Mutual Influence between Polyvinyl Chloride (Micro)Plastics and Black Soldier Fly Larvae (Hermetia illucens L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Substrate and PVC Preparation
2.2. Rearing of Black Soldier Fly Larvae
2.3. Determination of BSF Larval Growth Performance, Survival Rate and Bioconversion
2.4. Dry Matter Analysis
2.5. Assessment of PVC Degradation Caused by BSF Larvae
2.6. Assessment of Chemical Changes in the PVC Material
2.7. Determination of PVC Degradation
2.8. Statistical Analyses
3. Results and Discussion
3.1. Microplastic Characterisation
3.2. Influence of PVC Present in Rearing Substrates on BSF Larvae
3.2.1. Growth Performance and Survival Rate of BSF Larvae
3.2.2. Bioconversion by BSF Larvae Reared on (micro)plastics Containing Food Waste
3.3. Influence of BSF Larvae on PVC
3.3.1. Recovery of PVC after Rearing Tests
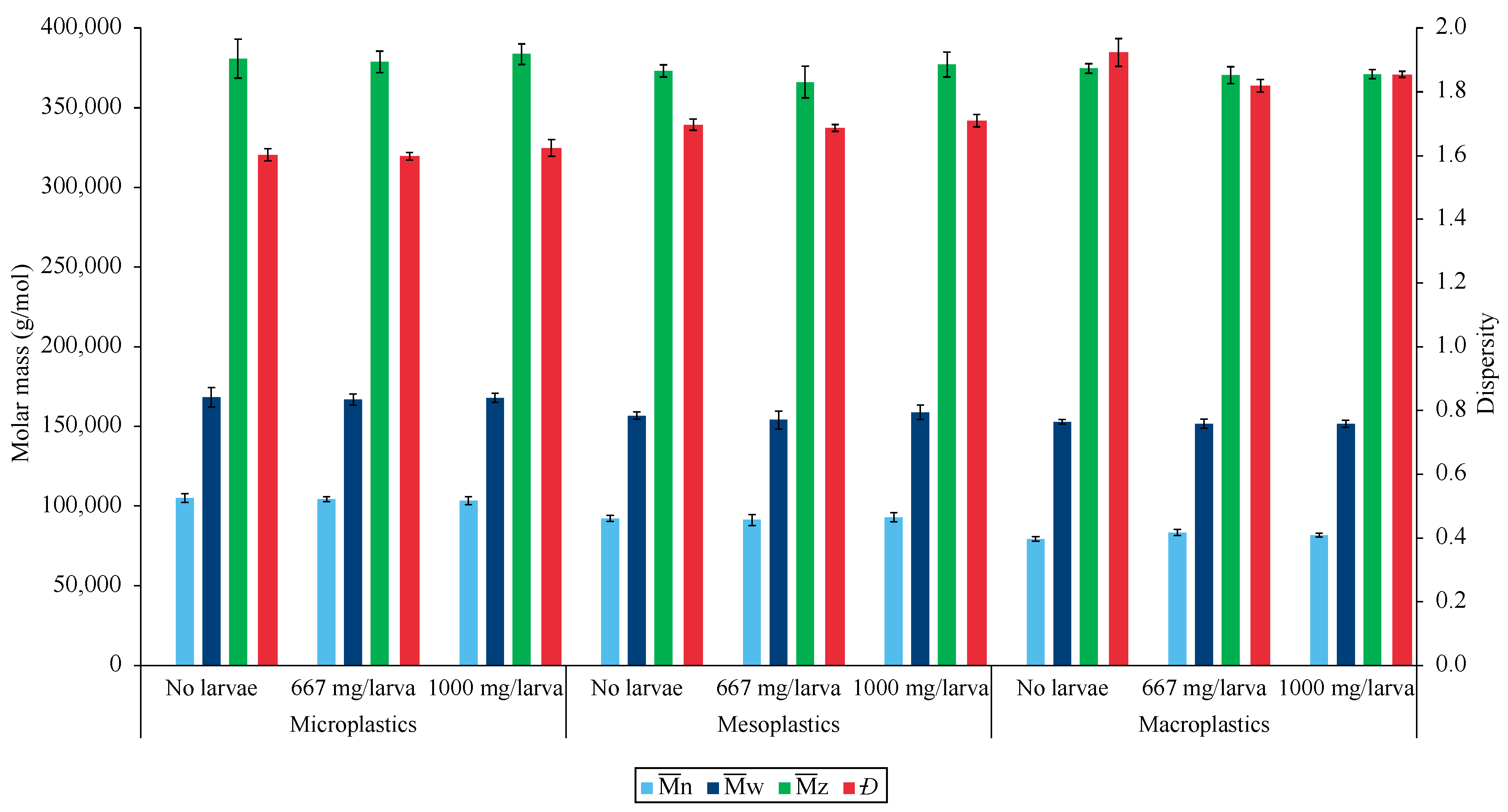
3.3.2. Changes in Chemical Composition
| Plastic Type | Sample Description | Recovery of PVC [%] | Characteristic Bands [cm−1] | Experimental Bands [cm−1] |
|---|---|---|---|---|
| Macroplastics (RS3) | No larvae/650 g substrate | 100.2 ± 1.0 | ), ), ), ), ), ), ) | ), ), ) ) 1728, 1195, 1094 (additives) |
| 650 larvae/650 g substrate | 99.6 ± 1.3 | |||
| 650 larvae/433 g substrate | 100.1 ± 0.7 | |||
| Control PVC | / | |||
| Mesoplastics (RS2) | No larvae/650 g substrate | 109.5 ± 7.6 * | ), ), ) ), ) 1728, 1195, 1094 (additives) | |
| 650 larvae/650 g substrate | 106.5 ± 7.0 * | |||
| 650 larvae/433 g substrate | 105.1 ± 4.9 * | |||
| Control PVC | / | |||
| Microplastics (RS1) | No larvae/650 g substrate | 109.7 ± 10.4 | ), ), ) ) 1732, 1098 (additives) | |
| 650 larvae/650 g substrate | 106.8 ± 9.1 * | |||
| 650 larvae/433 g substrate | 104.1 ± 4.2 | |||
| Control PVC | / |
3.3.3. PVC Degradation
3.3.4. Plastic Degradation Capacity of BSF Larvae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Growing at a Slower Pace, World Population Is Expected to Reach 9.7 Billion in 2050 and Could Peak at Nearly 11 Billion around 2100. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed on 26 March 2020).
- OECD/FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD/FAO: Paris, France, 2021. [Google Scholar]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and Supply of High-Quality Food Protein for Human Consumption: Sustainability, Challenges, and Innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Song, X.P.; Hansen, M.C.; Potapov, P.; Adusei, B.; Pickering, J.; Adami, M.; Lima, A.; Zalles, V.; Stehman, S.V.; Di Bella, C.M.; et al. Massive Soybean Expansion in South America since 2000 and Implications for Conservation. Nat. Sustain. 2021, 4, 784–792. [Google Scholar] [CrossRef]
