Development of High-Performance Fiber Cement: A Case Study in the Integration of Circular Economy in Product Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heat of Hydration Test
2.3. FC Sample Preparation
2.4. Characterization of the FC Samples
2.4.1. Mechanical Testing and Density Measurement
2.4.2. Durability and Recyclability Test
Freeze–Thaw Test, Heat Conductivity Test, and Flammability Test
Recyclability
3. Results and Discussion
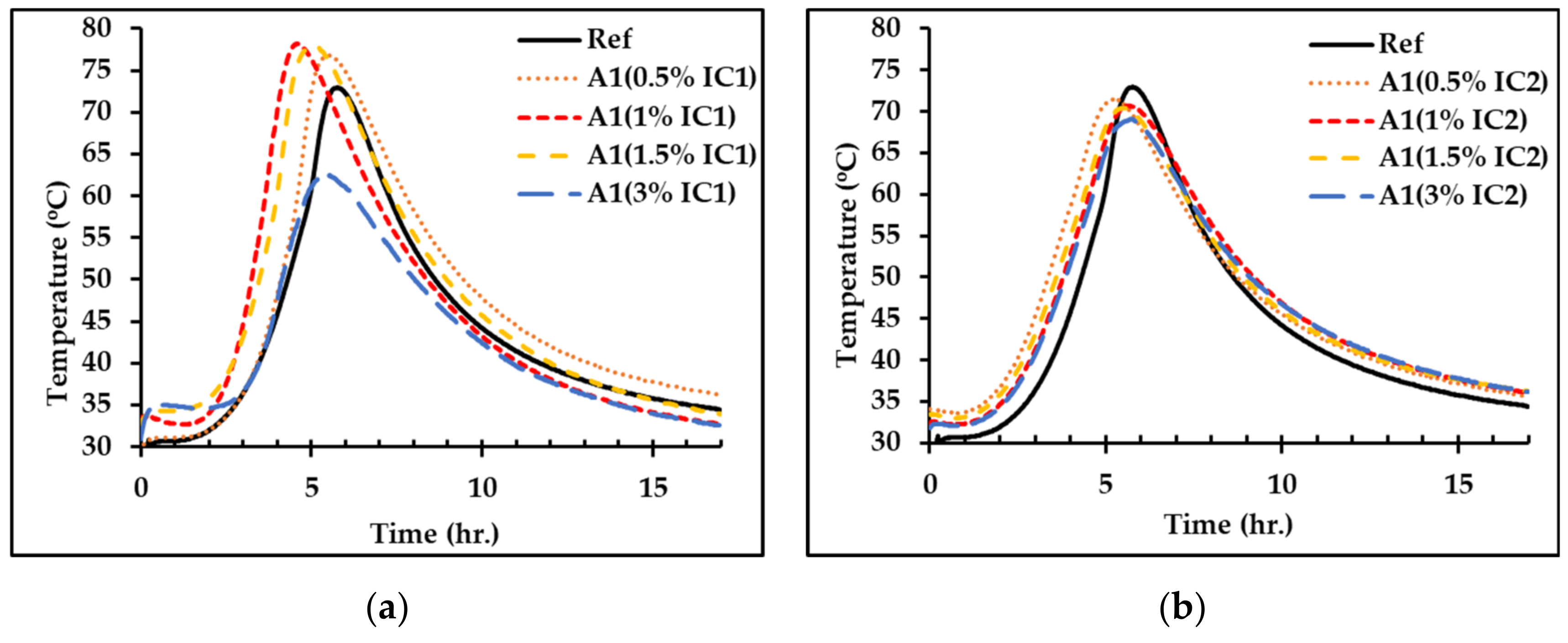
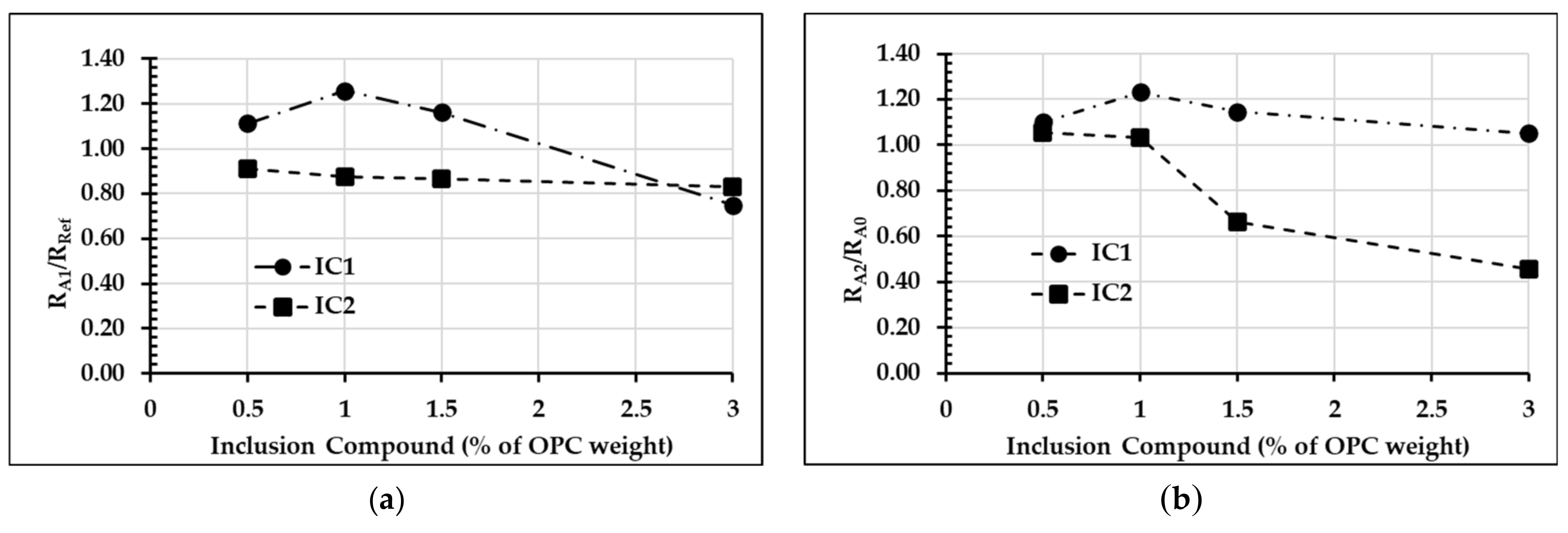
