A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications
Abstract
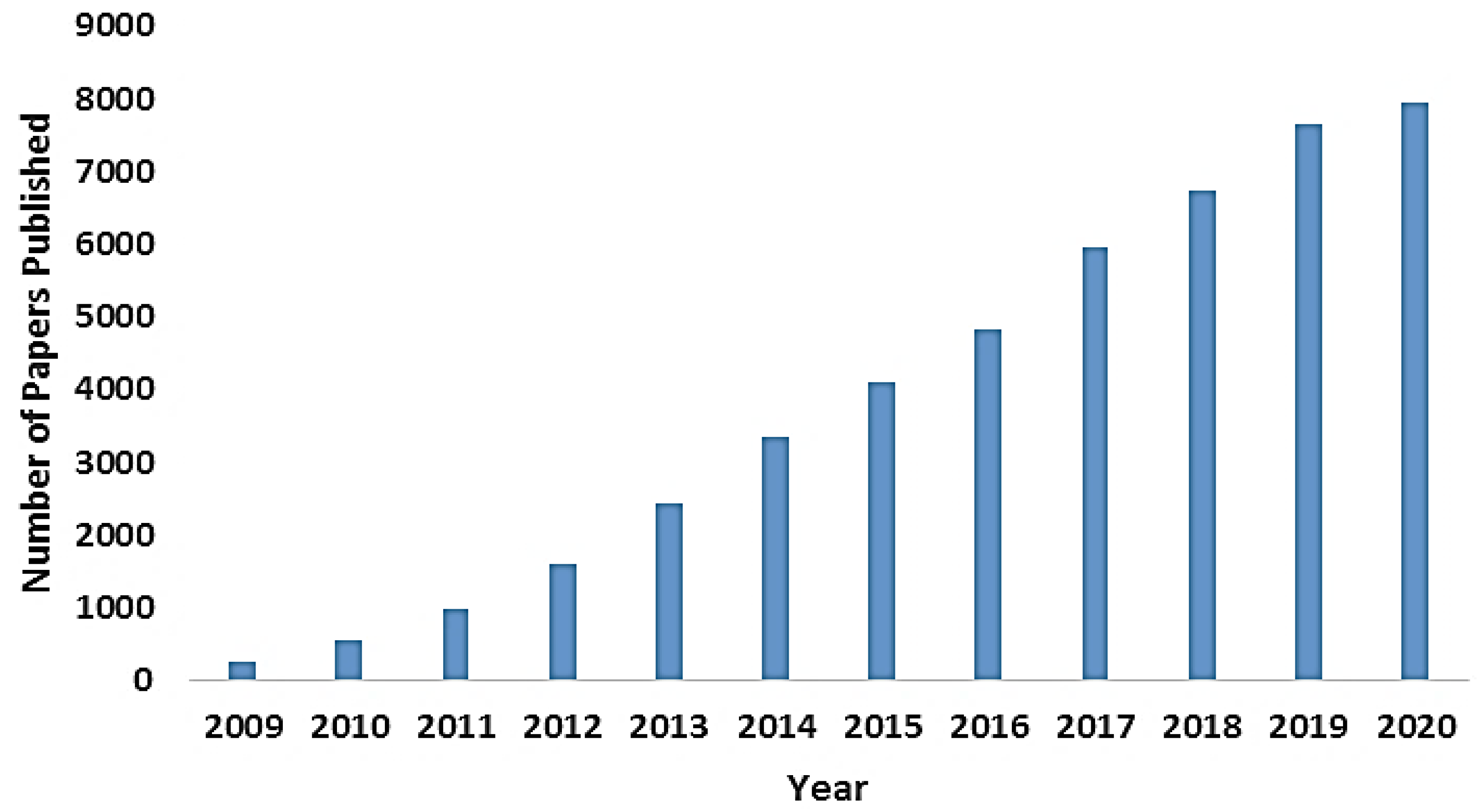
:1. Introduction
