The Characteristics of Dissolved Organic Matter and Soil Microbial Communities in the Soils of Larix principis-rupprechtii Mayr. Plantations in the Qinling Mountains, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Physicochemical Properties and DOM Analysis
2.4. PARAFAC Modeling
2.5. Soil Microbial PLFA Analysis
2.6. Statistical Analysis
3. Results
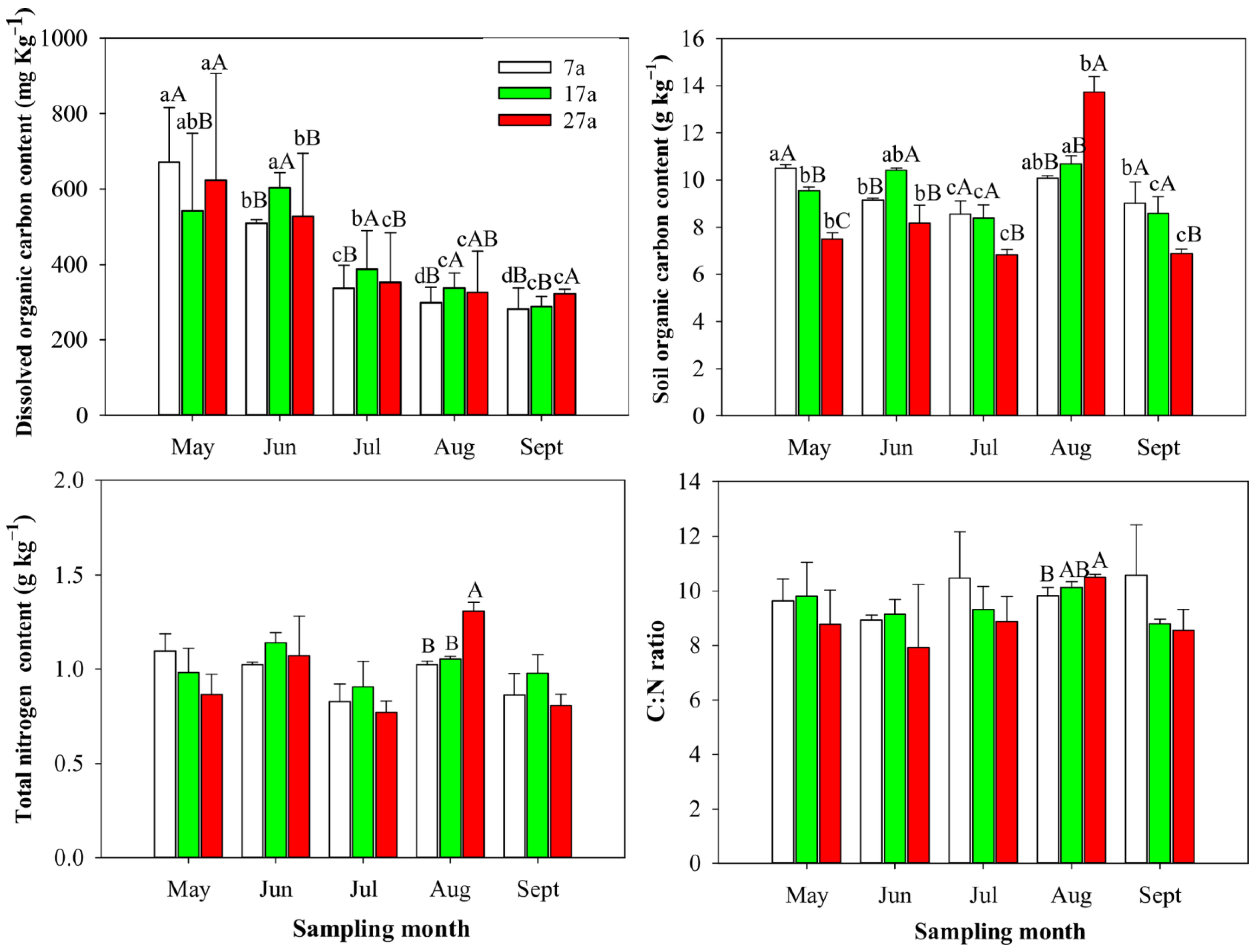
3.1. Seasonal Changes in the Soil Physicochemical Properties of Stands of Differently Ages
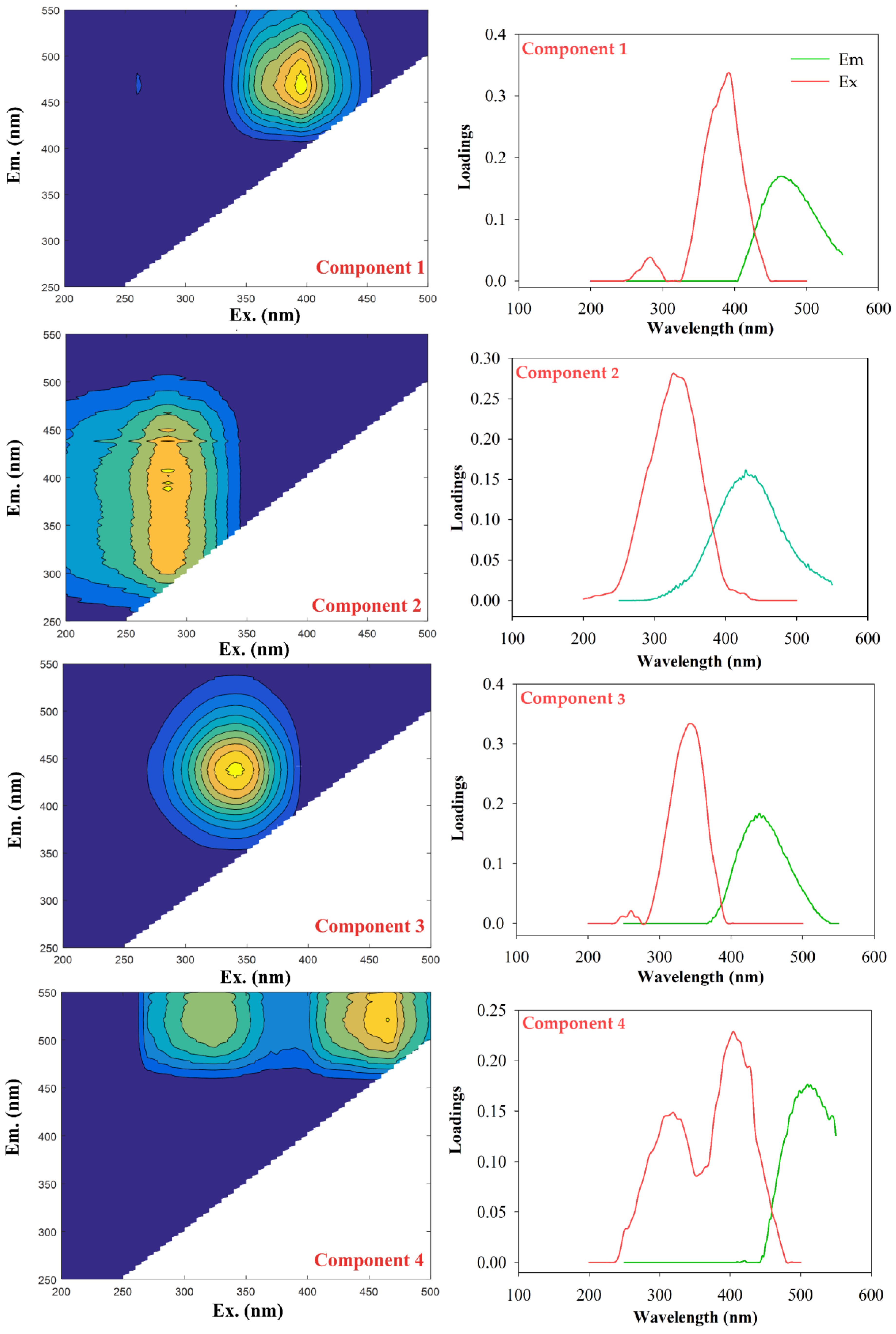
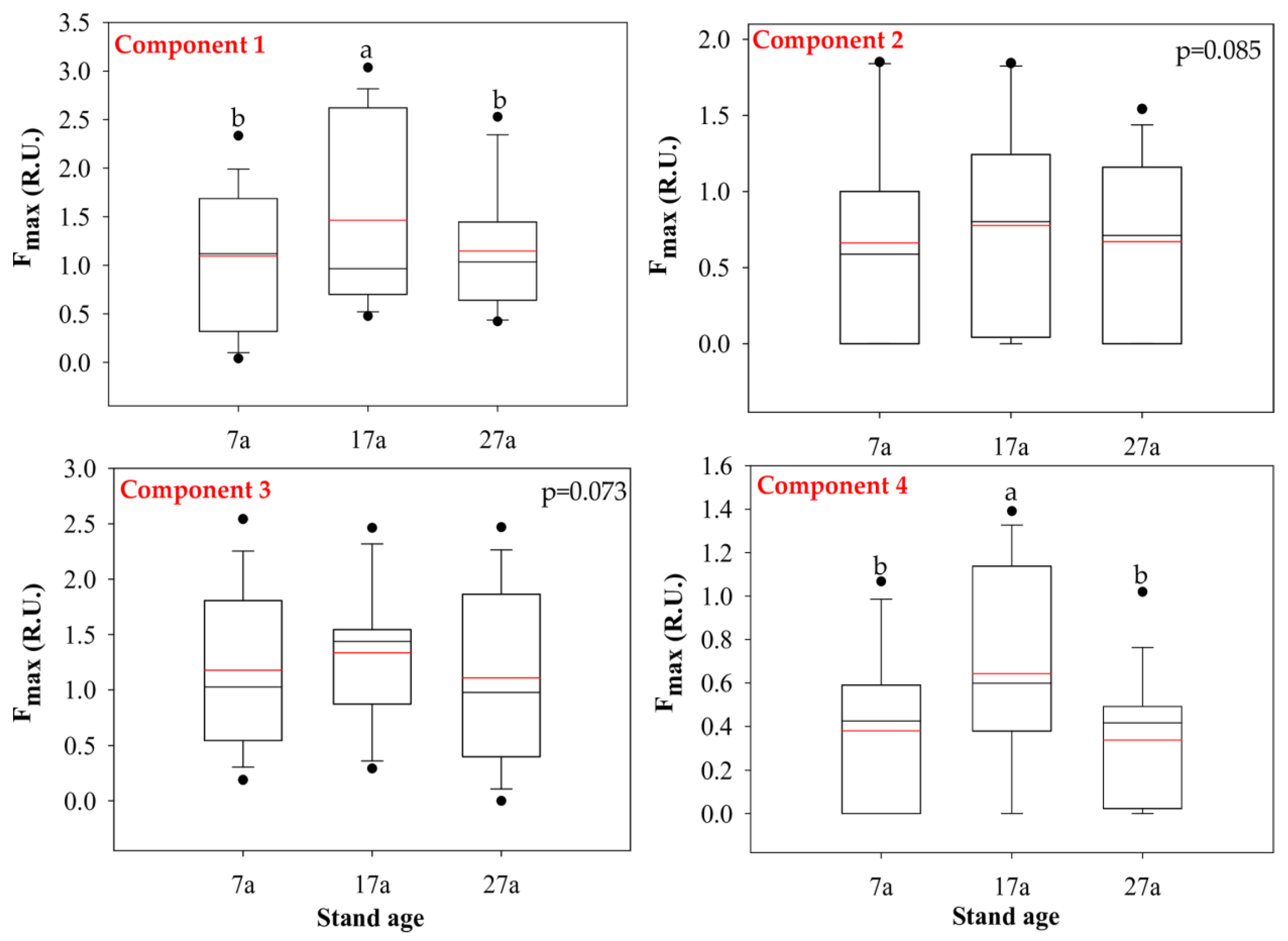
3.2. Characteristics of the Different Components Identified via EEM-PARAFAC Analysis
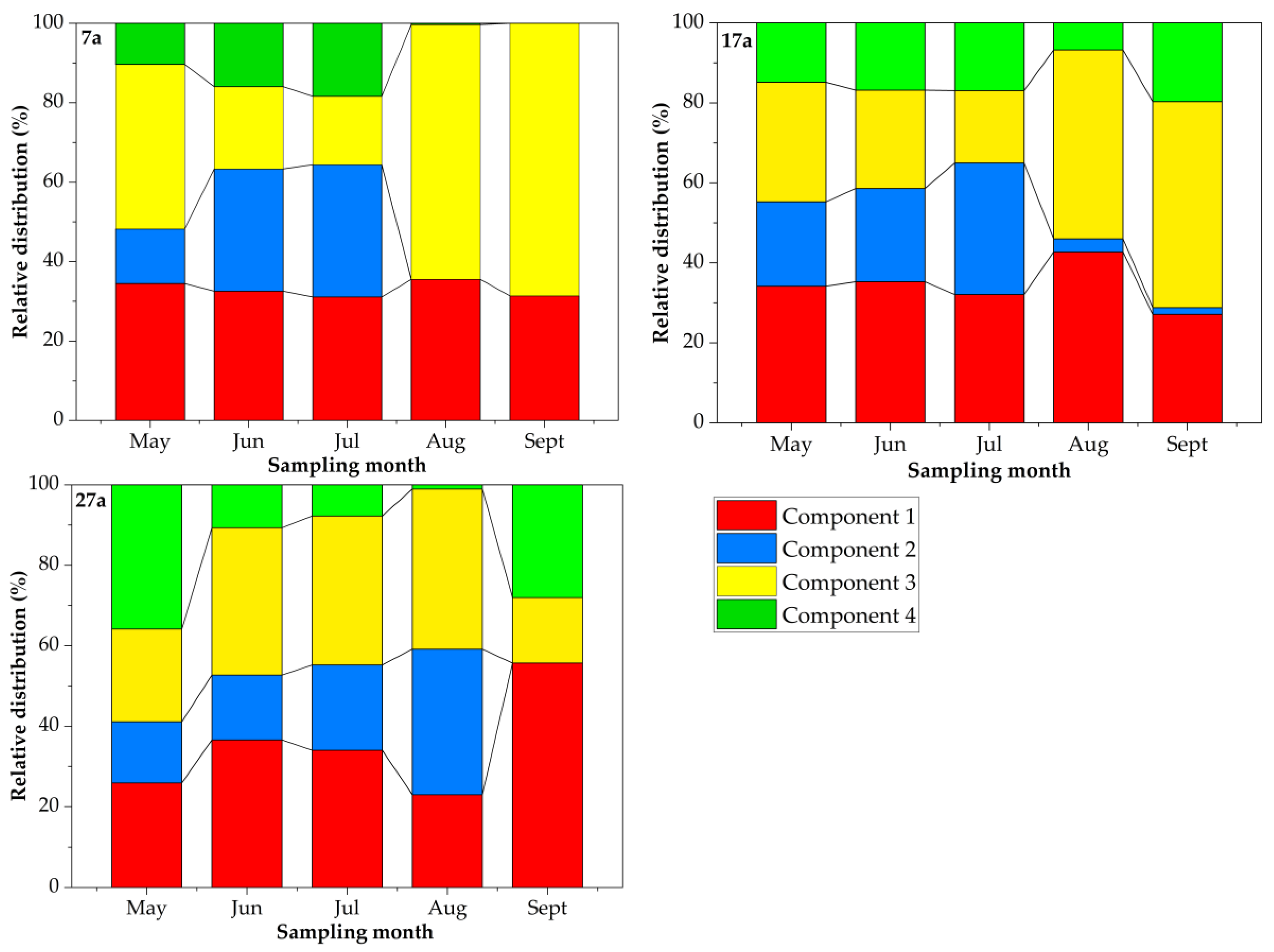
3.3. Relative Distribution of DOM Components
3.4. Variations in UV–Visible Absorbance and Fluorescence Spectra Indicators of DOM
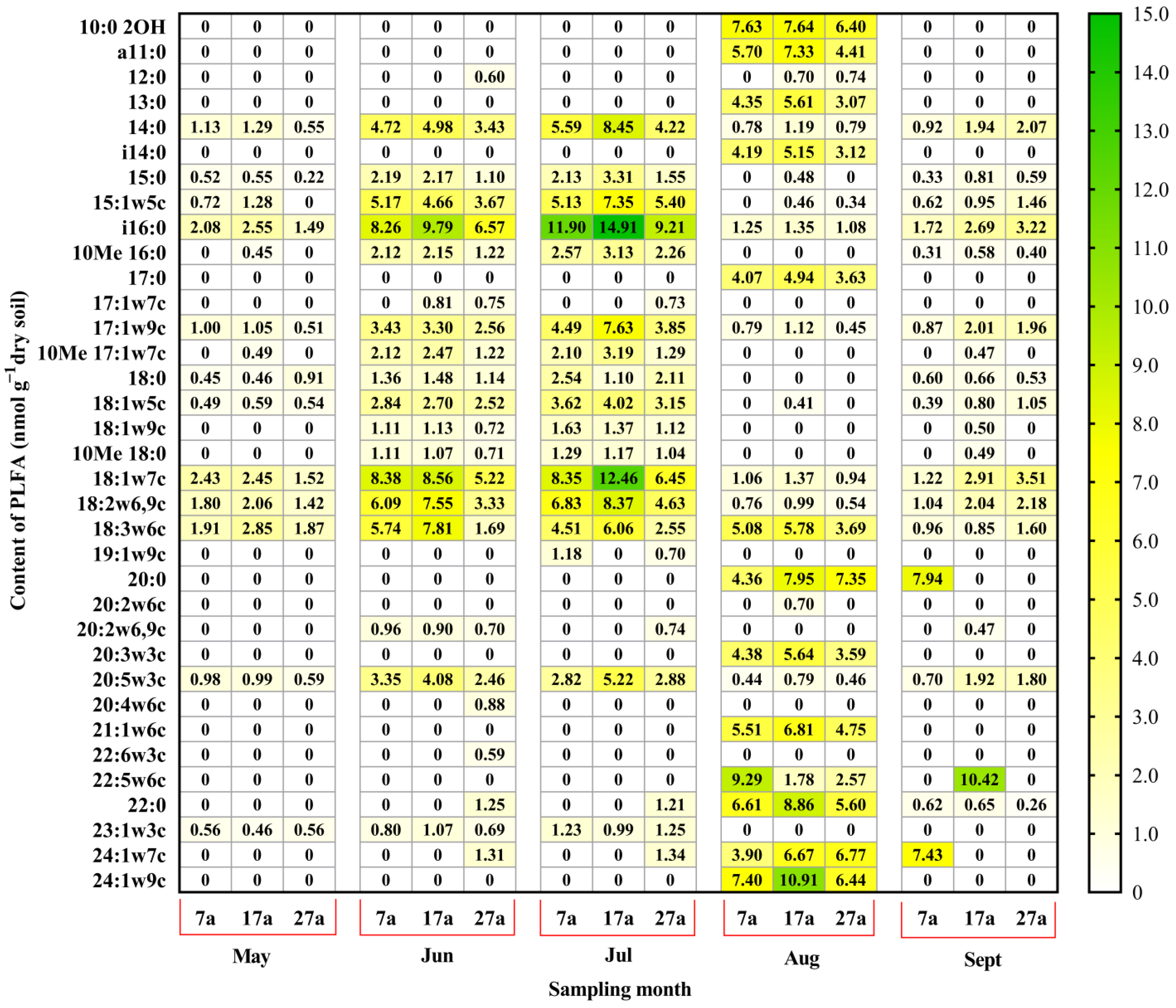
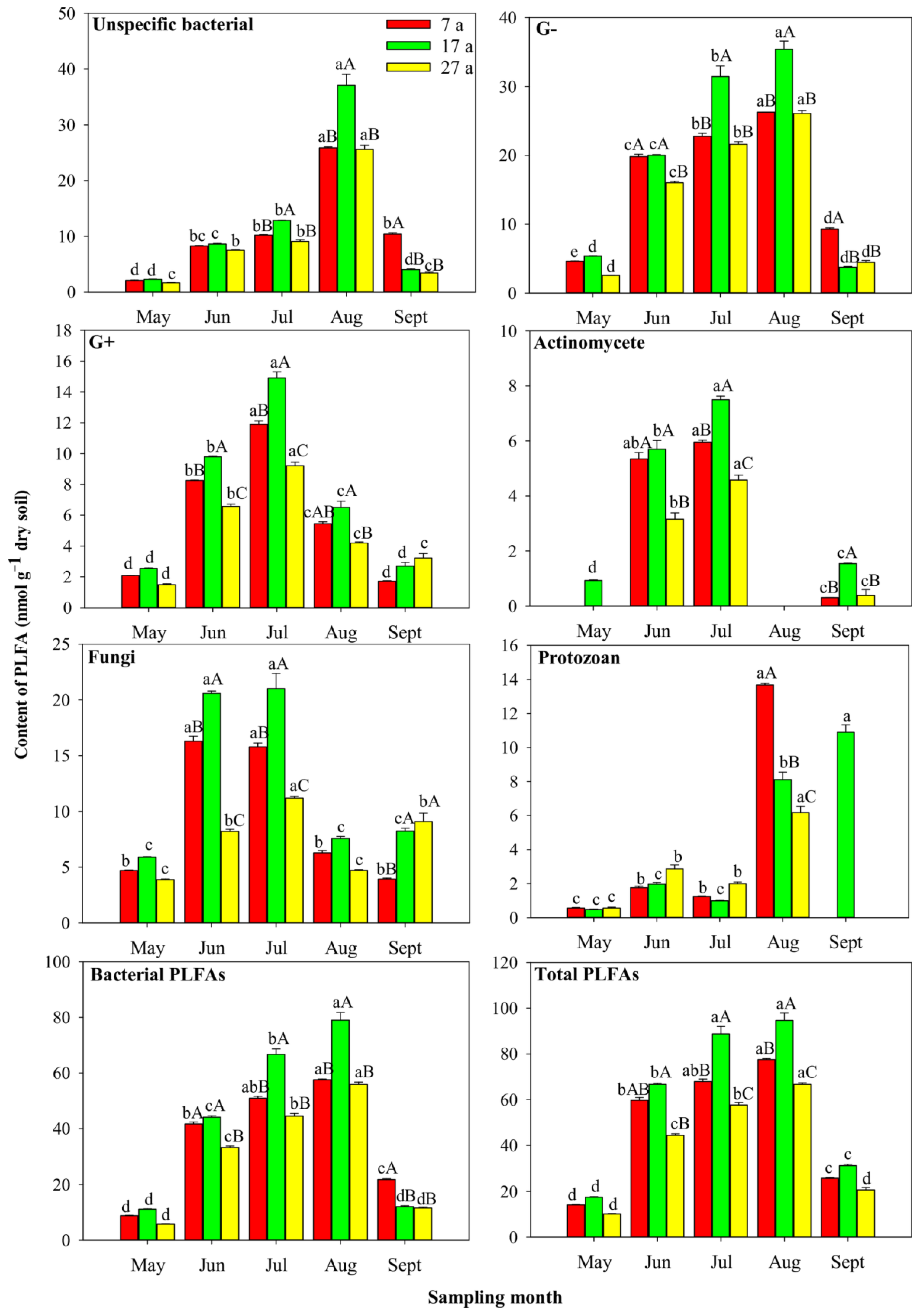
3.5. Seasonal Variations in Soil Microbial PLFAs in Stands of Different ages
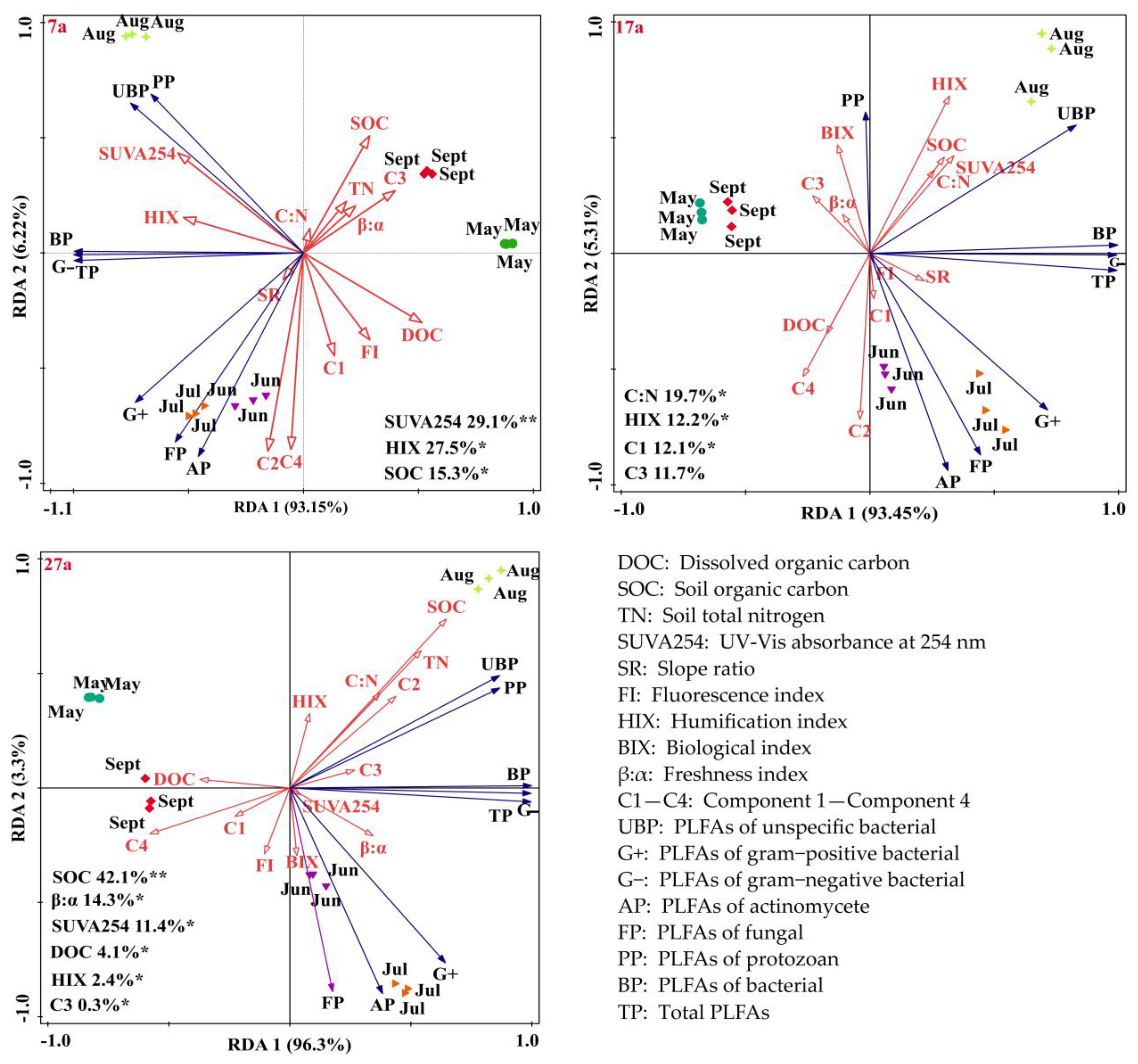
3.6. Correlations between the Soil Physicochemical Factors, Characteristics of DOM and Soil Microbial Community Composition
4. Discussion
4.1. Seasonal Variations in Soil Physicochemical Properties of Stands of Different Ages
4.2. Effects of Seasonal Variations on Soil DOM Characteristics of Stands of Different Ages
4.3. Effects of Seasonal Variations on Microbial Community Structure
4.4. Effect of Soil Environmental Factors on the Soil Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Jia, X.; Li, M.; Wu, H. Insight into the vertical characteristics of dissolved organic matter in 5-m soil profiles under different land-use types on the Loess Plateau. Sci. Total Environ. 2019, 692, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.M.; Engel, R.E.; D’Andrilli, J.; Chen, C.; Zabinski, C.; Miller, P.R.; Wallander, R. Bulk optical characterization of dissolved organic matter from semiarid wheat-based cropping systems. Geoderma 2017, 306, 40–49. [Google Scholar] [CrossRef]