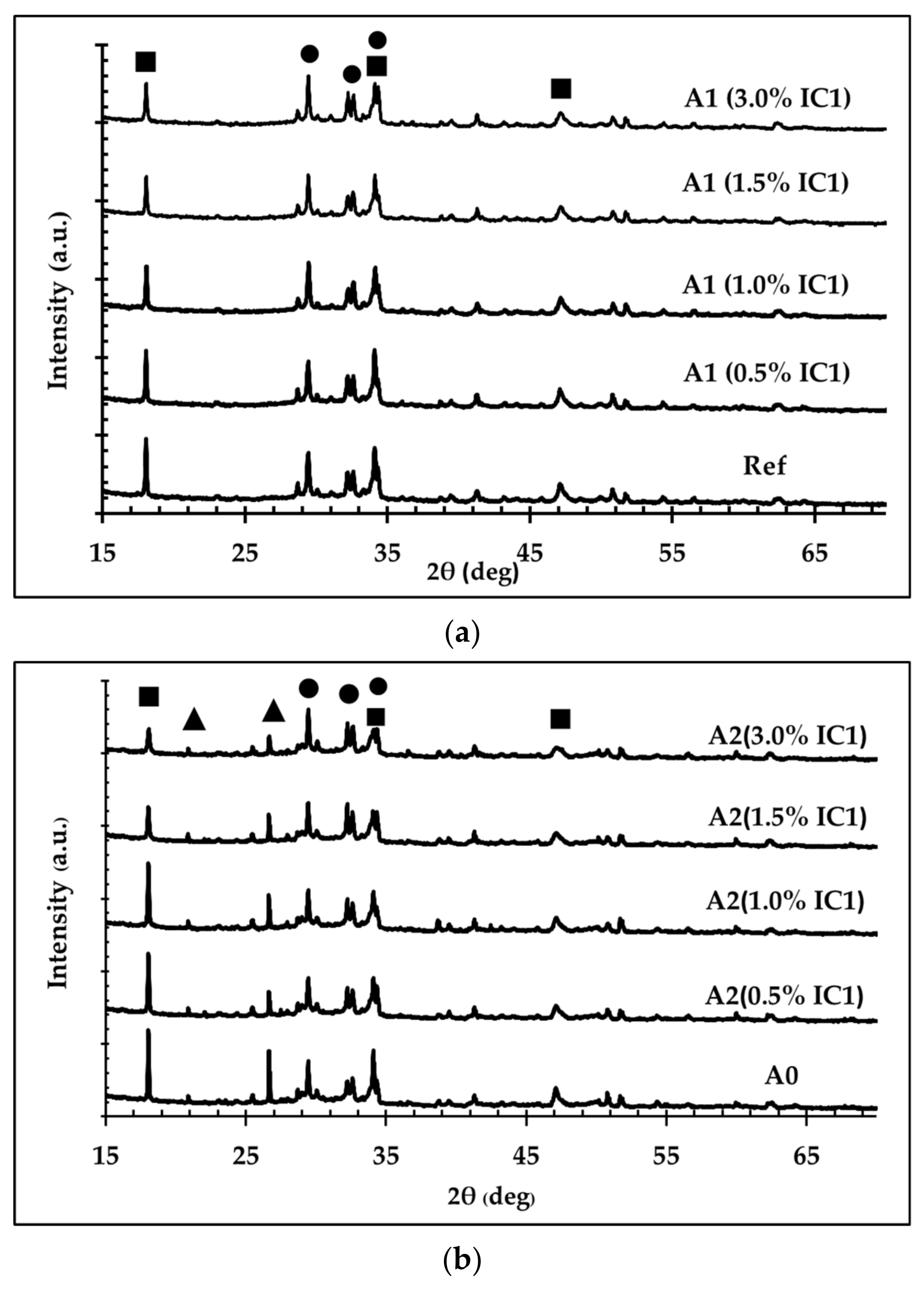
3.1. Effect of Inclusion Compounds on the Hydration Reaction of OPC
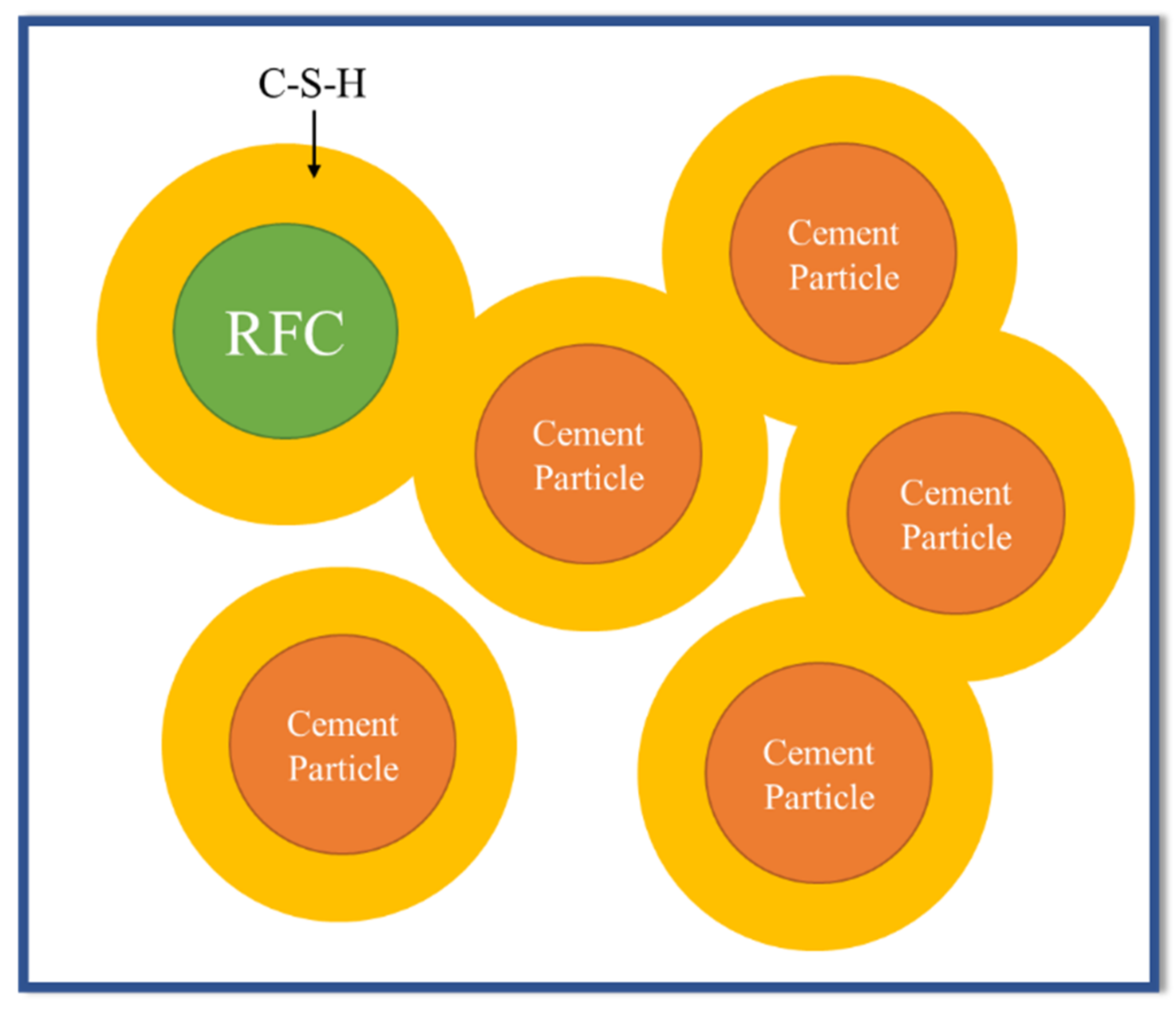
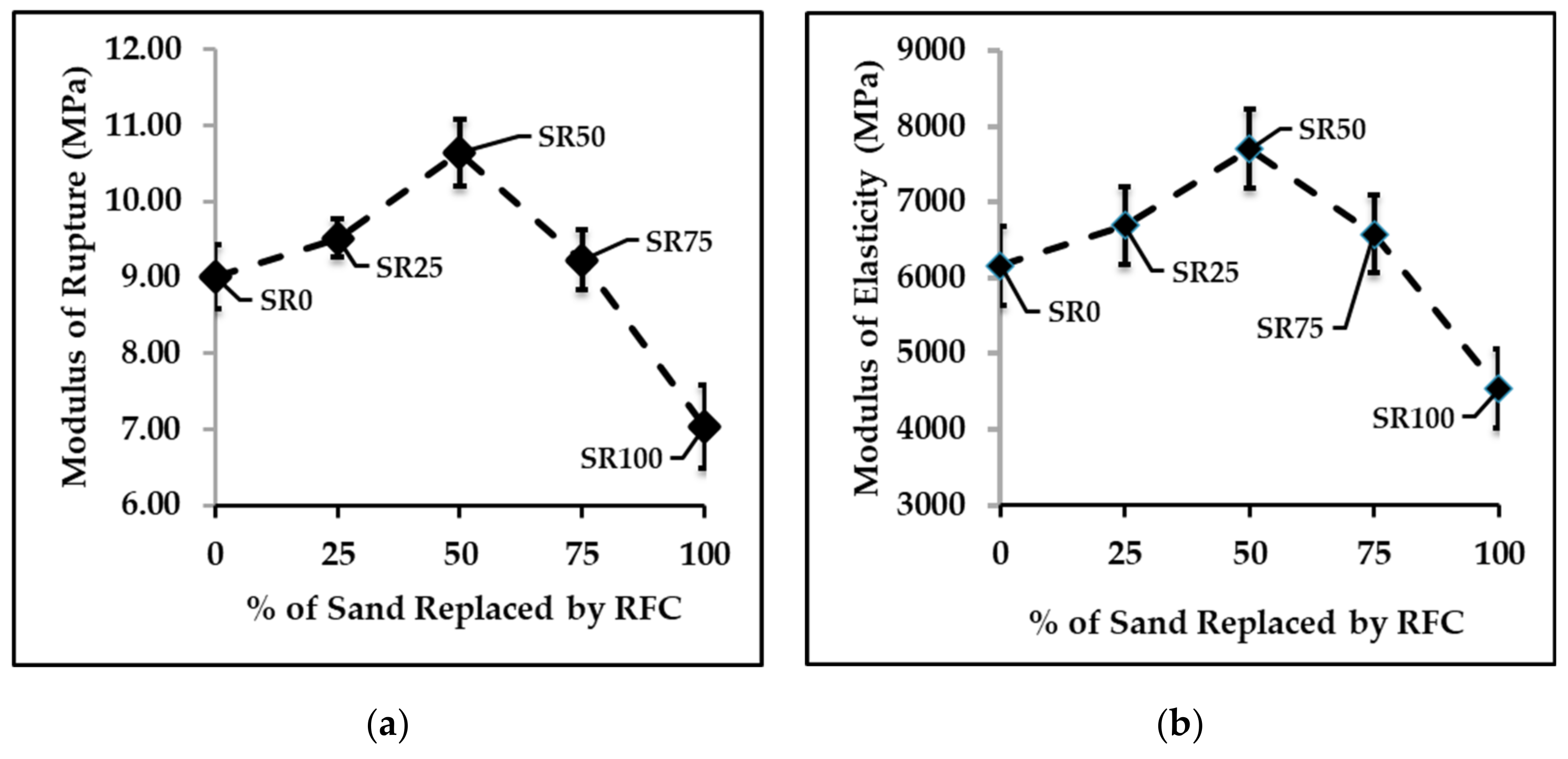
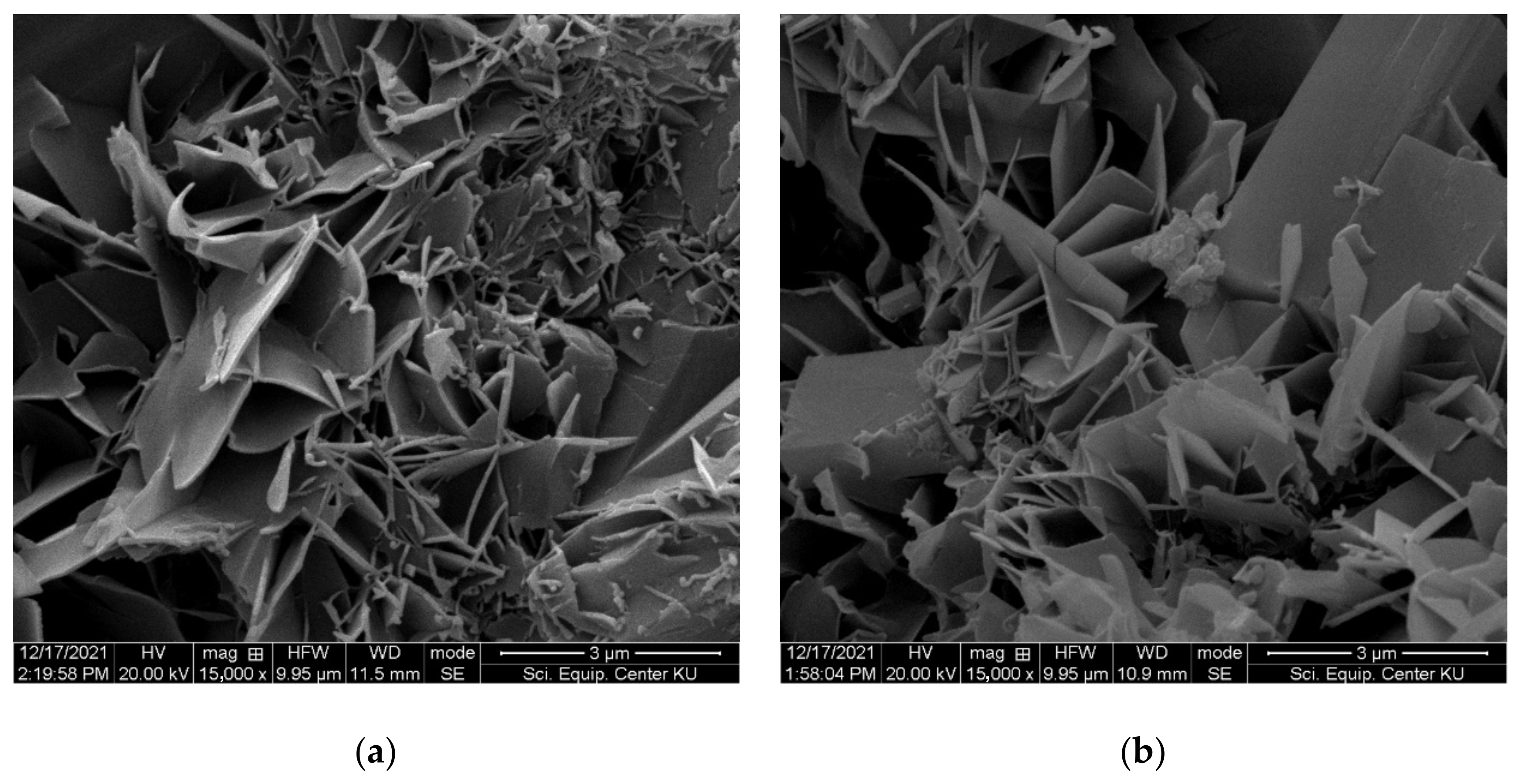
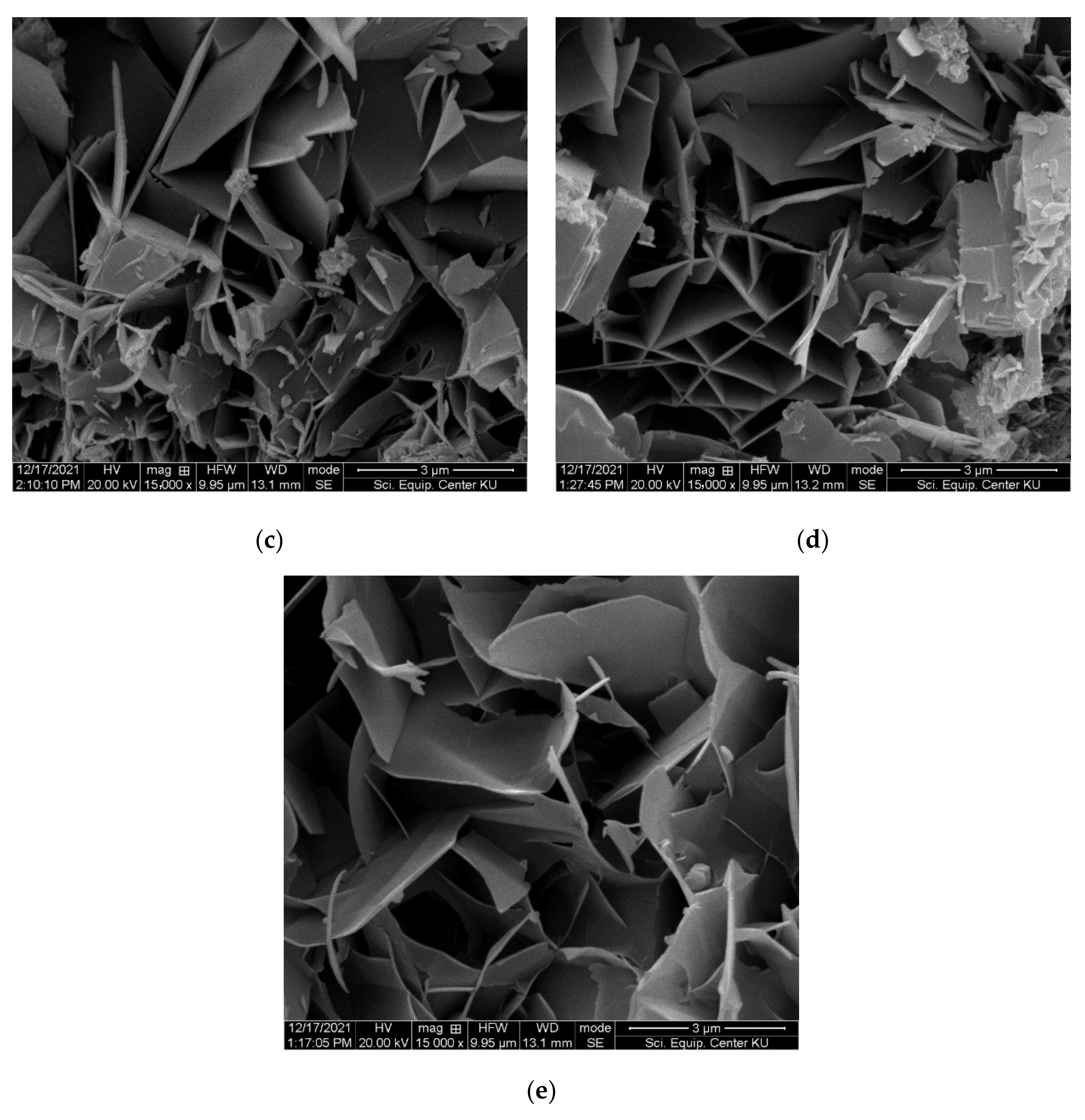
3.2. Mechanical and Physical Properties of FC Samples
3.3. Freeze–Thaw Resistance of FC Samples
3.4. Thermal Conductivity and Flammability of FC Samples
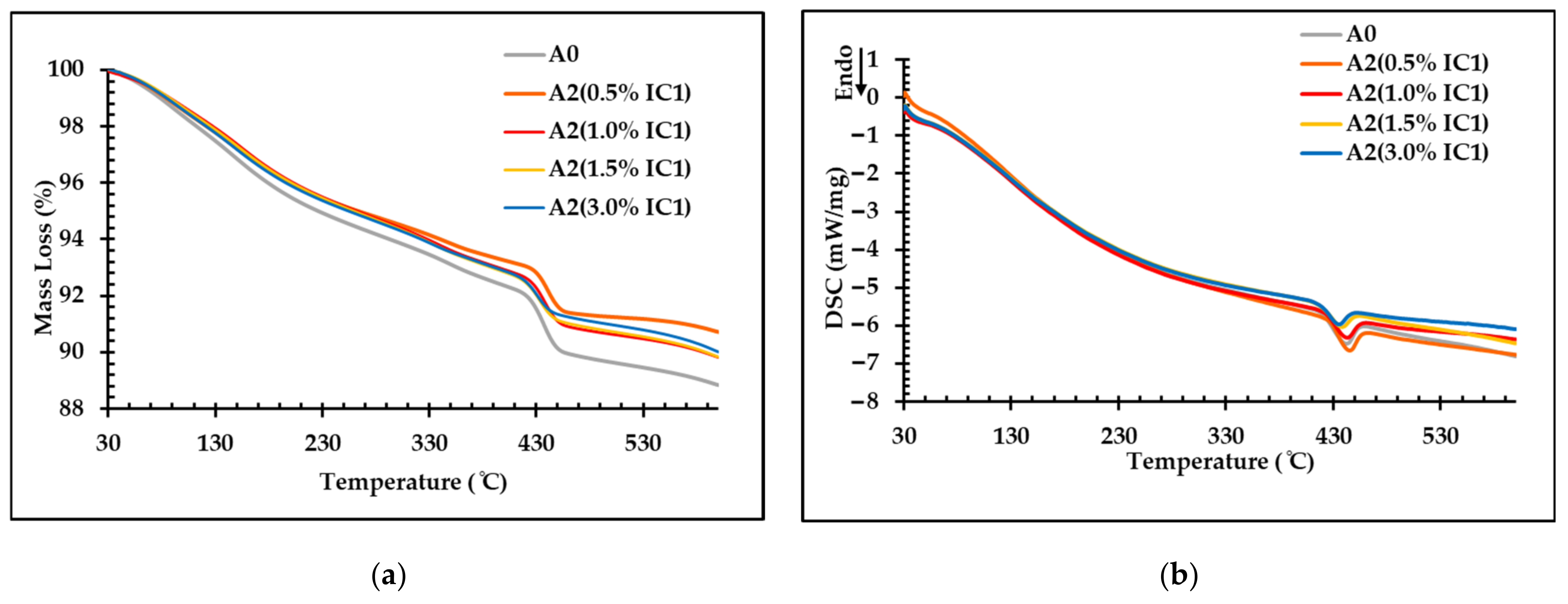
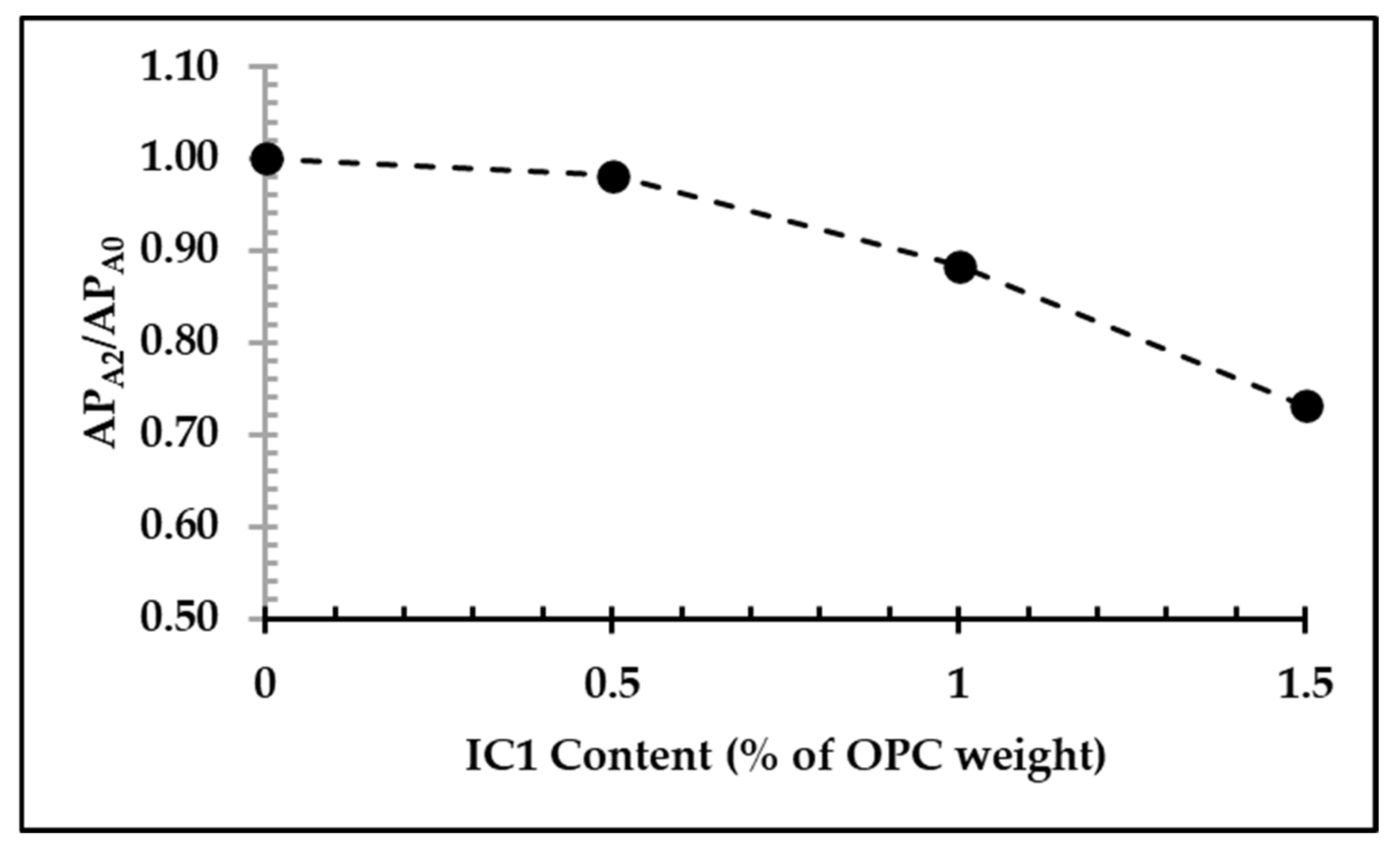