2. Graphene Basic Structure
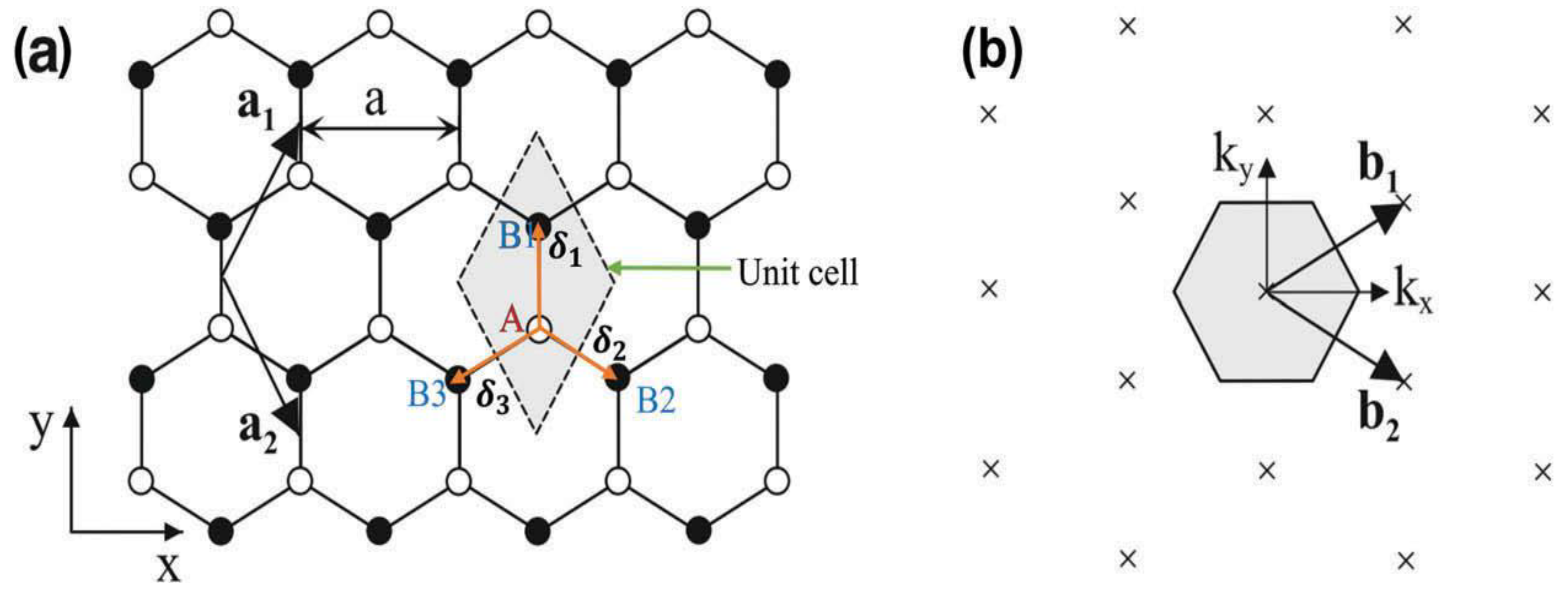
2.1. Basic Mechanical Structure of Graphene
2.2. Electrical Band Structure of Graphene