- Dai, P.; Luan, S.; Sui, J.; Cao, J.; Chen, B.; Meng, X.; Kong, J. Insight into Genetic Potential for Growth and Survival of the Pacific White Shrimp (Litopenaeus vannamei) in the Context of Low-Protein and Low-Fishmeal Diet Use. Aquac. Res. 2022, 53, 3337–3345. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0; World Bank: Washington, DC, USA, 2018; Volume 66, ISBN 9781464813290. [Google Scholar]
- Lievens, S.; Slegers, T.; Mees, M.A.; Thielemans, W.; Poma, G.; Covaci, A.; Van Der Borght, M. A Simple, Rapid and Accurate Method for the Sample Preparation and Quantification of Meso- and Microplastics in Food and Food Waste Streams. Environ. Pollut. 2022, 307, 1. [Google Scholar] [CrossRef] [PubMed]
- Deena, S.R.; Vickram, A.S.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K. Enhanced Biogas Production from Food Waste and Activated Sludge Using Advanced Techniques—A Review. Bioresour. Technol. 2022, 355, 127234. [Google Scholar] [CrossRef]
- Lievens, S.; Poma, G.; De Smet, J.; Van Campenhout, L.; Covaci, A.; Van Der Borght, M. Chemical Safety of Black Soldier Fly Larvae (Hermetia illucens), Knowledge Gaps and Recommendations for Future Research: A Critical Review Abstract. J. Insects Food Feed. 2021, 7, 383–396. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-Efficient Food Production to Reduce Global Warming and Ecodegradation: The Use of Edible Insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Sustainable Waste Management Using Black Soldier Fly Larva: A Review. Int. J. Environ. Sci. Technol. 2021, 1–26. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Fabbri, D.; Modugno, F. A Review on Challenges and Developments of Analytical Pyrolysis and Other Thermoanalytical Techniques for the Quali-Quantitative Determination of Microplastics. J. Anal. Appl. Pyrolysis 2020, 149, 104841. [Google Scholar] [CrossRef]
- Cole, M. A Novel Method for Preparing Microplastic Fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Fabi, G. From Macroplastic to Microplastic Litter: Occurrence, Composition, Source Identification and Interaction with Aquatic Organisms. Experiences from the Adriatic Sea Alessio. In Plastics in the Environment; IntechOpen: London, UK, 2016; p. 15. [Google Scholar]