- Karavanova, E. Dissolved organic matter: Fractional composition and sorbability by the soil solid phase (review of literature). Eurasian Soil Sci. 2013, 46, 833–844. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes that influence dissolved organic matter in the soil: A review. Sci. Agr. 2019, 77, e20180164. [Google Scholar] [CrossRef]
- Fahad, S.; Hasanuzzaman, M.; Alam, M.; Ullah, H.; Saeed, M.; Khan, I.A.; Adnan, M. Environment, Climate, Plant and Vegetation Growth; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Roesch, L.F.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yang, K.; Li, Z.; Schädler, M.; Yang, W.; Wu, F.; Tan, B.; Zhang, L.; Xu, Z. Effects of forest conversion on soil microbial communities depend on soil layer on the eastern Tibetan Plateau of China. PLoS ONE 2017, 12, e0186053. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Davis, A.S.; Yannarell, A.C. Interactions between allelochemicals and the microbial community affect weed suppression following cover crop residue incorporation into soil. Plant Soil 2016, 399, 357–371. [Google Scholar] [CrossRef]
- Yang, J.; Duan, Y.; Zhang, R.; Liu, C.; Wang, Y.; Li, M.; Ding, Y.; Awasthi, M.K.; Li, H. Connecting soil dissolved organic matter to soil bacterial community structure in a long-term grass-mulching apple orchard. Ind. Crop. Prod. 2020, 149, 112344. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Sun, X.; Chen, D.; Insam, H.; Koide, R.T.; Zhang, S. Effects of mixed-species litter on bacterial and fungal lignocellulose degradation functions during litter decomposition. Soil Biol. Biochem. 2020, 141, 107690. [Google Scholar] [CrossRef]
- Lutsenko, T.; Arzhanova, V.; Kim, N.Y. Transformation of dissolved organic matter in soils of the felled areas in fir-spruce forests (Primorskii Krai). Eurasian Soil Sci. 2006, 39, 604–610. [Google Scholar] [CrossRef]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.; Chen, F. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 2010, 8, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Wu, H.; Lin, L.; Shen, G.; Li, M. Heavy-metal pollution alters dissolved organic matter released by bloom-forming Microcystis aeruginosa. RSC Adv. 2017, 7, 18421–18427. [Google Scholar] [CrossRef]