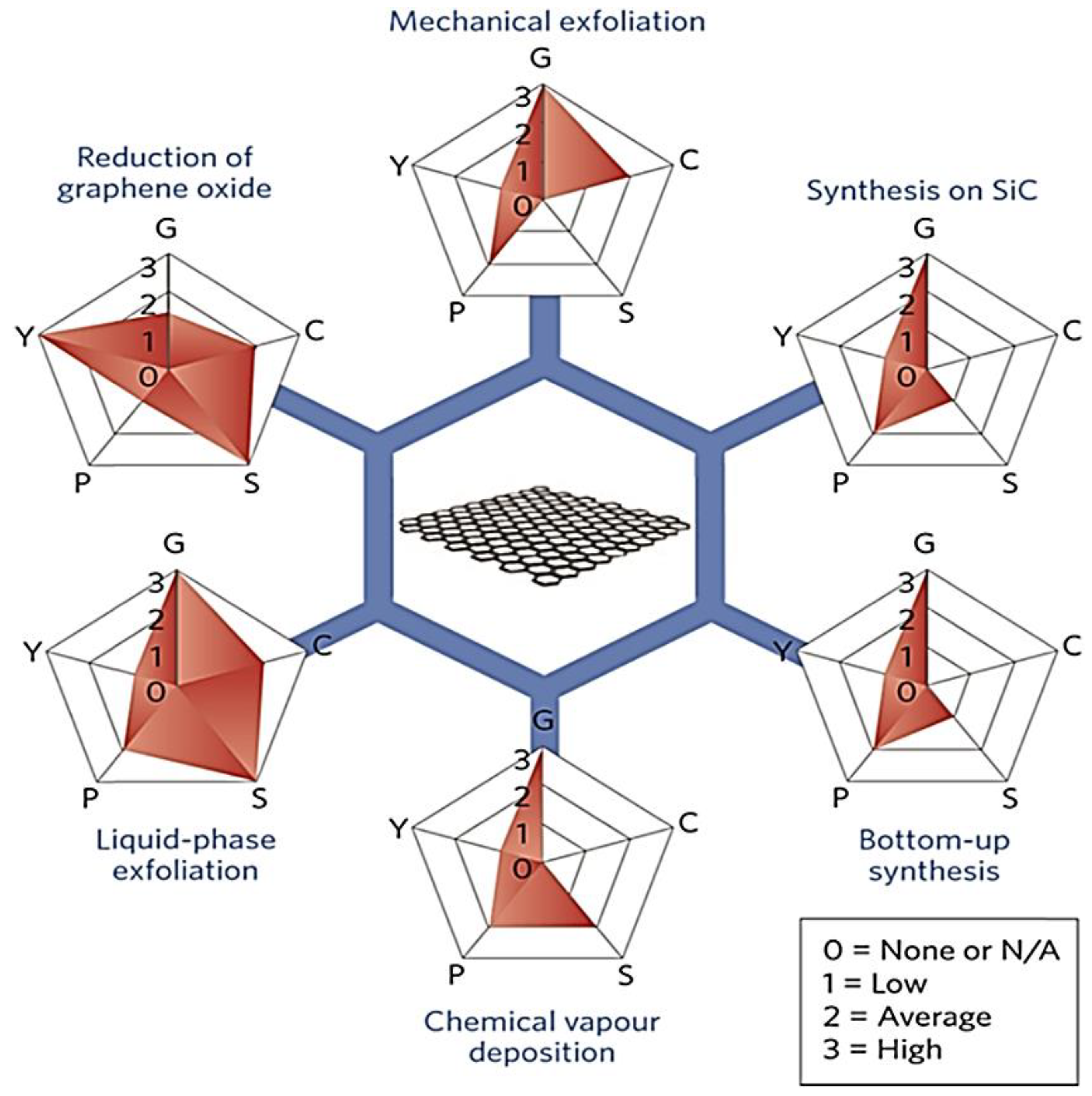
3. Preparation of Graphene Nanoparticles
3.1. Top-Down Process
3.1.1. Mechanical Exfoliation
