Speed Breeding Scheme of Hot Pepper through Light Environment Modification
Abstract
:1. Introduction
2. Materials and Methods
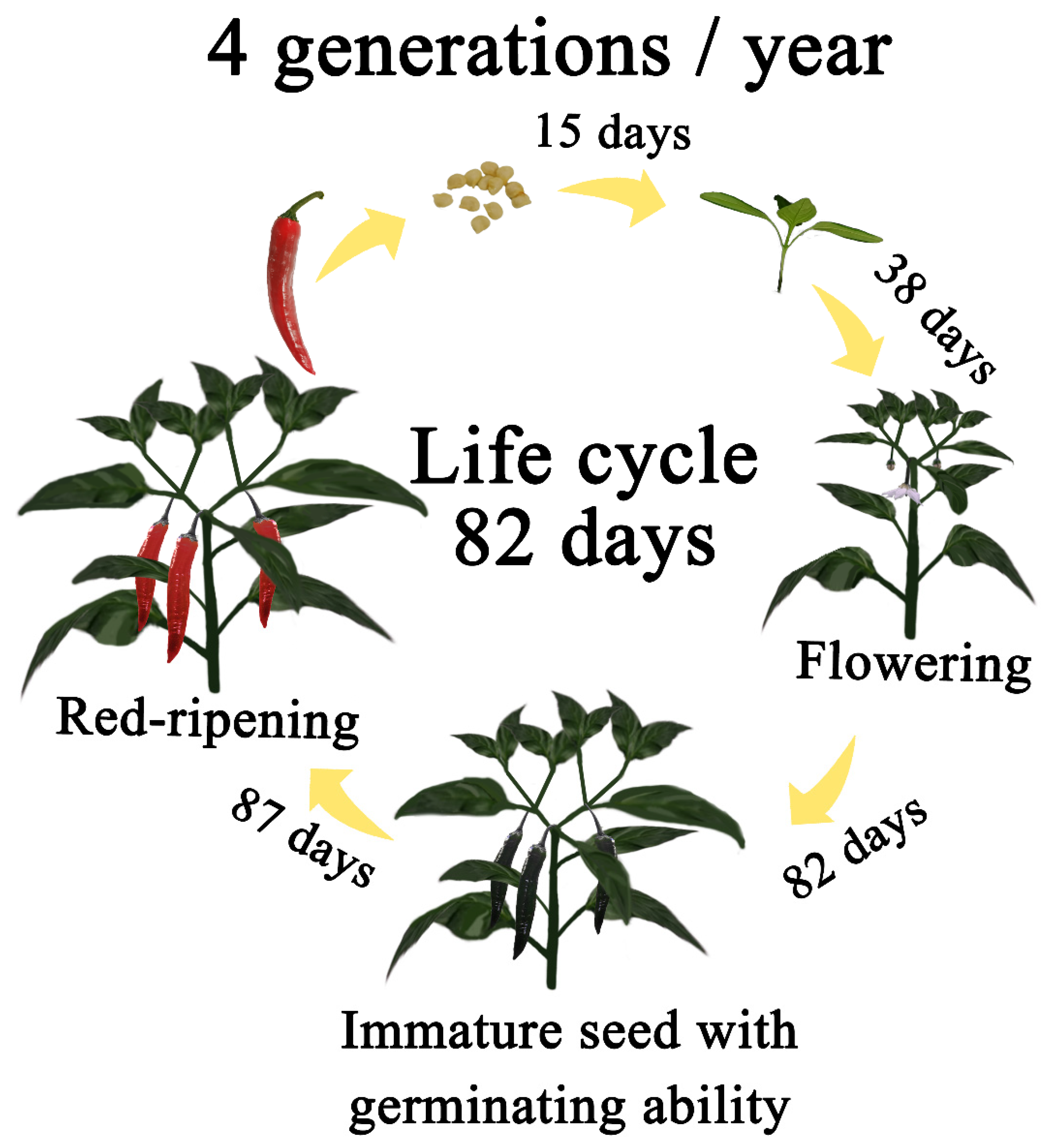
2.1. Plant Material and Growth Conditions
2.2. Growth and Morphology Evaluation
2.3. Sampling and Germination Ability Test
2.4. Statistical Analysis
3. Results
3.1. Effects of Light Intensity on the Growth and Development of Hot Peppers
3.2. Effects of the Photoperiod on the Growth and Development of Hot Peppers
3.3. Effects of the Red-to-Far-Red Ratio on the Growth and Development of Hot Peppers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samineni, S.; Sen, M.; Sajja, S.B.; Gaur, P.M. Rapid generation advance (RGA) in chickpea to produce up to seven generations per year and enable speed breeding. Crop. J. 2020, 8, 164–169. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Samantara, K.; Bohra, A.; Mohapatra, S.R.; Prihatini, R.; Asibe, F.; Singh, L.; Reyes, V.P.; Tiwari, A.; Maurya, A.K.; Croser, J.S.; et al. Breeding More Crops in Less Time: A Perspective on Speed Breeding. Biology 2022, 11, 275. [Google Scholar] [CrossRef]
- Friend, C.D.J.; Fisher, J.E.; Helson, V.A. The effect of light intensity and temperature on floral initiation and inflorescence development of Marquis wheat. J. Can. J. Bot. 1963, 41, 1663–1674. [Google Scholar] [CrossRef]
- Hikosaka, S.; Iyoki, S.; Hayakumo, M.; Goto, E. Effects of light intensity and amount of supplemental LED lighting on photosynthesis and fruit growth of tomato plants under artificial conditions. J. Agric. Meteorol. 2013, 69, 93–100. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Env. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Env. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Kristiansen, K. Light quality regulates flower initiation, differentiation and development of Campanula carpatica Jacq. “Karl Forster.” Sci. Hortic. 1988, 35, 275–283. [Google Scholar] [CrossRef]
- Sang, Y.K.; Yu, X.; Michaels, S.D. Regulation of Constans and Flowering Locus T expression in response to changing light quality. Plant Physiol. 2008, 148, 269–279. [Google Scholar] [CrossRef]
- Nishidate, K.; Kanayama, Y.; Nishiyama, M.; Yamamoto, T.; Hamaguchi, Y.; Kanahama, K. Far-red light supplemented with weak red light promotes flowering of Gypsophila paniculata. J. Japanese Soc. Hortic. Sci. 2012, 81, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Hulse-Kemp, A.M.; Ashrafi, H.; Plieske, J.; Lemm, J.; Stoffel, K.; Hill, T.; Luerssen, H.; Pethiyagoda, C.L.; Lawley, C.T.; Ganal, M.W.; et al. A HapMap leads to a Capsicum annuum SNP infinium array: A new tool for pepper breeding. Hortic. Res. 2016, 3, 16036. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistics Database. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 August 2021).