- Li, H.; C. Crabbe, M.J.; Xu, F.; Wang, W.; Ma, L.; Niu, R.; Gao, X.; Li, X.; Zhang, P.; Ma, X. Seasonal variations in carbon, nitrogen and phosphorus concentrations and C: N: P stoichiometry in different organs of a Larix principis-rupprechtii Mayr. plantation in the Qinling Mountains, China. PLoS ONE 2017, 12, e0185163. [Google Scholar]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Justine, M.F.; Yang, W.; Wu, F.; Tan, B.; Naeem Khan, M.; Li, Z. Dissolved organic matter in soils varies across a chronosequence of Pinus massoniana plantations. Ecosphere 2017, 8, e01764. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kloeppel, B.D.; Knoepp, J.; Zausen, G.L.; Jaffé, R. Effects of watershed history on dissolved organic matter characteristics in headwater streams. Ecosystems 2011, 14, 1110–1122. [Google Scholar] [CrossRef]
- Praise, S.; Ito, H.; Sakuraba, T.; Pham, D.V.; Watanabe, T. Water extractable organic matter and iron in relation to land use and seasonal changes. Sci. Total Environ. 2020, 707, 136070. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources: A Framework for International Classification, Correlation and Communication; World Soil Resources Reports 103; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Bremmer, J.; Mulvaney, C. Methods of Soil Analysis. Nitrogen-Total. Part 2. Chemical and Microbiollogical Properties, 2nd ed.; American Society of Agronomy. Inc.: Madison, WI, USA, 1982; pp. 295–624. [Google Scholar]
- Zhang, R.; Huang, Q.; Yan, T.; Yang, J.; Zheng, Y.; Li, H.; Li, M. Effects of intercropping mulch on the content and composition of soil dissolved organic matter in apple orchard on the loess plateau. J. Environ. Manage. 2019, 250, 109531. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.P.; Cory, R.M. Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils. Environ. Sci. Technol. 2016, 50, 3545–3553. [Google Scholar] [CrossRef]