3.1.2. Chemical Exfoliation
3.1.3. Reduction of Graphene Oxide
3.2. Bottom-Up Process
3.2.1. Pyrolysis Process
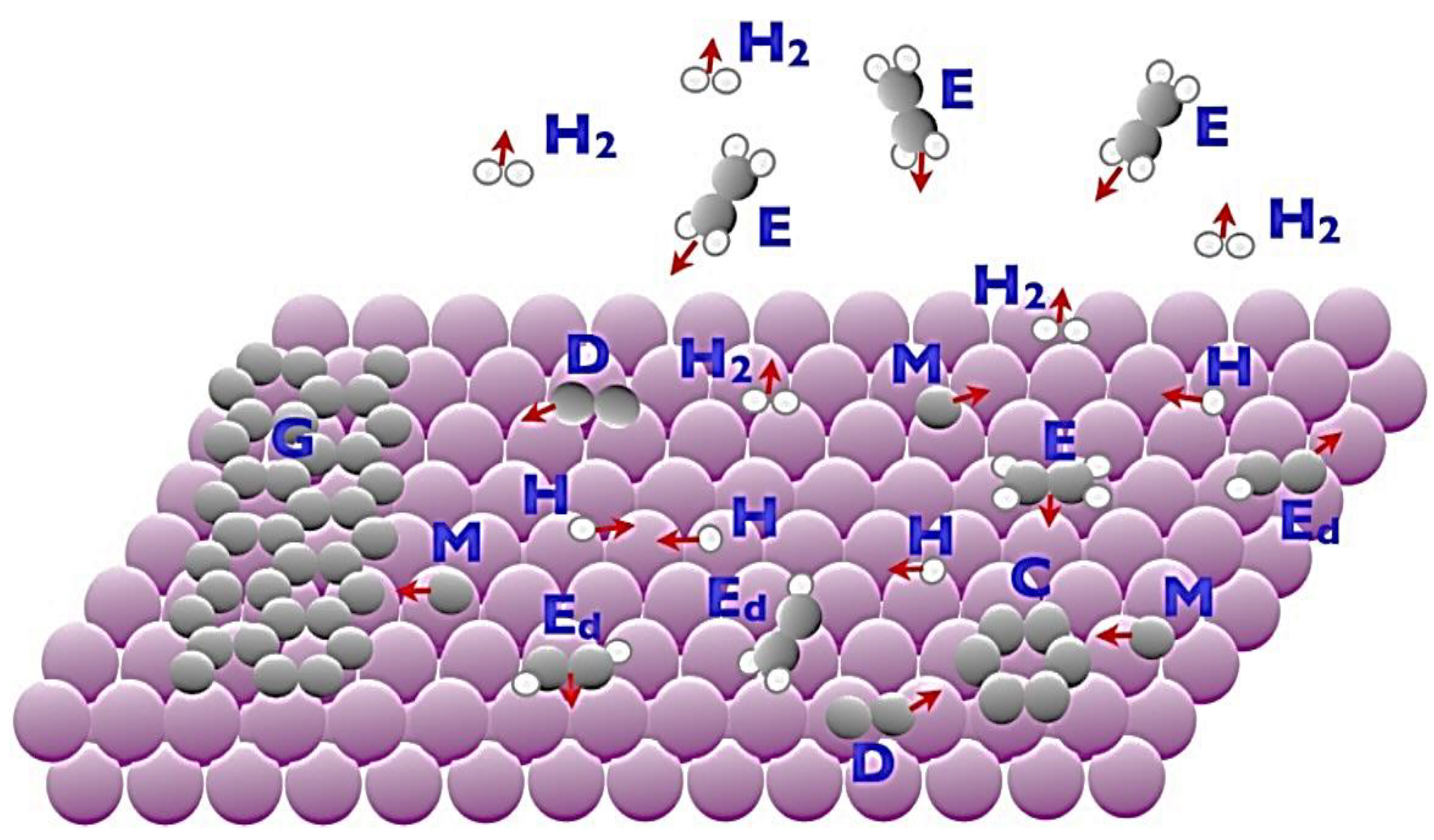
3.2.2. Chemical Vapor Deposition (CVD)
3.2.3. Epitaxial Growth
4. Properties of Graphene Nanoparticles