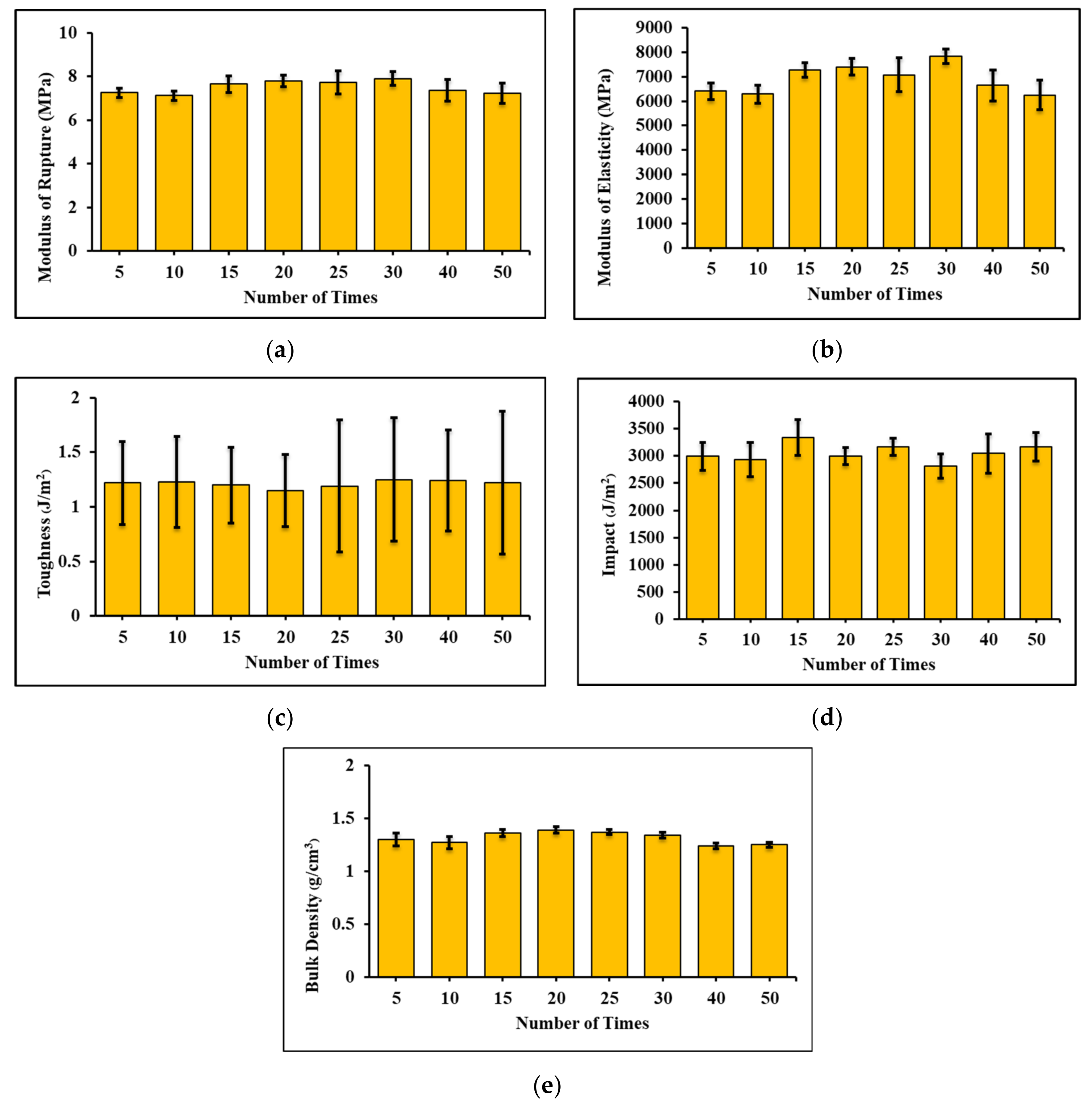
3.5. Recyclability of FC Samples and Their Environmental Impacts
4. Conclusions
- The circular economy (CE) model was employed for the creation of new high-performance FC, a fiber-reinforced construction material. Two inclusion compounds were investigated, with IC1 determined to be the most beneficial: a mixture of lithium silicate, sodium thiocyanate, alkylbenzene sulfonate, and hydrochloric acid. According to the heat of hydration test, using IC1 at 1 wt% in