholar] [CrossRef]
- UK Biodiversity Indicators 2020; Department for Environment, Food & Rural Affairs: London, UK, 2020.
- Martínez-Sastre, R.; García, D.; Miñarro, M.; Martín-López, B. Farmers’ perceptions and knowledge of natural enemies as providers of biological control in cider apple orchards. J. Environ. Manag. 2020, 266, 110589. [Google Scholar] [CrossRef]
- Cleveland, C.J.; Betke, M.; Federico, P.; Frank, J.D.; Hallam, T.G.; Horn, J.; López, J.D.; McCracken, G.F.; Medellín, R.A.; Moreno-Valdez, A.; et al. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front. Ecol. Environ. 2006, 4, 238–243. [Google Scholar] [CrossRef]
- Federico, P.; Hallam, T.G.; McCracken, G.; Purucker, S.T.; Grant, W.E.; Correa-Sandoval, A.N.; Westbrook, J.K.; Medellín, R.A.; Cleveland, C.J.; Sansone, C.G.; et al. Brazilian free-tailed bats as insect pest regulators in transgenic and conventional cotton crops. Ecol. Appl. 2008, 18, 826–837. [Google Scholar] [CrossRef]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Economic Importance of Bats in Agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef]
- Maas, B.; Clough, Y.; Tscharntke, T. Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol. Lett. 2013, 16, 1480–1487. [Google Scholar] [CrossRef]
- Maine, J.J.; Boyles, J.G. Bats initiate vital agroecological interactions in corn. Proc. Natl. Acad. Sci. USA 2015, 112, 12438–12443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig-Montserrat, X.; Torre, I.; López-Baucells, A.; Guerrieri, E.; Monti, M.M.; Ràfols-García, R.; Ferrer, X.; Gisbert, D.; Flaquer, C. Pest control service provided by bats in Mediterranean rice paddies: Linking agroecosystems structure to ecological functions. Mamm. Biol. 2015, 80, 237–245. [Google Scholar] [CrossRef]
- McLean, J.A.; Speakman, J.R. Energy budgets of lactating and non-reproductive Brown Long-Eared Bats (Plecotus auritus) suggest females use compensation in lactation. Funct. Ecol. 2002, 13, 360–372. [Google Scholar] [CrossRef]
- Speakman, J.R.; Racey, P.A. The Energetics of Pregnancy and Lactation in the Brown Long-Eared Bat, Plecotus Auritus. In Recent Advances in the Study of Bats; Racey, P.A., Rayner, J.M.V., Eds.; Cambridge University Press: Fenton, MB, USA, 1987; pp. 367–393. [Google Scholar]
- Margaritopoulos, J.T.; Voudouris, C.C.; Olivares, J.; Sauphanor, B.; Mamuris, Z.; Tsitsipis, J.A.; Franck, P. Dispersal ability in codling moth: Mark-release-recapture experiments and kinship analysis. Agric. For. Entomol. 2012, 14, 399–407. [Google Scholar] [CrossRef]
- Harris, S.; Yalden, D.W. Mammals of the British Isles Handbook; The Mammal Society: London, UK, 2008. [Google Scholar]
- Entwistle, A.C.; Racey, P.A.; Speakman, J.R. Social and Population Structure of a Gleaning Bat, Plecotus Auritus. J. Zool. 2000, 252, 11–17. [Google Scholar] [CrossRef]
- Shiel, C.; McAney, C.; Fairley, J. Analysis of the Diet of Natterer’s Bat Myotis Nattereri and the Common Long-eared Bat Plecotus Auritus in the West of Ireland. J. Zool. 1991, 223, 299–305. [Google Scholar] [CrossRef]
- Andriollo, T.; Gillet, F.; Michaux, J.R.; Ruedi, M. The menu varies with metabarcoding practices: A case study with the bat Plecotus auritus. PLoS ONE 2019, 14, e0219135. [Google Scholar] [CrossRef]
- Glen, D.M.; Milsom, N.F.; Wiltshire, C.W. The Effect of Predation by Blue-Tits (Parus caeruleus) on the Sex-Ratio of Codling Moth (Cydia pomonella). J. Appl. Ecol. 1981, 18, 133. [Google Scholar] [CrossRef]
- Plantwise Knowledge Bank: Codling Moth, Cydia Pomonella; Centre for Agriculture and Bioscience (CAB) International: Oxfordshire, UK, 2021.