- Manzur, J.P.; Oliva-Alarcón, M.; Rodríguez-Burruezo, A. In vitro germination of immature embryos for accelerating generation advancement in peppers (Capsicum annuum L.). Sci. Hortic. 2014, 170, 203–210. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Blackman, B.K. Changing responses to changing seasons: Natural variation in the plasticity of flowering time. Plant Physiol. 2017, 173, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian clock and photoperiodic flowering in arabidopsis: CONSTANS is a Hub for Signal integration. Plant Physiol. 2017, 173, 5–15. [Google Scholar] [CrossRef]
- Oh, W. Effects of temperature, photoperiod and light intensity on growth and flowering in Eustoma grandiflorum. Korean J. Hortic. Sci. Technol. 2015, 33, 349–355. [Google Scholar] [CrossRef]
- Haliapas, S.; Yupsanis, T.A.; Syros, T.D.; Kofidis, G.; Economou, A.S. Petunia x hybrida during transition to flowering as affected by light intensity and quality treatments. Acta Physiol. Plant. 2008, 30, 807–815. [Google Scholar] [CrossRef]
- Rizal, G.; Karki, S.; Alcasid, M.; Montecillo, F.; Acebron, K.; Larazo, N.; Garcia, R.; Slamet-Loedin, I.H.; Quick, W.P. Shortening the breeding cycle of sorghum, a model crop for research. Crop. Sci. 2014, 54, 520–529. [Google Scholar] [CrossRef]
- Mawphlang, O.I.L.; Kharshiing, E.V. Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef] [Green Version]
- González-Barrios, P.; Bhatta, M.; Halley, M.; Sandro, P.; Gutiérrez, L. Speed breeding and early panicle harvest accelerates oat (Avena sativa L.) breeding cycles. Crop. Sci. 2021, 61, 320–330. [Google Scholar] [CrossRef]
- Mobini, S.H.; Warkentin, T.D. A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). Vitr. Cell. Dev. Biol.—Plant 2016, 52, 530–536. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Harkess, R.L.; Telmadarrehei, T. The effect of light intensity and temperature on flowering and morphology of potted red Firespike. Horticulturae 2018, 4, 36. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Oh, J.; Park, E.; Song, K.; Bae, G.; Choi, G. PHYTOCHROME INTERACTING FACTOR8 Inhibits Phytochrome A-Mediated Far-Red Light Responses in Arabidopsis. Plant Cell. 2020, 32, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.H.; Lulsdorf, M.; Warkentin, T.D.; Vandenberg, A. Low red: Far-red light ratio causes faster in vitro flowering in lentil. Can. J. Plant Sci. 2016, 96, 908–918. [Google Scholar] [CrossRef]
- Song, Y.; Duan, X.; Wang, P.; Li, X.; Yuan, X.; Wang, Z.; Wan, L.; Yang, G.; Hong, D. Comprehensive speed breeding: A high-throughput and rapid generation system for long-day crops. Plant Biotechnol. J. 2022, 20, 13–15. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; He, R.; He, X.; Tan, J.; Chen, Y.; Li, Y.; Liu, R.; Huang, Y.; Liu, H. Speed Breeding Scheme of Hot Pepper through Light Environment Modification. Sustainability 2022, 14, 12225. https://doi.org/10.3390/su141912225
Liu K, He R, He X, Tan J, Chen Y, Li Y, Liu R, Huang Y, Liu H. Speed Breeding Scheme of Hot Pepper through Light Environment Modification. Sustainability. 2022; 14(19):12225. https://doi.org/10.3390/su141912225
Chicago/Turabian StyleLiu, Kaizhe, Rui He, Xinyang He, Jiehui Tan, Yongkang Chen, Yamin Li, Rongyun Liu, Yanwu Huang, and Houcheng Liu. 2022. "Speed Breeding Scheme of Hot Pepper through Light Environment Modification" Sustainability 14, no. 19: 12225. https://doi.org/10.3390/su141912225
APA StyleLiu, K., He, R., He, X., Tan, J., Chen, Y., Li, Y., Liu, R., Huang, Y., & Liu, H. (2022). Speed Breeding Scheme of Hot Pepper through Light Environment Modification. Sustainability, 14(19), 12225. https://doi.org/10.3390/su141912225