4.1. Electronics Properties
4.2. Mechanical Properties
4.3. Optical Properties
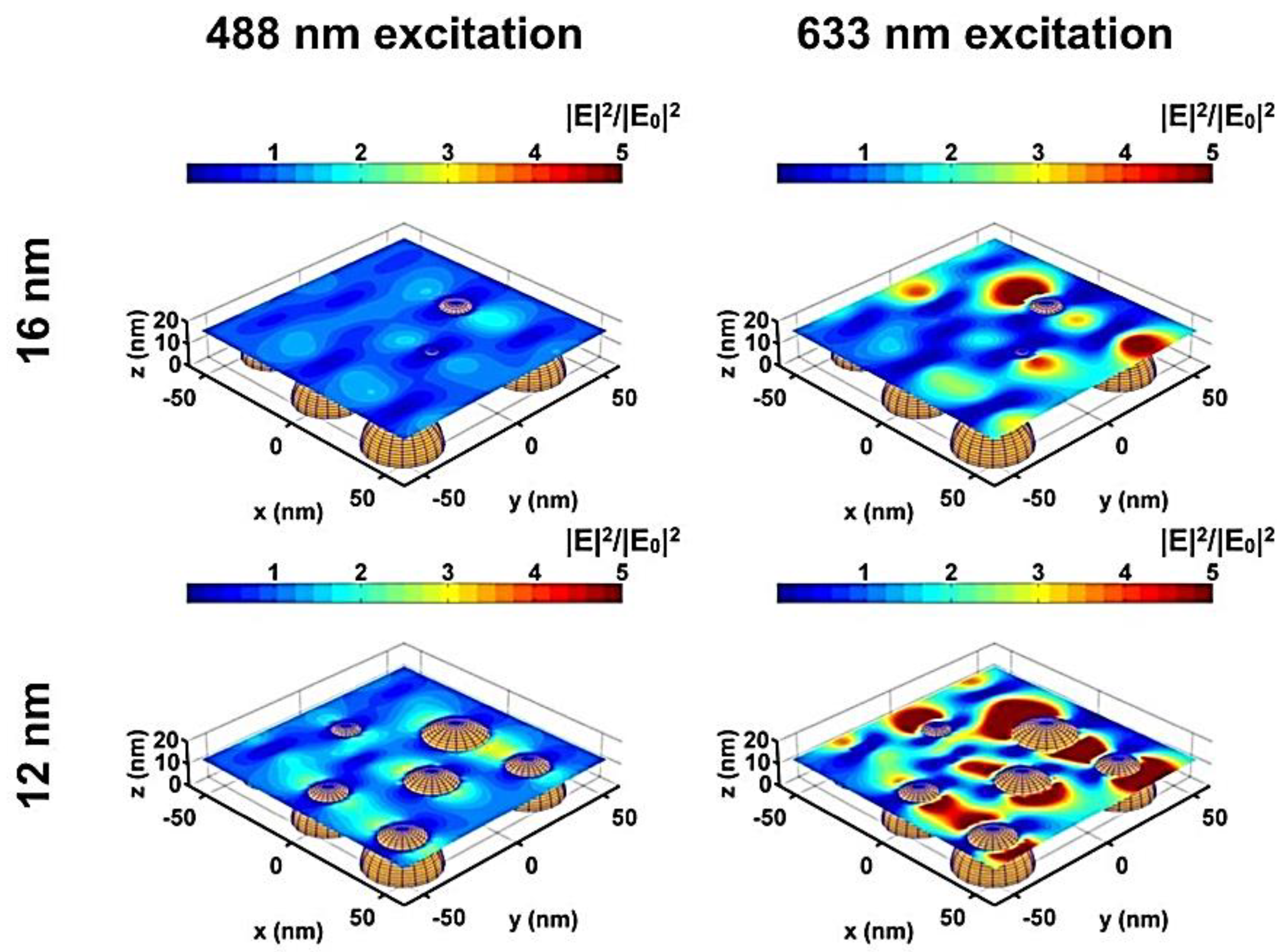
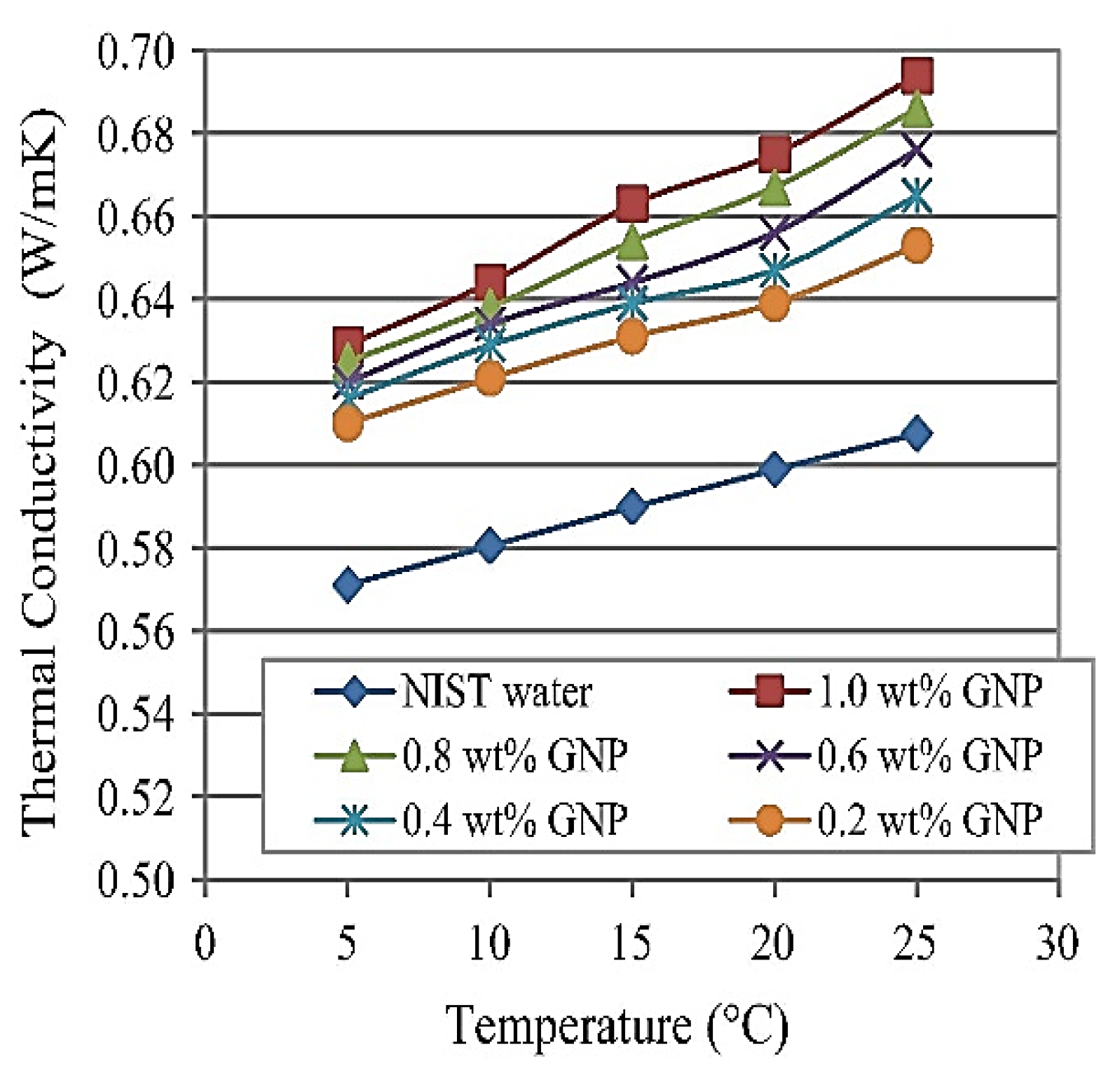