- Tiefenbacher, A.; Weigelhofer, G.; Klik, A.; Pucher, M.; Santner, J.; Wenzel, W.; Eder, A.; Strauss, P. Short-term effects of fertilization on dissolved organic matter in soil leachate. Water 2020, 12, 1617. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, X.; Li, M.; Wu, H. Impacts of biochar addition on soil dissolved organic matter characteristics in a wheat-maize rotation system in Loess Plateau of China. Chemosphere 2017, 186, 986–993. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Li, S.; Hou, X.; Shi, Y.; Huang, T.; Yang, H.; Huang, C. Rapid photodegradation of terrestrial soil dissolved organic matter (DOM) with abundant humic-like substances under simulated ultraviolet radiation. Environ. Monit. Assess. 2020, 192, 1–15. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, B.; Zhou, L.; Wang, H.; Xu, J.; Kemmitt, S.; Brookes, P.C. Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis. Appl. Soil Ecol. 2009, 43, 234–240. [Google Scholar] [CrossRef]
- Veum, K.S.; Lorenz, T.; Kremer, R.J. Phospholipid fatty acid profiles of soils under variable handling and storage conditions. Agron. J. 2019, 111, 1090–1096. [Google Scholar] [CrossRef]
- Zhang, S.; Jian, Y.; Yan, B.; Jin, J.; Wu, J.; Liang, C.; Liu, J. Land Consolidation with Seedling Cultivation Could Decrease Soil Microbial PLFA Diversity. Phyton-Int. J. Exp. Bot. 2022, 91, 1745–1756. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, N.; Zeng, H.; Wang, W. Effects of soil depth and plant–soil interaction on microbial community in temperate grasslands of northern China. Sci. Total Environ. 2018, 630, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, J.; Abbas, T.; Zhang, Q.; Cai, M.; Tahir, M.; Wu, D.; Di, H. Immobilization of exchangeable Cd in soil using mixed amendment and its effect on soil microbial communities under paddy upland rotation system. Chemosphere 2021, 262, 127828. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sui, X.; Hu, Y.; Feng, F. Microbial community structure and the relationship with soil carbon and nitrogen in an original Korean pine forest of Changbai Mountain, China. BMC Microbiol. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; D’amore, D.V.; Edwards, R.T.; White, D. Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds. Biogeochemistry 2009, 95, 277–293. [Google Scholar] [CrossRef]