- Ustabasi, G.S.; Baysal, A. Science of the Total Environment Bacterial Interactions of Microplastics Extracted from Toothpaste under Controlled Conditions and the Influence of Seawater. Sci. Total Environ. 2020, 703, 135024. [Google Scholar] [CrossRef]
- Visakh, P.M.; Darie-Nita, R.N. Polyvinylchloride-Based Blends: Preparation, Characterization and Applications; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9783030784546. [Google Scholar]
- Hogsette, J.A. New Diets for Production of House Flies and Stable Flies (Diptera: Muscidae) in the Laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Waldbauer, G.P. The Consumption and Utilization of Food by Insects. Adv. Insect Physiol. 1968, 5, 229–288. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of Feedstock on Larval Development and Process Efficiency in Waste Treatment with Black Soldier Fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Kinasih, I.; Putra, R.E.; Permana, A.D.; Gusmara, F.F.; Nurhadi, M.Y.; Anitasari, R.A. Growth Performance of Black Soldier Fly Larvae (Hermetia illucens) Fed on Some Plant Based Organic Wastes. Hayati J. Biosci. 2018, 25, 79–84. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing Substrate Impacts Growth and Macronutrient Composition of Hermetia illucens. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, C.; Kim, M.; Chung, H. Effects of Microplastics and Salinity on Food Waste Processing by Black Soldier Fly (Hermetia illucens) Larvae. J. Ecol. Environ. 2020, 44, 7. [Google Scholar] [CrossRef]
- Romano, N.; Fischer, H. Microplastics Affected Black Soldier Fly (Hermetia illucens) Pupation and Short Chain Fatty Acids. J. Appl. Entomol. 2021, 145, 731–736. [Google Scholar] [CrossRef]
- Harnden, L.M.; Tomberlin, J.K. Effects of Temperature and Diet on Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), Development. Forensic Sci. Int. 2016, 266, 109–116. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of Rearing Substrate on Growth Performance, Waste Reduction Efficiency and Chemical Composition of Black Soldier Fly (Hermetia illucens) Larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, Y.; Rehman, K.U.; Yu, Z.; Zhang, J.; Li, W.; Li, Q.; Tomberlin, J.K.; Zheng, L. Dynamic Effects of Initial pH of Substrate on Biological Growth and Dynamic Effects of Initial pH of Substrate on Biological Growth and Metamorphosis of Black Soldier Fly (Diptera: Stratiomyidae). Physiol. Ecol. 2018, 47, 159–165. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Meijer, N.; Nijkamp, M.M.; Schmitt, E.; van Loon, J.J.A. Chemical Food Safety of Using Former Foodstuffs for Rearing Black Soldier Fly Larvae (Hermetia illucens) for Feed and Food Use. J. Insects Food Feed 2020, 6, 475–488. [Google Scholar] [CrossRef]
