Interaction of Microplastic Presence and Oxidative Stress in Freshwater Fish: A Regional Scale Research, East Anatolia of Türkiye (Erzurum & Erzincan & Bingöl)
Abstract
:1. Introduction
- Determination of MP abundance and characteristics in dominant fish species at the determined stations;
- Evaluation and characterization of MPs found in gastrointestinal system (GIS);
- Determination of the effect of MP presence on oxidative damage in liver tissues by evaluation of oxidative stress marker levels.
2. Materials and Methods
2.1. Fish Sampling
2.2. Laboratory Studies
2.3. Fish Sampling Analyses
3. Results
3.1. Average MP, Total MP and Physical Characteristics in Fish Samples
3.2. Abundance of Microplastics in Fish
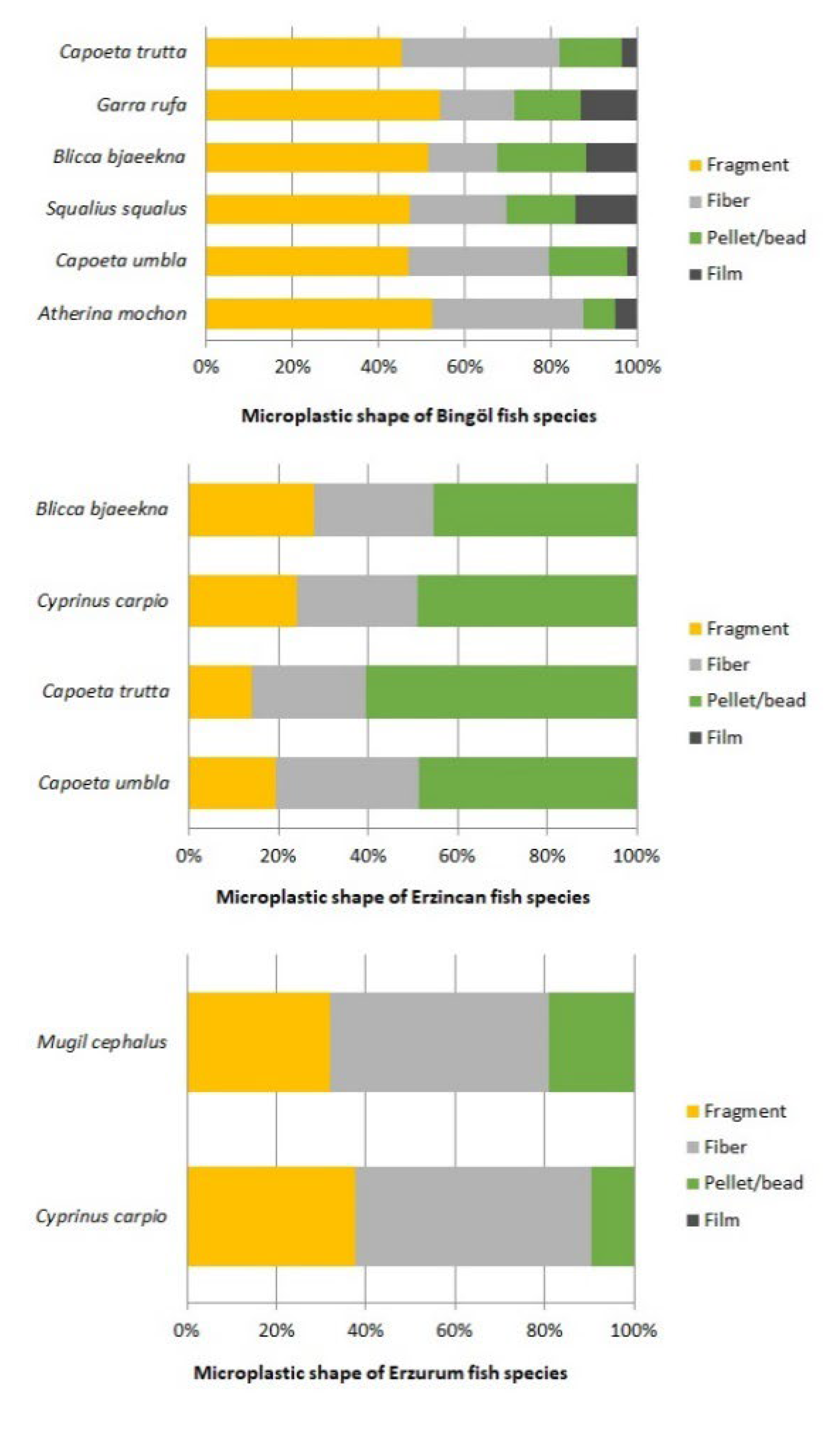
3.3. Physical Characterization of Microplastics
3.4. Characterization of Microplastics
| Polymer Type of Bingöl, Erzincan and Erzurum | Microplastic Percentage (%) |
|---|---|
| Polypropylene (PP) | 31.8 |
| Polyester (PET) | 20.0 |
| Polysulfide rubber (PCR) | 7.1 |
| Polychloroprene chlorine (PCP) | 8.2 |
| Polyvinyl chloride (PVC) | 8.2 |
| Polyisobutylene rubber (PIB) | 8.2 |
| Ethylene propylene (EPR) | 4.7 |
| Ethyl-acrylate (EA) | 5.9 |
| Polyacrylate (PA) | 5.9 |

3.5. Enzyme Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in aquatic ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Atamanalp, M.; Köktürk, M.; Uçar, A.; Duyar, H.A.; Özdemir, S.; Parlak, V.; Esenbuğa, N.; Alak, G. Microplastics in tissues (brain, gill, muscle and gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch. Environ. Contam. Toxicol. 2021, 81, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Microplastics Are Contaminants of Emerging Concern in Freshwater Environments: An Overview; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 1–23. [Google Scholar]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Roebroek, C.T.; Harrigan, S.; Van Emmerik, T.H.; Baugh, C.; Eilander, D.; Prudhomme, C.; Pappenberger, F. Plastic in global rivers: Are floods making it worse? Environ. Res. Lett. 2021, 16, 025003. [Google Scholar] [CrossRef]
- Simon-Sánchez, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Erdoğan, Ş. Microplastic pollution in freshwater ecosystems: A case study from Turkey. J. Fish. 2020, 37, 213–221. [Google Scholar]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; A Van Franeker, J.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plasticpellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Mallik, A.; Xavier, K.M.; Naidu, B.C.; Nayak, B.B. Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Sci. Total Environ. 2021, 779, 146433. [Google Scholar] [CrossRef]