a mixture of ordinary Portland cement (OPC), the hydration reaction rate was highest, represented by the highest heat-generation rate. The improved hydration-reaction rate was from the reaction between lithium silicate in IC1 and calcium hydroxide, the product from the hydration reaction between tricalcium silicate and water.
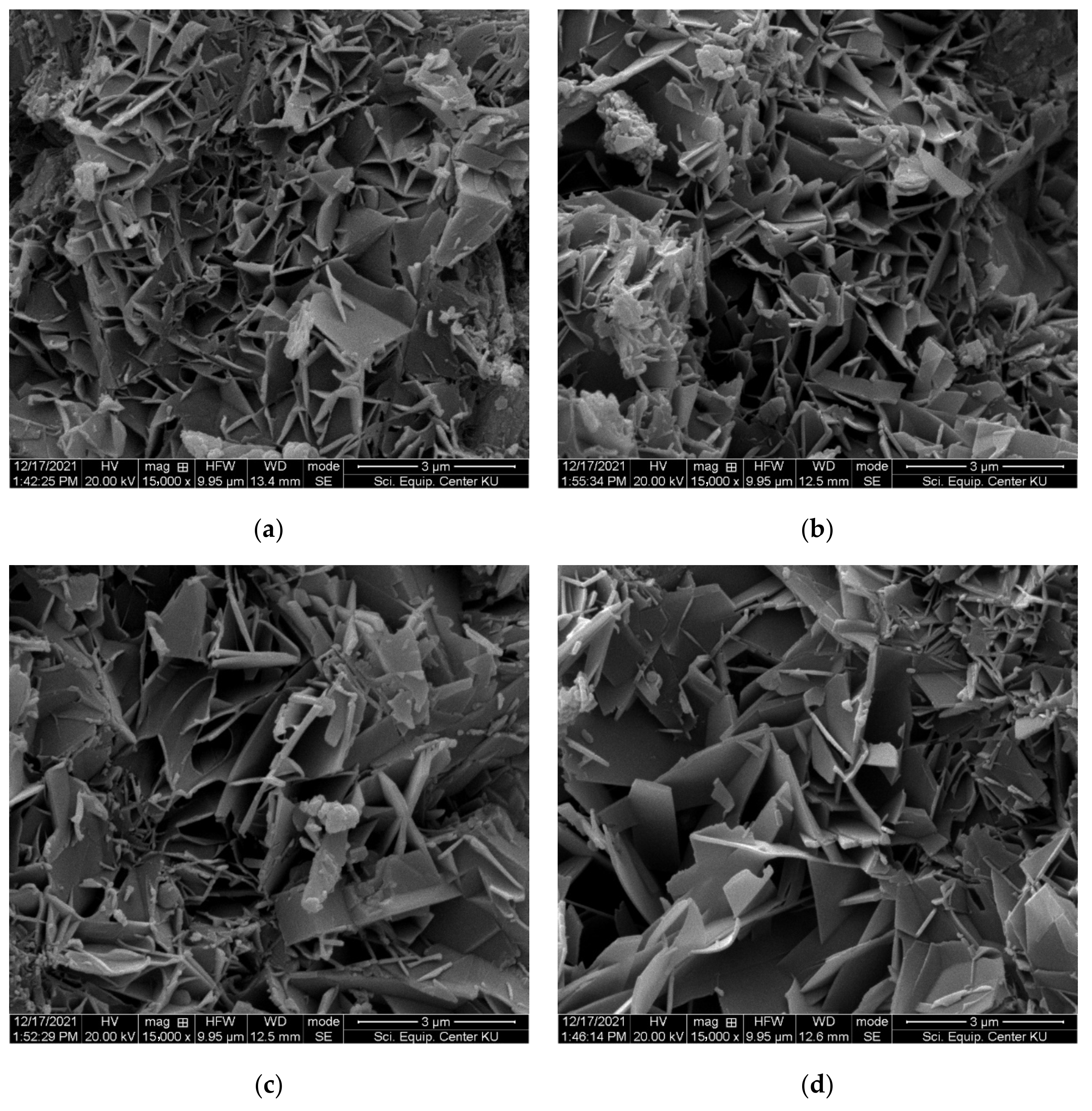
- The results from mechanical and physical property testing showed that the FC samples produced with IC1 as the chemical admixture and the partial replacement of sand by recycled fiber cement (RFC) had the properties required by the industrial requirements. Up to 50% of the sand can be replaced with RFC, and the samples passed the durability test including the freeze-and-thaw and flammability tests. At 50% sand replacement, the variation in tobermorite size, the crystalline phase of calcium silicate hydrate (C-S-H), improves properties including the modulus of rupture (MOR), modulus of elasticity (MOE), toughness, and bulk density of FC samples.