- Palma, L.; Ceballos, S.J.; Johnson, P.C.; Niemeier, D.; Pitesky, M.; VanderGheynst, J.S. Cultivation of Black Soldier Fly Larvae on Almond Byproducts: Impacts of Aeration and Moisture on Larvae Growth and Composition. J. Sci. Food Agric. 2018, 98, 5893–5900. [Google Scholar] [CrossRef]
- Dzepe, D.; Nana, P.; Mube, H.; Mitsue, J.; Ornela, K.; Tchuinkam, T.; Djouaka, R. Feeding Strategies for Small-scale Rearing Black Soldier Fly Larvae (Hermetia illucens) as Organic Waste Recycler. SN Appl. Sci. 2021, 3, 252. [Google Scholar] [CrossRef]
- Shishkov, O.; Hu, M.; Johnson, C.; Hu, D.L. Black Soldier Fly Larvae Feed by Forming a Fountain around Food. J. R. Soc. Interface 2019, 16, 20180735. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food by-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed]
- Msangi, J.W.; Mweresa, C.K.; Ndong’a, M.F.O. Using Organic Wastes as Feed Substrate for Black Soldier Fly Larvae. J. Insects Food Feed 2022, 8, 357–366. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional Value of the Black Soldier Fly (Hermetia illucens L.) and Its Suitability as Animal Feed—A Review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth Rates of Black Soldier Fly Larvae Fed on Fresh Human Faeces and Their Implication for Improving Sanitation. Trop. Med. Int. Heal. 2014, 19, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Srikanth, B.H.; Kumari, K. Determining the Black Soldier Fly Larvae Performance for Plant-Based Food Waste Reduction and the Effect on Biomass Yield. Waste Manag. 2021, 130, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of Biowaste Macronutrients, Microbes, and Chemicals in Black Soldier Fly Larval Treatment: A Review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Widya Yuwono, N.; Nisa, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black Soldier Fly Larvae (BSFL) and Their Affinity for Organic Waste Processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Solano, N.M.S.; Gutiérrez, F.R.; Zurbrügg, C.; Tockner, K. Biological Treatment of Municipal Organic Waste Using Black Soldier Fly Larvae. Waste Biomass Valorization 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Verleye, G.A.L.; Roeges, N.P.G.; De Moor, M.O. Easy Identification of Plastics and Rubbers; Smithers Rapra Publishing: Shrewsbury, UK, 2001; ISBN 1859572685. [Google Scholar]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, H.; Zhu, H.; Cheng, M.; Li, Y.; Wang, S. Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation. Polymers 2018, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeong, D.; Park, K.H.; Yu, J.H.; Jung, S. Efficient Adsorption on Benzoyl and Stearoyl Cellulose to Remove Phenanthrene and Pyrene from Aqueous Solution. Polymers 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kurose, K.; Nishijima, W.; Okada, M. Separation of Polyvinyl Chloride from Plastic Mixture by Froth Flotation after Surface Modification with Ozone. Ozone Sci. Eng. 2007, 29, 373–377. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Krimm, S.; Liangj, C.Y. Infrared Spectra of High Polymers. IV. Polyvinyl Chloride, Polyvinylidene Chloride, and Copolymers*. J. Polym. Sci. 1956, 112, 95–112. [Google Scholar] [CrossRef]
- Kuan, Z.J.; Chan, B.K.N.; Gan, S.K.E. Worming the Circular Economy for Biowaste and Plastics: Hermetia illucens, Tenebrio molitor, and Zophobas morio. Sustainability 2022, 14, 1594. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.M.R.; Grove, H.C.; Smith, C.M.; Cassone, B.J. A Very Hungry Caterpillar: Polyethylene Metabolism and Lipid Homeostasis in Larvae of the Greater Wax Moth (Galleria mellonella). Environ. Sci. Technol. 2020, 54, 14706–14715. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef]
- Kim, W.T.; Bae, S.W.; Park, H.C.; Park, K.H.; Lee, S.B.; Choi, Y.C.; Han, S.M.; Koh, Y.H. The Larval Age and Mouth Morphology of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2010, 21, 185–187. [Google Scholar]

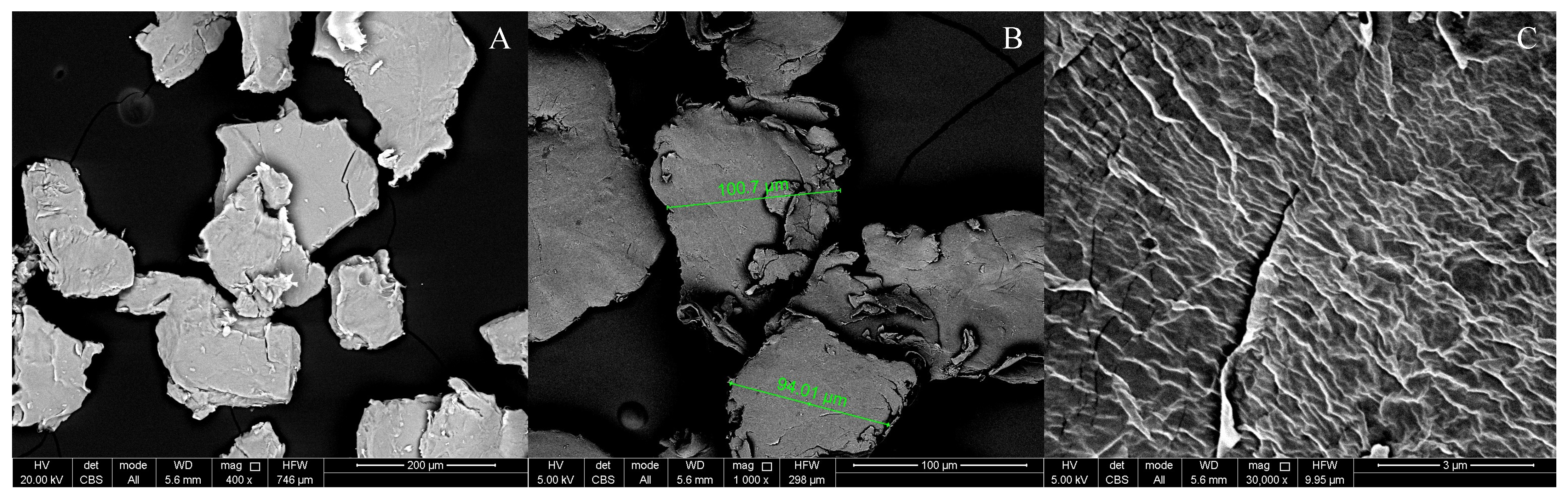

| Substrate Name | Code | Substrate | PVC Particles Content and Size | Number of Larvae | Feed Amount [g] |
|---|---|---|---|---|---|
| Control substrate 1 | CS1 | Gainesville diet | None | 650 | 650 |
| 433 | |||||
| Control substrate 2 | CS2 | Artificial food waste | None | 650 | 650 |
| 433 | |||||
| Research substrate 1 | RS1 | Artificial food waste | w = 1.0% (≈279 µm) | 0 | 650 |
| 650 | 650 | ||||
| 650 | 433 | ||||
| Research substrate 2 | RS2 | Artificial food waste | w = 1.0% (5 mm × 5 mm) | 0 | 650 |
| 650 | 650 | ||||
| 650 | 433 | ||||