- Fellman, J.B.; D’Amore, D.V.; Hood, E.; Boone, R.D. Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry 2008, 88, 169–184. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, G.; Wang, E. Global subsoil organic carbon turnover times dominantly controlled by soil properties rather than climate. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Burton, J.; Chen, C.; Xu, Z.; Ghadiri, H. Gross nitrogen transformations in adjacent native and plantation forests of subtropical Australia. Soil Biol. Biochem. 2007, 39, 426–433. [Google Scholar] [CrossRef]
- Du, C.; Xu, C.Y.; Jian, J.S.; He, W.X.; Hou, L.; Geng, Z.C. Seasonal dynamics of bacterial communities in a Betula albosinensis forest. Eur. J. Soil Sci. 2018, 69, 666–674. [Google Scholar] [CrossRef]
- Sanchez, F.G. Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. Forest Ecol. Manage. 2001, 152, 85–96. [Google Scholar] [CrossRef]
- Li, H.; Crabbe, M.J.C.; Xu, F.; Wang, W.; Niu, R.; Gao, X.; Zhang, P.; Chen, H. Seasonal variations in carbon, nitrogen and phosphorus concentrations and C: N: P stoichiometry in the leaves of differently aged Larix principis-rupprechtii Mayr. plantations. Forests 2017, 8, 373. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, Y.-H.; Zhang, Z.-T.; Huang, W.-L.; Li, L.-P.; Li, J.; Liu, J.; Zheng, Y.; Mo, J.-M.; Zhang, W. Dissolved organic matter characteristics in soils of tropical legume and non-legume tree plantations. Soil Biol. Biochem. 2020, 148, 107880. [Google Scholar] [CrossRef]
- Harrison, R.B.; Footen, P.W.; Strahm, B.D. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. Forest Sci. 2011, 57, 67–76. [Google Scholar]