- The FC samples have good recyclability because the properties of the samples remain the same after recycling 5 to 50 times. Based on these properties, the possible applications of this recycled FC are in floor boards, wall boards, and roof tiles. Each month, an FC factory can consume up to 9000 tons of sand. Sand is a non-renewable natural resource. For an FC factory, substituting sand with RFC by 50% could reduce the demand for sand by up to 4500 tons/month. According to results from the SimaPro software, using RFC as the sand replacement at a 50% level could reduce greenhouse gas emissions by up to 1,719,000 kg of CO2e/month at each FC factory. In addition, the possible negative environmental impacts from the CDW disposal are also eliminated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taned Mahattanalai. Construction Contractor. Available online: https://www.krungsri.com/getmedia/61eb4685-3b57-442a-9a9a-d7a8bb9f6cf7/IO_Construction_Contractor_210205_EN_EX.pdf.aspx (accessed on 1 December 2021).
- Jain, S.; Singhal, S.; Pandey, S. Environmental life cycle assessment of construction and demolition waste recycling: A case of urban India. Resour. Conserv. Recycl. 2020, 155, 104642. [Google Scholar] [CrossRef]
- Jin, R.; Yuan, H.; Chen, Q. Science mapping approach to assisting the review of construction and demolition waste management research published between 2009 and 2018. Resour. Conserv. Recycl. 2019, 140, 175–188. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Sustainable Management of Construction and Demolition Materials; EPA: Washington, DC, USA, 2018. [Google Scholar]
- Wu, H.; Duan, H.; Zheng, L.; Wang, J.; Niu, Y.; Zhang, G. Demolition waste generation and recycling potentials in a rapidly developing flagship megacity of South China: Prospective scenarios and implications. Constr. Build. Mater. 2016, 113, 1007–1016. [Google Scholar] [CrossRef]
- Luangcharoenrat, C.; Intrachooto, S.; Peansupap, V.; Sutthinarakorn, W. Factors Influencing Construction Waste Generation in Building Construction: Thailand’s Perspective. Sustainability 2019, 11, 3638. [Google Scholar] [CrossRef]
- Cherubini, F.; Bargigli, S.; Ulgiati, S. Life cycle assessment (LCA) of waste management strategies: Landfilling, sorting plant and incineration. Energy 2009, 34, 2116–2123. [Google Scholar] [CrossRef]
- Li, J.; Xia, R.; Li, J.; Chen, G. Environmental Impact Assessment of Construction and Demolition Waste Recycling in Shenzhen. In Proceedings of the 20th International Symposium on Advancement of Construction Management and Real Estate; Springer: Singapore, 2017; pp. 1101–1110. [Google Scholar]
- Wang, J.; Wu, H.; Tam, V.W.Y.; Zuo, J. Considering life-cycle environmental impacts and society’s willingness for optimizing construction and demolition waste management fee: An empirical study of China. J. Clean. Prod. 2019, 206, 1004–1014. [Google Scholar] [CrossRef]
- Thongkamsuk, P.; Sudasna, K.; Tondee, T. Waste generated in high-rise buildings construction: A current situation in Thailand. Energy Procedia 2017, 138, 411–416. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, H.; Zhang, H.; Duan, H.; Wang, J.; Jiang, W.; Dong, B.; Liu, G.; Zuo, J.; Song, Q. Characterizing the generation and flows of construction and demolition waste in China. Constr. Build. Mater. 2017, 136, 405–413. [Google Scholar] [CrossRef]
- Li, R.Y.M.; Du, H. Sustainable Construction Waste Management in Australia: A Motivation Perspective. In Construction Safety and Waste Management: An Economic Analysis; Li, R.Y.M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–30. [Google Scholar]
- Ellen MacArthur Foundation. What Is a Circular Economy? Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 1 September 2021).
- Akhimien, N.G.; Latif, E.; Hou, S.S. Application of circular economy principles in buildings: A systematic review. J. Build. Eng. 2021, 38, 102041. [Google Scholar] [CrossRef]
- Kongkajun, N.; Laitila, E.A.; Ineure, P.; Prakaypan, W.; Cherdhirunkorn, B.; Chakartnarodom, P. Soil-cement bricks produced from local clay brick waste and soft sludge from fiber cement production. Case Stud. Constr. Mater. 2020, 13, e00448. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Wu, R.; Yang, D.; Ma, Z. The utilization of active recycled powder from various construction wastes in preparing ductile fiber-reinforced cementitious composites: A case study. Case Stud. Constr. Mater. 2021, 15, e00650. [Google Scholar] [CrossRef]
- Mistri, A.; Bhattacharyya, S.K.; Dhami, N.; Mukherjee, A.; Barai, S.V. A review on different treatment methods for enhancing the properties of recycled aggregates for sustainable construction materials. Constr. Build. Mater. 2020, 233, 117894. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Munir, M.J.; Wu, Y.-F.; Patnaikuni, I.; Zhou, Y.; Xing, F. Influence of different treatment methods on the mechanical behavior of recycled aggregate concrete: A comparative study. Cem. Concr. Compos. 2019, 104, 103398. [Google Scholar] [CrossRef]
- Sonprasarn, P.; Chakartnarodom, P.; Ineure, P.; Prakaypan, W. Effects of the chemical treatment on coal-fired bottom ash for the utilization in fiber-reinforced cement. J. Met. Mater. Miner. 2019, 29, 55–60. [Google Scholar] [CrossRef]
- Sonprasarn, P.; Chakartnarodom, P.; Kongkajun, N.; Prakaypan, W. Microstructure and mechanical performance of fiber-reinforced cement composites made with nucleating-agent activated coal-fired power plant bottom ash. Solid State Phenom. 2020, 302, 85–92. [Google Scholar] [CrossRef]
- Ren, G.; Tian, Z.; Wu, J.; Gao, X. Effects of combined accelerating admixtures on mechanical strength and microstructure of cement mortar. Constr. Build. Mater. 2021, 304, 124642. [Google Scholar] [CrossRef]
- Pahuswanno, P.; Chakartnarodom, P.; Ineure, P.; Prakaypan, W. The influences of chemical treatment on recycled rejected fiber cement used as fillers in the fiber cement products. J. Met. Mater. Miner. 2019, 29, 66–70. [Google Scholar] [CrossRef]
- Pahuswanno, P.; Chakartnarodom, P.; Kongkajun, N.; Prakaypan, W. Feasibility study of using modified recycled fiber-cement for the production of high performance fiber-cement composites. Solid State Phenom. 2020, 302, 93–99. [Google Scholar] [CrossRef]
- Ram, V.G.; Kishore, K.C.; Kalidindi, S.N. Environmental benefits of construction and demolition debris recycling: Evidence from an Indian case study using life cycle assessment. J. Clean. Prod. 2020, 255, 120258. [Google Scholar] [CrossRef]
- Dokter, G.; Thuvander, L.; Rahe, U. How circular is current design practice? Investigating perspectives across industrial design and architecture in the transition towards a circular economy. Sustain. Prod. Consum. 2021, 26, 692–708. [Google Scholar] [CrossRef]
- European Commission. Sustainable Product Policy. Available online: https://joint-research-centre.ec.europa.eu/scientific-activities-z/sustainable-product-policy_en (accessed on 22 September 2022).