- Jovanović, B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Kang, P.; Ji, B.; Zhao, Y.; Wei, T. How can we trace microplastics in wastewater treatment plants: A review of the current knowledge on their analysis approaches. Sci. Total Environ. 2020, 745, 140943. [Google Scholar] [CrossRef]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Processes Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sobhan, F.; Uddin, M.N.; Sharifuzzaman, S.M.; Chowdhury, S.R.; Sarker, S.; Chowdhury, M.S.N. Microplastics in fishes from the Northern Bay of Bengal. Sci. Total Environ. 2019, 690, 821–830. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.-L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Alak, G.; Köktürk, M.; Atamanalp, M. Evaluation of different packaging methods and storage temperature on MPs abundance and fillet quality of rainbow trout. J. Hazard. Mater. 2021, 420, 126573. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Uddin, S.; Lyons, B. Evidence of microplastics (MP) in gut content of major consumed marine fish species in the State of Kuwait (of the Arabian/Persian Gulf). Mar. Pollut. Bull. 2020, 154, 111052. [Google Scholar] [CrossRef]
- Sun, Y.I.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Academic Press: Cambridge, MA, USA, 1974; pp. 673–684. [Google Scholar]
- Beutler, H.O. A new enzymatic method for determination of sulphite in food. Food Chem. 1984, 15, 157–164. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism; A Manual of Biochemical Methods 12; Academic Press: London, UK, 1971; pp. 68–70. [Google Scholar]
- Luo, P.; Ren, Z.; Wu, X.; Zhang, H.; Zhang, H.; Feng, J. Structural and biochemical mechanism responsible for the stay-green phenotype in common wheat. Chin. Sci. Bull. 2006, 51, 2595–2603. [Google Scholar] [CrossRef]
- Gupta, R.; Dubey, D.K.; Kannan, G.M.; Flora, S.J.S. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol. Int. 2007, 31, 44–56. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, Q.; Sun, Y.; Sun, X.; Lin, H. Environmental implications of microplastic pollution in the Northwestern Pacific Ocean. Mar. Pollut. Bull. 2019, 146, 215–224. [Google Scholar] [CrossRef]
- Bertoldi, C.; Lara, L.Z.; Fernanda, A.D.L.; Martins, F.C.; Battisti, M.A.; Hinrichs, R.; Fernandes, A.N. First evidence of microplastic contamination in the freshwater of Lake Guaíba, Porto Alegre, Brazil. Sci. Total Environ. 2021, 759, 143503. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Scordo, F.; Seitz, C.; Manstretta, G.M.M.; Ronda, A.C.; Arias, A.H.; Tomba, J.P.; Silva, L.I.; Perillo, G.M.E.; Piccolo, M.C. First evidence of microplastics in nine lakes across Patagonia (South America). Sci. Total Environ. 2020, 733, 139385. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, Y.; Liu, X.; Wang, J. Microplastic pollution in vegetable farmlands of suburb Wuhan, central China. Environ. Pollut. 2020, 257, 113449. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lu, H.; Tian, P.; Xue, Y.; Lu, J.; Tang, M.; Feng, W. Analysis of microplastics in a remote region of the Tibetan Plateau: Implications for natural environmental response to human activities. Sci. Total Environ. 