- Graf, B.; Höhn, H.; Höpli, H.; Kuske, S. Predicting the phenology of codling moth, Cydia pomonella, for sustainable pest management in Swiss apple orchards. Entomol. Exp. Appl. 2018, 166, 618–627. [Google Scholar] [CrossRef]
- Kuyulu, A.; Genc, H.B.; Codling Moth, L.R. Cydia pomonella (L.) (Lepidoptera: Tortricidae) on Its Natural Host “Green Immature Apple” Malus domestica (Borkh; Rosaceae); Türk Tarim Ve Doga Bilimleri Derg: Rosales, Philippines, 2019; Volume 6. [Google Scholar]
- Glen, D.M.; Milsom, N.F. Survival of mature larvae of codling moth (Cydia pomonella) on apple trees and ground. Ann. Appl. Biol. 1978, 90, 133–146. [Google Scholar] [CrossRef]
- Glen, D.M. Predation of Codling Moth Eggs, Cydia pomonella, the Predators Responsible and Their Alternative Prey. J. Appl. Ecol. 1977, 14, 445–456. [Google Scholar] [CrossRef]
- Adams, C.G.; Schenker, J.H.; McGhee, P.S.; Gut, L.J.; Brunner, J.F.; Miller, J.R. Maximizing Information Yield from Pheromone-Baited Monitoring Traps: Estimating Plume Reach, Trapping Radius, and Absolute Density of Cydia pomonella (Lepidoptera: Tortricidae) in Michigan Apple. J. Econ. Entomol. 2017, 110, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Alford, D.V.; Carden, P.W.; Dennis, E.B.; Gould, H.J.; Vernon, J.D.R. Monitoring codling and tortrix moths in United Kingdom apple orchards using pheromone traps. Ann. Appl. Biol. 1979, 91, 165–178. [Google Scholar] [CrossRef]
- Glenn, P.A. Codling-moth Investigations of the State Entomologist’s Office. Ill. Nat. Hist. Surv. Bull. 1915, 14, 219–289. [Google Scholar] [CrossRef]
- Sæthre, M.-G.; Hofsvang, T. Effect of Temperature on Oviposition Behavior, Fecundity, and Fertility in Two Northern European Populations of the Codling Moth (Lepidoptera: Tortricidae). Environ. Entomol. 2002, 31, 804–815. [Google Scholar] [CrossRef]
- Apples & Orchards. The National Association of Cider Makers. Available online: https://cideruk.com/orchards-apples/ (accessed on 14 September 2022).
- Waitrose & Partners. Available online: https://www.waitrose.com/ecom/products/essential-loose-braeburn-apples/088640-45525-45526 (accessed on 14 June 2021).
- Pesticide Fact Sheet: Chlorantraniliprole; United States Environmental Protection Agency: Washington, DC, USA, 2008.
- Leach, A.; Mumford, J. Pesticide Environmental Accounting: A method for assessing the external costs of individual pesticide applications. Environ. Pollut. 2008, 151, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.; Petzoldt, C.; Degnil, J.; Tette, J. A Method to Measure the Environmental Impacts of Pesticides. N. Y. Food Life Sci. Bull. 1992, 139, 1–8. [Google Scholar]
- Taylor, P.J.; Grass, I.; Alberts, A.J.; Joubert, E.; Tscharntke, T. Economic value of bat predation services—A review and new estimates from macadamia orchards. Ecosyst. Serv. 2018, 30, 372–381. [Google Scholar] [CrossRef]
- Corcoran, A.J.; Conner, W.E. Bats jamming bats: Food competition through sonar interference. Science 2014, 346, 745–747. [Google Scholar] [CrossRef]
- Ricci, B.; Franck, P.; Bouvier, J.-C.; Casado, D.; Lavigne, C. Effects of hedgerow characteristics on intra-orchard distribution of larval codling moth. Agric. Ecosyst. Environ. 2011, 140, 395–400. [Google Scholar] [CrossRef]
- Sauphanor, B.; Severac, G.; Maugin, S.; Toubon, J.F.; Capowiez, Y. Exclusion netting may alter reproduction of the codling moth (Cydia pomonella) and prevent associated fruit damage to apple orchards. Entomol. Exp. et Appl. 2012, 145, 134–142. [Google Scholar] [CrossRef]
- Solomon, M.E.; Glen, D.M. Prey Density and Rates of Predation by Tits Parus Spp. on Larvae of Codling Moth Cydia pomonella (L.) under Bark. J. Appl. Ecol. 1979, 16, 49–59. [Google Scholar] [CrossRef]