- Gao, J.; Liang, C.; Shen, G.; Lv, J.; Wu, H. Spectral characteristics of dissolved organic matter in various agricultural soils throughout China. Chemosphere 2017, 176, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Jun, X.; Ya-nan, Z.; Xuan-jing, C.; Ke, W.; Chun-li, X.; Dan-ping, L.; Yue-qiang, Z.; Ding-yong, W.; Xiao-jun, S. Effect on Soil DOM Content and Structure Characteristics in Different Soil Layers by Long-Term Fertilizations. Spectrosc. Spect. Anal. 2018, 38, 2250–2255. [Google Scholar]
- Wu, M.; Li, P.; Li, G.; Petropoulos, E.; Feng, Y.; Li, Z. The chemodiversity of paddy soil dissolved organic matter is shaped and homogenized by bacterial communities that are orchestrated by geographic distance and fertilizations. Soil Biol. Biochem. 2021, 161, 108374. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limno. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Perdrial, J.N.; McIntosh, J.; Harpold, A.; Brooks, P.D.; Zapata-Rios, X.; Ray, J.; Meixner, T.; Kanduc, T.; Litvak, M.; Troch, P.A. Stream water carbon controls in seasonally snow-covered mountain catchments: Impact of inter-annual variability of water fluxes, catchment aspect and seasonal processes. Biogeochemistry 2014, 118, 273–290. [Google Scholar] [CrossRef]
- Yano, Y.; McDowell, W.H.; Kinner, N.E. Quantification of biodegradable dissolved organic carbon in soil solution with flow-through bioreactors. Soil Sci. Soc. Am. J. 1998, 62, 1556–1564. [Google Scholar] [CrossRef]
- Fan, L.; Tarin, M.W.K.; Zhang, Y.; Han, Y.; Rong, J.; Cai, X.; Chen, L.; Shi, C.; Zheng, Y. Patterns of soil microorganisms and enzymatic activities of various forest types in coastal sandy land. Glob. Ecol. Conserv. 2021, 28, e01625. [Google Scholar] [CrossRef]
- Mao, R.; Li, S. Temporal controls on dissolved organic carbon biodegradation in subtropical rivers: Initial chemical composition versus stoichiometry. Sci. Total Environ. 2019, 651, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-P.; He, B.-H.; Zeng, Q.-P.; Li, N.-J.; Yang, L. Effects of seasonal variation on soil microbial community structure and enzyme activity in a Masson pine forest in Southwest China. J. Mt. Sci.-Engl. 2020, 17, 1398–1409. [Google Scholar] [CrossRef]