4.4. Thermal Properties
4.5. Raman Spectroscopy of Graphene
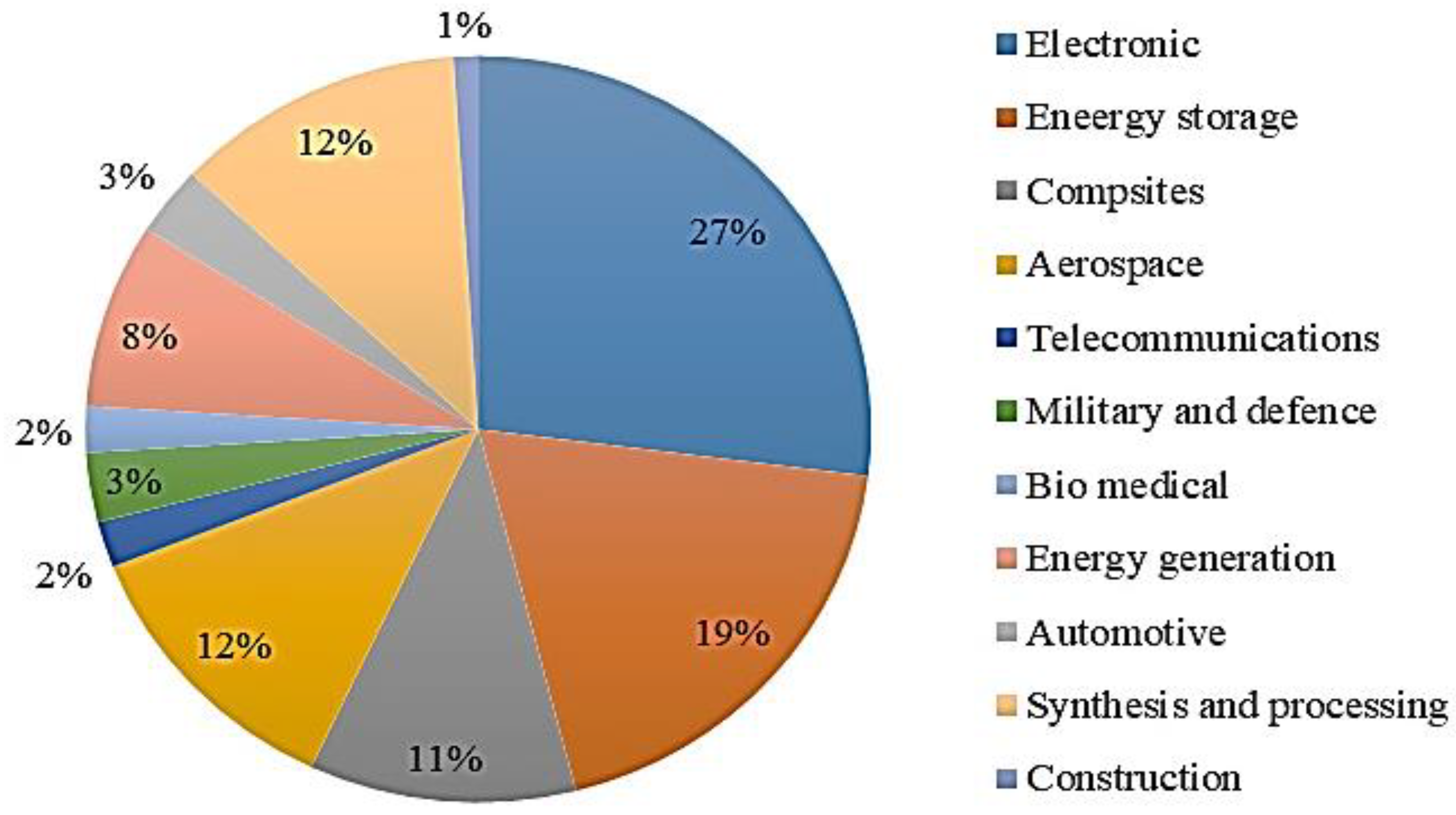
5. Applications of Graphene Nanoparticles