- Olimpi, E.M.; Philpott, S.M. Agroecological farming practices promote bats. Agric. Ecosyst. Environ. 2018, 265, 282–291. [Google Scholar] [CrossRef]
- Farber, S.C.; Costanza, R.; Wilson, M.A. Economic and ecological concepts for valuing ecosystem services. Ecol. Econ. 2002, 41, 375–392. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Muradian, R. In markets we trust? Setting the boundaries of Market-Based Instruments in ecosystem services governance. Ecol. Econ. 2015, 117, 217–224. [Google Scholar] [CrossRef]
- de Roincé, C.B.; Lavigne, C.; Ricard, J.-M.; Franck, P.; Bouvier, J.-C.; Garcin, A.; Symondson, W.O.C. Predation by generalist predators on the codling moth versus a closely-related emerging pest the oriental fruit moth: A molecular analysis. Agric. For. Entomol. 2012, 14, 260–269. [Google Scholar] [CrossRef]
- Crosby, J. The Social and Cultural Value of the Apple and the Orchard in Victorian England. Ph.D. Thesis, University of Essex, Colchester, UK, 2020. [Google Scholar]



| C. pomonella Parameter | Baseline Value | Range | Author(s) | Methods of Obtaining | Country of Study |
|---|---|---|---|---|---|
| Adult lifespan | 21.9 days (female) (at mean 22 °C) | 16.1–27.7 days | Graf et al. [68] | Captive study of individuals in climate chambers at six constant temperatures. | Switzerland |
| Number of eggs per female moth | 186.7 | 161.8–241.9 | Graf et al. [68] | As above. | Switzerland |
| Egg viability | 0.778 | 0.717–0.839 | Kuyulu & Genc [69] | Rearing of C. pomonella on Malus domestica cv. ‘Gala’ in laboratory conditions | Turkey |
| Egg mortality (inc. arthropod predation) | 0.49 | 0.12–0.86 | Glen [71] | Field experiment. Known number of eggs glued to known locations over regular intervals in a cider apple orchard. | South-West England |
| Gestation | 8.5 days | 7–10 days | AHDB, DEFRA [30,51] | Unknown | UK |
| Larvae mortality | 0.92 | 0.241–0.926 Kuyulu & Genc [69] | Glen & Milsom [70] | Field experiment. Apple trees kept in different conditions with some excluding ground predatory beetles or birds. | South-West England |
| Adult body mass | Males = 0.0164 g, Females = 0.02785 g | Unknown | Kuyulu & Genc [69] | As above. | Turkey |
| Larvae lifespan | 27.15 days | 13–47.1 days | Graf et al. [68] | As above. | Switzerland |
| Conditions for C. pomonella Reproduction | Low Reproduction | Stochastic Mode Reference | High Reproduction |
|---|---|---|---|
| Percentage crop loss reduction | 82.45–83.94% | 81.06–83.68% | 75.07–77.00% |
| Gross avoided crop loss | 868–884 apples | 1066–2718 apples | 3751–3847 apples |
| Avoided costs | |||
| Crop loss (non-reprod.) Crop loss (pregnant) | GBP 156.24 GBP 159.12 | GBP 191.88–489.24 GBP 221.58–324.90 | GBP 657.18 GBP 692.46 |
| Pesticide application | GBP 114.54 | GBP 114.54 | GBP 114.54 |
| Hidden pesticide cost | GBP 2.28 | GBP 2.28 | GBP 2.28 |
| Total | GBP 273.06–275.94 | GBP 308.70–606.06 | GBP 774.00–809.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, F.; Ament, J. Pluralistic Valuation of Codling Moth Regulation by Brown Long-Eared Bats in English Apple Orchards. Sustainability 2022, 14, 11966. https://doi.org/10.3390/su141911966
Murphy F, Ament J. Pluralistic Valuation of Codling Moth Regulation by Brown Long-Eared Bats in English Apple Orchards. Sustainability. 2022; 14(19):11966. https://doi.org/10.3390/su141911966
Chicago/Turabian StyleMurphy, Francis, and Joe Ament. 2022. "Pluralistic Valuation of Codling Moth Regulation by Brown Long-Eared Bats in English Apple Orchards" Sustainability 14, no. 19: 11966. https://doi.org/10.3390/su141911966
APA StyleMurphy, F., & Ament, J. (2022). Pluralistic Valuation of Codling Moth Regulation by Brown Long-Eared Bats in English Apple Orchards. Sustainability, 14(19), 11966. https://doi.org/10.3390/su141911966