- Gong, B.; Chen, S.; Lan, W.; Huang, Y.; Zhu, X. Antibacterial and antitumor potential of actinomycetes isolated from mangrove soil in the Maowei Sea of the southern coast of China. Iran. J. Pharm. Res. 2018, 17, 1339. [Google Scholar]
- Kong, T.; Wu, X.; Zhao, X.; Liu, M.; Huang, J.; Zhang, D. Soil microbial quantity and soil enzyme activity characteristics of riparian zone in upper reachesof Hunhe river. Bull. Soil Water Conserv. 2014, 34, 123–128. (In Chinese) [Google Scholar]
- Li, W.; Lv, X.; Ruan, J.; Yu, M.; Song, Y.-B.; Yu, J.; Dong, M. Variations in soil bacterial composition and diversity in newly formed coastal wetlands. Front. Microbiol. 2019, 9, 3256. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- De Vries, F.T.; Manning, P.; Tallowin, J.R.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.; de Moraes Sa, J.C.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils-Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Global Change Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef] [PubMed]
- Goffin, A.; Guérin, S.; Rocher, V.; Varrault, G. Towards a better control of the wastewater treatment process: Excitation-emission matrix fluorescence spectroscopy of dissolved organic matter as a predictive tool of soluble BOD5 in influents of six Parisian wastewater treatment plants. Environ. Sci. Pollut. Res. 2018, 25, 8765–8776. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.; Khan, S.J. Organic Matter Fluorescence in Municipal Water Recycling Schemes: Toward a Unified PARAFAC Model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef]
- Guo, W.; Xu, J.; Wang, J.; Wen, Y.; Zhuo, J.; Yan, Y. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. J. Environ. Sci. 2010, 22, 1728–1734. [Google Scholar] [CrossRef]
- Qin, X.-Q.; Yao, B.; Jin, L.; Zheng, X.-Z.; Ma, J.; Benedetti, M.F.; Li, Y.; Ren, Z.-L. Characterizing Soil Dissolved Organic Matter in Typical Soils from China Using Fluorescence EEM–PARAFAC and UV–Visible Absorption. Aquat. Geochem. 2020, 26, 71–88. [Google Scholar] [CrossRef]