2020, 739, 140087. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Y.; Shen, M. Investigation on microplastic pollution of Dongting Lake and its affiliated rivers. Mar. Pollut. Bull. 2020, 160, 111555. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake–Qinghai Lake. Environ. Pollut. 2018, 235, 899–906. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef] [PubMed]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [CrossRef]
- Horton, A.A.; Barnes, D.K. Microplastic pollution in a rapidly changing world: Implications for remote and vulnerable marine ecosystems. Sci. Total Environ. 2020, 738, 140349. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Piñon-Colin, T.; Rodriguez-Jimenez, R.; Rogel-Hernandez, E.; Alvarez-Andrade, A.; Wakida, F.T. Microplastics in stormwater runoff in a semiarid region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef] [PubMed]
- Talbot, R.; Granek, E.; Chang, H.; Wood, R.; Brander, S. Spatial and temporal variations of microplastic concentrations in Portland’s freshwater ecosystems. Sci. Total Environ. 2022, 833, 155143. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef]
- Atamanalp, M.; Köktürk, M.; Parlak, V.; Ucar, A.; Arslan, G.; Alak, G. A new record for the presence of microplastics in dominant fish species of the Karasu River Erzurum, Turkey. Environ. Sci. Pollut. Res. 2022, 29, 7866–7876. [Google Scholar] [CrossRef]
- Mattsson, K.; Björkroth, F.; Karlsson, T.; Hassellöv, M. Nanofragmentation of expanded polystyrene under simulated environmental weathering (thermooxidative degradation and hydrodynamic turbulence). Front. Mar. Sci. 2021, 7, 1252. [Google Scholar] [CrossRef]
- Krause, S.; Baranov, V.; Nel, H.A.; Drummond, J.D.; Kukkola, A.; Hoellein, T.; Smith, G.H.S.; Lewandowski, J.; Bonet, B.; Packman, A.I.; et al. Gathering at the top? Environmental controls of microplastic uptake and biomagnification in freshwater food webs. Environ. Pollut. 2021, 268, 115750. [Google Scholar] [CrossRef]
- Assas, M.; Qiu, X.; Chen, K.; Ogawa, H.; Xu, H.; Shimasaki, Y.; Oshima, Y. Bioaccumulation and reproductive effects of fluorescent microplastics in medaka fish. Mar. Pollut. Bull. 2020, 158, 111446. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. In Analysis of Nanoplastics and Microplastics in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 119–148. [Google Scholar]
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total Environ. 2020, 726, 138682. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Yuan, F.; Zhao, H.; Sun, H.; Zhao, J.; Sun, Y. Abundance, morphology, and removal efficiency of microplastics in two wastewater treatment plants in Nanjing, China. Environ. Sci. Pollut. Res. 2021, 28, 9327–9337. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T.H. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Roch, S.; Walter, T.; Ittner, L.D.; Friedrich, C.; Brinker, A. A systematic study of the microplastic burden in freshwater fishes of south-western Germany—Are we searching at the right scale? Sci. Total Environ. 2019, 689, 1001–1011. [Google Scholar] [CrossRef]