| Research substrate 3 | RS3 | Artificial food waste | w = 5.0% (5.0 cm × 5.0 cm) | 0 | 650 |
| 650 | 650 | ||||
| 650 | 433 |
| Substrates | Maximum Mass [g] | Time to Reach Max. Mass [Days] | FCR | ECD | WRR |
|---|---|---|---|---|---|
| 1000 mg/larva | |||||
| CS1 | 0.1493 ± 0.0344 A,B | 16 ± 1 | 11.74 ± 5.25 A | 0.23 ± 0.07 A | 0.44 ± 0.12 A |
| CS2 | 0.1996 ± 0.0159 B | 16 ± 0 | 5.26 ± 0.59 B | 0.34 ± 0.08 B,C | 0.58 ± 0.09 B,C |
| RS1 | 0.2134 ± 0.0203 B | 16 ± 0 | 4.65 ± 0.14 B | 0.39 ± 0.07 C | 0.57 ± 0.11 A,B,C |
| RS2 | 0.2020 ± 0.0200 B | 16 ± 0 | 5.42 ± 0.70 B,C | 0.29 ± 0.02 A,B,C | 0.65 ± 0.05 B,C,D |
| RS3 | 0.2202 ± 0.0049 B | 16 ± 1 | 5.53 ± 0.69 B,C | 0.23 ± 0.02 A,B | 0.78 ± 0.05 D,E |
| 667 mg/larva | |||||
| CS1 | 0.1297 ± 0.0137 A | 16 ± 1 | 8.46 ± 1.45 C | 0.22 ± 0.06 A | 0.56 ± 0.08 B |
| CS2 | 0.1748 ± 0.0150 B | 15 ± 1 | 4.59 ± 0.54 B | 0.35 ± 0.14 B,C | 0.68 ± 0.12 C,D |
| RS1 | 0.1786 ± 0.0131 B | 15 ± 1 | 3.97 ± 0.21 B | 0.40 ± 0.09 C | 0.65 ± 0.13 B,C,D |
| RS2 | 0.1789 ± 0.0036 B | 16 ± 1 | 4.31 ± 0.30 B | 0.29 ± 0.04 A,B,C | 0.81 ± 0.13 D,E |
| RS3 | 0.1884 ± 0.0094 B | 15 ± 1 | 4.23 ± 0.19 B | 0.26 ± 0.01 A,B | 0.90 ± 0.01 E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lievens, S.; Poma, G.; Frooninckx, L.; Van der Donck, T.; Seo, J.W.; De Smet, J.; Covaci, A.; Van Der Borght, M. Mutual Influence between Polyvinyl Chloride (Micro)Plastics and Black Soldier Fly Larvae (Hermetia illucens L.). Sustainability 2022, 14, 12109. https://doi.org/10.3390/su141912109
Lievens S, Poma G, Frooninckx L, Van der Donck T, Seo JW, De Smet J, Covaci A, Van Der Borght M. Mutual Influence between Polyvinyl Chloride (Micro)Plastics and Black Soldier Fly Larvae (Hermetia illucens L.). Sustainability. 2022; 14(19):12109. https://doi.org/10.3390/su141912109
Chicago/Turabian StyleLievens, Siebe, Giulia Poma, Lotte Frooninckx, Tom Van der Donck, Jin Won Seo, Jeroen De Smet, Adrian Covaci, and Mik Van Der Borght. 2022. "Mutual Influence between Polyvinyl Chloride (Micro)Plastics and Black Soldier Fly Larvae (Hermetia illucens L.)" Sustainability 14, no. 19: 12109. https://doi.org/10.3390/su141912109
APA StyleLievens, S., Poma, G., Frooninckx, L., Van der Donck, T., Seo, J. W., De Smet, J., Covaci, A., & Van Der Borght, M. (2022). Mutual Influence between Polyvinyl Chloride (Micro)Plastics and Black Soldier Fly Larvae (Hermetia illucens L.). Sustainability, 14(19), 12109. https://doi.org/10.3390/su141912109