- Song, Z.; Lu, Z.; Lai, Z. The effect of lithium silicate impregnation on the compressive strength and pore structure of foam concrete. Constr. Build. Mater. 2021, 277, 122316. [Google Scholar] [CrossRef]
- Wise, T.; Ramachandran, V.S.; Polomark, G.M. The effect of thiocyanates on the hydration of portland cement at low temperatures. Thermochim. Acta 1995, 264, 157–171. [Google Scholar] [CrossRef]
- Costa, M.F.; de Oliveira, A.M.; Oliveira Junior, E.N.d. Biodegradation of linear alkylbenzene sulfonate (LAS) by Penicillium chrysogenum. Bioresour. Technol. Rep. 2020, 9, 100363. [Google Scholar] [CrossRef]
- Carvalho, J.M.F.; Oliveira, M.B.; Moreira, L.P.S.H.; Mendes, J.C.; Pereira, C.A. Use of Alkylbenzene Sulfonate (LAS) and Polycarboxylate-Ether (PCE) as Reagents in Iron Ore Flotation. Holos 2017, 6, 116–125. [Google Scholar] [CrossRef]
- Mohit, M.; Ranjbar, A.; Sharifi, Y. Mechanical and microstructural properties of mortars incorporating ceramic waste powder exposed to the hydrochloric acid solution. Constr. Build. Mater. 2021, 271, 121565. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Acidic corrosion of hydrated cement based materials. Part 1. Mechanism of the phenomenon. Ceram.-Silik. 2000, 44, 114–120. [Google Scholar]
- Chandra, S. Hydrochloric acid attack on cement mortar—An analytical study. Cem. Concr. Res. 1988, 18, 193–203. [Google Scholar] [CrossRef]
- Ismail, A.H.; Kusbiantoro, A.; Chin, S.C.; Muthusamy, K.; Islam, M.; Tee, K.F. Pozzolanic reactivity and strength activity index of mortar containing palm oil clinker pretreated with hydrochloric acid. J. Clean. Prod. 2020, 242, 118565. [Google Scholar] [CrossRef]
- Han, J.; Wang, K.; Shi, J.; Wang, Y. Mechanism of triethanolamine on Portland cement hydration process and microstructure characteristics. Constr. Build. Mater. 2015, 93, 457–462. [Google Scholar] [CrossRef]
- Flatt, R.; Schober, I. 7—Superplasticizers and the rheology of concrete. In Understanding the Rheology of Concrete; Roussel, N., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 144–208. [Google Scholar]
- Steven, G. Evaluation of Calcium Formate and Sodium Formate as Accelerating Admixtures for Portland Cement Concrete. ACI J. Proc. 1983, 80, 439–444. [Google Scholar] [CrossRef]
- ASTM C186-98; Standard Test Method for Heat of Hydration of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM C1185; Standard Test Methods for Sampling and Testing Non-Asbestos Fiber-Cement Flat Sheet, Roofing and Siding Shingles, and Clapboards. ASTM International: West Conshohocken, PA, USA, 2016.
- BS EN 12467; Fiber-Cement Flat Sheets—Product Specification and Test Methods. The British Standard Institution: London, UK, 2012.
- ASTM C177; Standard Test Method for Steady-State Heat Flux Measurements and Thermal Transmission Properties by Means of the Guarded-Hot-Plate Apparatus. ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 11925-2; Reaction to fire tests—Ignitability of products subjected to direct impingement of flame—Part 2: Single-Flame Source Test. International Organization for Standardization: London, UK, 2020.
- Dwivedi, V.; Das, S.; Singh, N.; Rai, S.; Gajbhiye, N. Portland cement hydration in the presence of admixtures—Black gram pulse and superplasticizer. Mater. Res. 2008, 11, 427–431. [Google Scholar] [CrossRef]
- Chakartnarodom, P.; Prakaypan, W.; Ineure, P.; Kongkajun, N.; Chuankrerkkul, N. Feasibility Study of Using Basalt Fibers as the Reinforcement Phase in Fiber-Cement Products. Key Eng. Mater. 2018, 766, 252–257. [Google Scholar] [CrossRef]
- Maljaee, H.; Paiva, H.; Madadi, R.; Tarelho, L.A.C.; Morais, M.; Ferreira, V.M. Effect of cement partial substitution by waste-based biochar in mortars properties. Constr. Build. Mater. 2021, 301, 124074. [Google Scholar] [CrossRef]
- Harrisson, A.M. 4—Constitution and Specification of Portland Cement. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P.C., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 87–155. [Google Scholar]
- Chaipanich, A.; Nochaiya, T. Thermal analysis and microstructure of Portland cement-fly ash-silica fume pastes. J. Therm. Anal. Calorim. 2009, 99, 487–493. [Google Scholar] [CrossRef]
- Nochaiya, T.; Wongkeo, W.; Pimraksa, K.; Chaipanich, A. Microstructural, physical, and thermal analyses of Portland cement–fly ash–calcium hydroxide blended pastes. J. Therm. Anal. Calorim. 2009, 100, 101–108. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Sha, W.; O’Neill, E.A.; Guo, Z. Differential scanning calorimetry study of ordinary Portland cement. Cem. Concr. Res. 1999, 29, 1487–1489. [Google Scholar] [CrossRef]
- Marchon, D.; Flatt, R.J. 8—Mechanisms of cement hydration. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 129–145. [Google Scholar]
- Zhou, W.; Duan, L.; Tang, S.; Chen, E.; Hanif, A. Modeling the evolved microstructure of cement pastes governed by diffusion through barrier shells of C–S–H. J. Mater. Sci. 2019, 54, 4680–4700. [Google Scholar] [CrossRef]
- Rahimi-Aghdam, S.; Bažant, Z.P.; Abdolhosseini Qomi, M.J. Cement hydration from hours to centuries controlled by diffusion through barrier shells of C-S-H. J. Mech. Phys. Solids 2017, 99, 211–224. [Google Scholar] [CrossRef]
- ASTM C1186; Standard Specification for Flat Fiber-Cement Sheets. ASTM International: West Conshohocken, PA, USA, 2016.
- TIS 1427; Fibre-Cement Sheets: Flat Sheets. Thai Industrial Standard Institute: Bangkok, Thailand, 2018.
- Wu, Y.; Pan, X.; Li, Q.; Yu, H. Crystallization and phase transition of tobermorite synthesized by hydrothermal reaction from dicalcium silicate. Int. J. Appl. Ceram. Technol. 2020, 17, 1213–1223. [Google Scholar] [CrossRef]
- Bell, N.S.; Venigalla, S.; Gill, P.M.; Adair, J.H. Morphological Forms of Tobermorite in Hydrothermally Treated Calcium Silicate Hydrate Gels. J. Am. Ceram. Soc. 1996, 79, 2175–2178. [Google Scholar] [CrossRef]
- Chakartnarodom, P.; Kongkajun, N.; Chuankrerkkul, N.; Ineure, P.; Prakaypan, A.W. Reducing water absorption of fiber-cement composites for exterior applications by crystal modification method. J. Met. Mater. Miner. 2019, 29, 90–98. [Google Scholar] [CrossRef]
- Wongkeo, W.; Thongsanitgarn, P.; Pimraksa, K.; Chaipanich, A. Compressive strength, flexural strength and thermal conductivity of autoclaved concrete block made using bottom ash as cement replacement materials. Mater. Des. 2012, 35, 434–439. [Google Scholar] [CrossRef]
- Askeland, D.R.; Fulay, P.P.; Wrigh, W.J. The Science and Engineering of Materials, 6th ed.; Cengage Learning: Stamford, CT, USA, 2011. [Google Scholar]
- Jelitto, H.; Schneider, G. Fracture Toughness of Porous Materials—Experimental Methods and Data. Data Brief 2019, 23, 103709. [Google Scholar] [CrossRef]
- Marinescu, I.D.; Pruteanu, M. Chapter 2—Deformation and Fracture of Ceramic Materials. In Handbook of Ceramics Grinding and Polishing; Marinescu, I.D., Doi, T.K., Uhlmann, E., Eds.; William Andrew Publishing: Boston, MA, USA, 2015; pp. 50–66. [Google Scholar]
- Lautenberger, C.; Torero, J.; Fernandez-Pello, C. 1—Understanding materials flammability. In Flammability Testing of Materials Used in Construction, Transport and Mining; Apte, V.B., Ed.; Woodhead Publishing: Sawston, UK, 2006; pp. 1–21. [Google Scholar]
- Kim, Y.K. 8—Natural fibre composites (NFCs) for construction and automotive industries. In Handbook of Natural Fibres; Kozłowski, R.M., Ed.; Woodhead Publishing: Sawston, UK, 2012; Volume 2, pp. 254–279. [Google Scholar]
- Canosa, G.; Alfieri, P.V.; Giydice, C.A. Nano lithium silicates as flame-retardant impregnants for Pinus radiata. J. Fire Sci. 2011, 29, 431–441. [Google Scholar] [CrossRef]
- McCarter, R.J.; Gaithersburg, M. Flame Retarding Cellulosic Materials Withsodium or Potassium Thiocyanate. Available online: https://patentimages.storage.googleapis.com/29/58/8b/f99358cb0b36de/US4302345.pdf (accessed on 1 June 2022).