- Yılmaz, F.; Solak, K. Feeding Organisms Living in Capoeta trutta (Heckel;1843) in the Tigris River and Changes in these Organisms According to Month and Age. Turk. J. Zool. 1999, 23, 973–978. [Google Scholar]
- Koyun, M. Biogeographical Distribution of Garra rufa (HECKEL, 1843) in freshwater Sources of Turkey. Sci. J Bingöl. Univ. 2011, 1, 5–8. [Google Scholar]
- Welden, N.A.; Abylkhani, B.; Howarth, L.M. The effects of trophic transfer and environmental factors on microplastic uptake by plaice, Pleuronectes plastessa, and spider crab, Maja squinado. Environ. Pollut. 2018, 239, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Uçar, A.; Parlak, V.; Özgeriş, F.B.; Yeltekin, A.; Alak, G.; Atamanalp, M. Determination of Fipronil toxicity by different biomarkers in gill and liver tissue of rainbow trout (Oncorhynchus mykiss). In Vitro Cell. Dev. Biol. Anim. 2020, 56, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Cao, W.; Liu, X.; Jiang, F.; Ding, J.; Yin, X.; Sun, C. Distribution characteristics of microplastics in the seawater and sediment: A case study in Jiaozhou Bay, China. Sci. Total Environ. 2019, 674, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Chmelova, L.; Sehonova, P.; Blahova, J.; Doubkova, V.; Plhalova, L.; Fiorino, E.; Vojtek, L.; Vicenova, M.; Siroka, Z.; et al. The effects of a therapeutic formalin bath on selected immunological and oxidative stress parameters in common carp (Cyprinus carpio). Sci. Total Environ. 2019, 653, 1120–1127. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Wilhelm Filho, D.; Althoff, S.L.; Dafré, A.L.; Boveris, A. Antioxidant defenses, longevity and ecophysiology of South American bats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of oxidative stress in zebrafish embryos. J. Vis. Exp. 2014, 89, e51328. [Google Scholar] [CrossRef] [PubMed]
- Sreejai, R.; Jaya, D.S. Studies on the changes in lipid peroxidation and antioxidants in fishes exposed to hydrogen sulfide. Toxicol. Int. 2010, 17, 71–77. [Google Scholar]
- De Oliveira Ramos, C.; Campos, K.K.D.; de Paula Costa, G.; Cangussu, S.D.; Talvani, A.; Bezerra, F.S. Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul. Toxicol. Pharmacol. 2018, 98, 50–57. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Mukherjee, M.; Das, D.; Chakraborty, S.B. Effectiveness of melatonin to restore fish brain activity in face of permethrin induced toxicity. Environ. Pollut. 2020, 266, 115230. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Trestrail, C.; Nugegoda, D.; Shimeta, J. Invertebrate responses to microplastic ingestion: Reviewing the role of the antioxidant system. Sci. Total Environ. 2020, 734, 138559. [Google Scholar] [CrossRef]