- Gao, J.; Shi, Z.; Wu, H.; Lv, J. Fluorescent characteristics of dissolved organic matter released from biochar and paddy soil incorporated with biochar. RSC Adv. 2020, 10, 5785–5793. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Shen, G.; Qu, D.; Li, K.; Li, M. Composition of Extracellular and Intracellular Polymeric Substances Produced by Scenedesmus and Microcystis. Environ. Eng. Sci. 2017, 34, 887–894. [Google Scholar] [CrossRef]
- Singh, S.; Inamdar, S.; Scott, D. Comparison of Two PARAFAC Models of Dissolved Organic Matter Fluorescence for a Mid-Atlantic Forested Watershed in the USA. J. Ecosyst. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Singh, S.; D’Sa, E.J.; Swenson, E.M. Chromophoric dissolved organic matter (CDOM) variability in Barataria Basin using excitation–emission matrix (EEM) fluorescence and parallel factor analysis (PARAFAC). Sci. Total Environ. 2010, 408, 3211–3222. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, Y.; Crabbe, M.J.C.; Chen, H. The Characteristics of Dissolved Organic Matter and Soil Microbial Communities in the Soils of Larix principis-rupprechtii Mayr. Plantations in the Qinling Mountains, China. Sustainability 2022, 14, 11968. https://doi.org/10.3390/su141911968
Li H, Yang Y, Crabbe MJC, Chen H. The Characteristics of Dissolved Organic Matter and Soil Microbial Communities in the Soils of Larix principis-rupprechtii Mayr. Plantations in the Qinling Mountains, China. Sustainability. 2022; 14(19):11968. https://doi.org/10.3390/su141911968
Chicago/Turabian StyleLi, Hailiang, Yang Yang, M. James C. Crabbe, and Haikui Chen. 2022. "The Characteristics of Dissolved Organic Matter and Soil Microbial Communities in the Soils of Larix principis-rupprechtii Mayr. Plantations in the Qinling Mountains, China" Sustainability 14, no. 19: 11968. https://doi.org/10.3390/su141911968
APA StyleLi, H., Yang, Y., Crabbe, M. J. C., & Chen, H. (2022). The Characteristics of Dissolved Organic Matter and Soil Microbial Communities in the Soils of Larix principis-rupprechtii Mayr. Plantations in the Qinling Mountains, China. Sustainability, 14(19), 11968. https://doi.org/10.3390/su141911968