- The Thai Ministry of Energy. Notification of the Ministry of Energy: Criteria and Method of Calculation for Building Energy Efficiency Standard. Available online: https://download.asa.or.th/03media/04law/eca/ma64-02.pdf (accessed on 1 June 2022).
- Chakartnarodom, P.; Prakaypan, W.; Kongkajun, N. Recycling of the Roof Tile Waste as a Raw Material for Roof Tile Manufacturing Process. In Proceedings of the Thailand Research Expo: Symposium 2018, Bangkok, Thailand, 9–13 August 2018. [Google Scholar]








| Oxide | Composition (wt.%) | |||
|---|---|---|---|---|
| Portland Cement | Sand | Gypsum | RFC | |
| MgO | 1.21 | 0.01 | 0.19 | 0.62 |
| Al2O3 | 5.98 | 1.10 | 0.18 | 2.90 |
| SiO2 | 19.8 | 95.46 | 0.14 | 40.41 |
| K2O | 0.34 | 0.27 | 0.05 | 0.43 |
| CaO | 64.45 | 0.24 | 23.92 | 38.31 |
| Fe2O3 | 3.18 | 0.26 | 0.13 | 1.76 |
| SO3 | 2.73 | 0.00 | 72.74 | 15.28 |
| TiO2 | 0.25 | 0.05 | 0.01 | 0.15 |
| Other | 2.06 | 2.61 | 2.64 | 0.14 |
| Formula | Raw Material (g) | |||
|---|---|---|---|---|
| OPC | RFC | Water | IC (IC1 or IC2) | |
| Ref | 650 | - | 200 | - |
| A0 | 487.50 | 162.50 | 200 | - |
| A1 | 650 | - | 200 | 3.25, 6.50, 9.75, 19.50 (0.5, 1, 1.5, and 3% of OPC weight) |
| A2 | 487.50 | 162.50 | 200 | 3.25, 6.50, 9.75, 19.50 (0.5, 1, 1.5, and 3% of OPC weight) |
| Formula | % of Sand Replacement | Material (wt.%) | IC1 (% of OPC Weight) | Water (Water-to-Cement Ratio) | ||||
|---|---|---|---|---|---|---|---|---|
| OPC | Sand | RFC | Gypsum | Cellulose Fibers | ||||
| SR0 | 0 | 34.75 | 34.75 | 0.00 | 25.00 | 5.50 | 1 | 0.35 |
| SR25 | 25 | 34.75 | 26.06 | 8.69 | 25.00 | 5.50 | 1 | 0.35 |
| SR50 | 50 | 34.75 | 17.38 | 17.38 | 25.00 | 5.50 | 1 | 0.35 |
| SR75 | 75 | 34.75 | 8.69 | 26.06 | 25.00 | 5.50 | 1 | 0.35 |
| SR100 | 100 | 34.75 | 0.00 | 34.75 | 25.00 | 5.50 | 1 | 0.35 |
| Formula | Inclusion Compound (IC) | Tinitial (°C) | Tmax (°C) | t (h:min) | R (°C/min) | |
|---|---|---|---|---|---|---|
| Type | % OPC Weight | |||||
| Ref | None | 0.0 | 27.7 | 73.0 | 05:45 | 0.131 |
| A0 | None | 0.0 | 25.9 | 62.3 | 06:26 | 0.094 |
| A1 | IC1 | 0.5 | 29.3 | 76.8 | 05:25 | 0.146 |
| 1.0 | 33.3 | 78.2 | 04:32 | 0.165 | ||
| 1.5 | 32.7 | 77.9 | 04:56 | 0.153 | ||
| 3.0 | 30.9 | 62.7 | 05:23 | 0.098 | ||
| A1 | IC2 | 0.5 | 34.1 | 71.5 | 05:12 | 0.120 |
| 1.0 | 32.3 | 70.7 | 05:34 | 0.115 | ||
| 1.5 | 33.5 | 70.4 | 05:25 | 0.114 | ||
| 3.0 | 31.8 | 69.1 | 05:41 | 0.109 | ||
| A2 | IC1 | 0.5 | 29.7 | 65.5 | 05:45 | 0.104 |
| 1.0 | 30.0 | 68.1 | 05:28 | 0.116 | ||
| 1.5 | 33.2 | 67.0 | 05:13 | 0.108 | ||
| 3.0 | 32.5 | 63.6 | 05:14 | 0.099 | ||
| A2 | IC2 | 0.5 | 32.1 | 64.8 | 05:29 | 0.099 |
| 1.0 | 28.2 | 59.3 | 05:20 | 0.097 | ||
| 1.5 | 31.3 | 49.4 | 04:49 | 0.063 | ||
| 3.0 | 34.6 | 47.8 | 05:06 | 0.043 | ||
| Material | Thermal Conductivity (W/m-K) |
|---|---|
| Concrete roof tile | 0.993 |
| Brick wall | 0.473–1.102 |
| Hollow concrete block wall | 0.546 |
| Concrete-slab wall | 1.442 |
| Asbestos-reinforced FC (wall) | 0.397 |
| Asbestos-reinforced FC (corrugated roof tile) | 0.384–0.441 |
| Flame Type | Sample | |
|---|---|---|
| SR0 | SR50 | |
| 45° Surface ignition | No ignition | No ignition |
| 90° Vertical edge | No ignition | No ignition |
| 180° Bottom edge | No ignition | No ignition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakartnarodom, P.; Wanpen, S.; Prakaypan, W.; Laitila, E.A.; Kongkajun, N. Development of High-Performance Fiber Cement: A Case Study in the Integration of Circular Economy in Product Design. Sustainability 2022, 14, 12263. https://doi.org/10.3390/su141912263
Chakartnarodom P, Wanpen S, Prakaypan W, Laitila EA, Kongkajun N. Development of High-Performance Fiber Cement: A Case Study in the Integration of Circular Economy in Product Design. Sustainability. 2022; 14(19):12263. https://doi.org/10.3390/su141912263
Chicago/Turabian StyleChakartnarodom, Parinya, Sarunya Wanpen, Wichit Prakaypan, Edward A. Laitila, and Nuntaporn Kongkajun. 2022. "Development of High-Performance Fiber Cement: A Case Study in the Integration of Circular Economy in Product Design" Sustainability 14, no. 19: 12263. https://doi.org/10.3390/su141912263
APA StyleChakartnarodom, P., Wanpen, S., Prakaypan, W., Laitila, E. A., & Kongkajun, N. (2022). Development of High-Performance Fiber Cement: A Case Study in the Integration of Circular Economy in Product Design. Sustainability, 14(19), 12263. https://doi.org/10.3390/su141912263