- Capo, X.; Rubio, M.; Solomando, A.; Alomar, C.; Compa, M.; Sureda, A.; Deudero, S. Microplastic intake and enzymatic responses in Mytilus galloprovincialis reared at the vicinities of an aquaculture station. Chemosphere 2021, 280, 130575. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Enseñat, M.; Alou, E.; Tauler, P.; Deudero, S.; Pons, A. Enzymatic antioxidant response of a labrid fish (Coris julis) liver to environmental caulerpenyne. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| City | Species (n = 7) | Average Fish Weight (g) | Average Fish Length (cm) | Average Gastrointestinal Tract Weight (g) | Average Number of MP | Total MP |
|---|---|---|---|---|---|---|
| Bingöl | Atherina mochon | 51.60 ± 17.69 | 18.97 ± 2.62 | 1.83 ± 1.27 | 11.71 ± 4.99 | 82 |
| Capoeta umbla | 124.76 ± 38.74 | 22.24 ± 2.32 | 12.45 ± 2.89 | 7.00 ± 5.29 | 49 | |
| Squalius squalus | 38.51 ± 8.60 | 14.71 ± 1.09 | 2.11 ± 0.32 | 15.14 ± 5.70 | 106 | |
| Blicca bjoerkna | 39.88 ± 21.28 | 14.46 ± 2.97 | 1.08 ± 0.54 | 13.29 ± 9.88 | 93 | |
| Garra rufa | 19.46 ± 3.30 | 11.29 ± 1.00 | 1.30 ± 0.50 | 13.14 ± 2.41 | 92 | |
| C. trutta | 44.49 ± 13.38 | 14.81 ± 1.70 | 5.36 ± 2.86 | 12.86 ± 1.21 | 90 | |
| Erzincan | C. umbla | 36.02 ± 35.61 | 14.24 ± 4.24 | 3.34 ± 3.68 | 9.43 ± 2.23 | 66 |
| C. trutta | 63.86 ± 14.07 | 16.63 ± 0.45 | 11.01 ± 4.19 | 6.00 ± 1.63 | 42 | |
| Cyprinus carpio | 229.45 ± 180.19 | 24.90 ± 7.42 | 26.50 ± 21.31 | 7.86 ± 2.97 | 55 | |
| B. bjoerkna | 63.06 ± 29.11 | 17.74 ± 2.96 | 6.21 ± 1.90 | 8.43 ± 2.64 | 59 | |
| Erzurum | Cyprinus carpio | 228.82 ± 163.14 | 24.99 ± 5.11 | 29.67 ± 24.72 | 7.57 ± 3.51 | 53 |
| Mugil cephalus | 213.34 ± 65.62 | 24.13 ± 2.61 | 18.42 ± 6.24 | 6.71 ± 2.69 | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atamanalp, M.; Kokturk, M.; Kırıcı, M.; Ucar, A.; Kırıcı, M.; Parlak, V.; Aydın, A.; Alak, G. Interaction of Microplastic Presence and Oxidative Stress in Freshwater Fish: A Regional Scale Research, East Anatolia of Türkiye (Erzurum & Erzincan & Bingöl). Sustainability 2022, 14, 12009. https://doi.org/10.3390/su141912009
Atamanalp M, Kokturk M, Kırıcı M, Ucar A, Kırıcı M, Parlak V, Aydın A, Alak G. Interaction of Microplastic Presence and Oxidative Stress in Freshwater Fish: A Regional Scale Research, East Anatolia of Türkiye (Erzurum & Erzincan & Bingöl). Sustainability. 2022; 14(19):12009. https://doi.org/10.3390/su141912009
Chicago/Turabian StyleAtamanalp, Muhammed, Mine Kokturk, Mahinur Kırıcı, Arzu Ucar, Muammer Kırıcı, Veysel Parlak, Ahmet Aydın, and Gonca Alak. 2022. "Interaction of Microplastic Presence and Oxidative Stress in Freshwater Fish: A Regional Scale Research, East Anatolia of Türkiye (Erzurum & Erzincan & Bingöl)" Sustainability 14, no. 19: 12009. https://doi.org/10.3390/su141912009