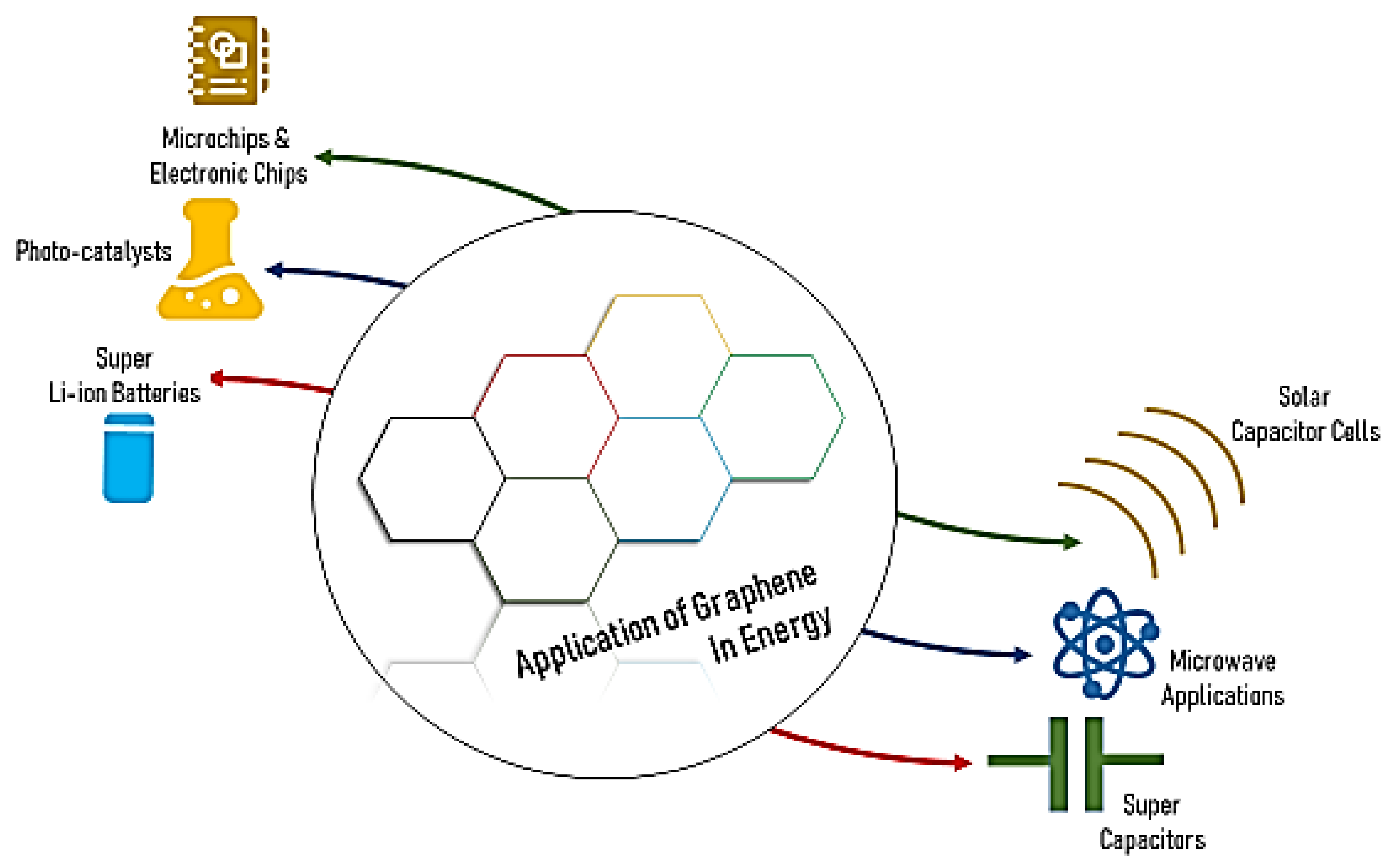
5.1. Applications of Graphene in Energy
5.2. Graphene’s Applications in Medicine
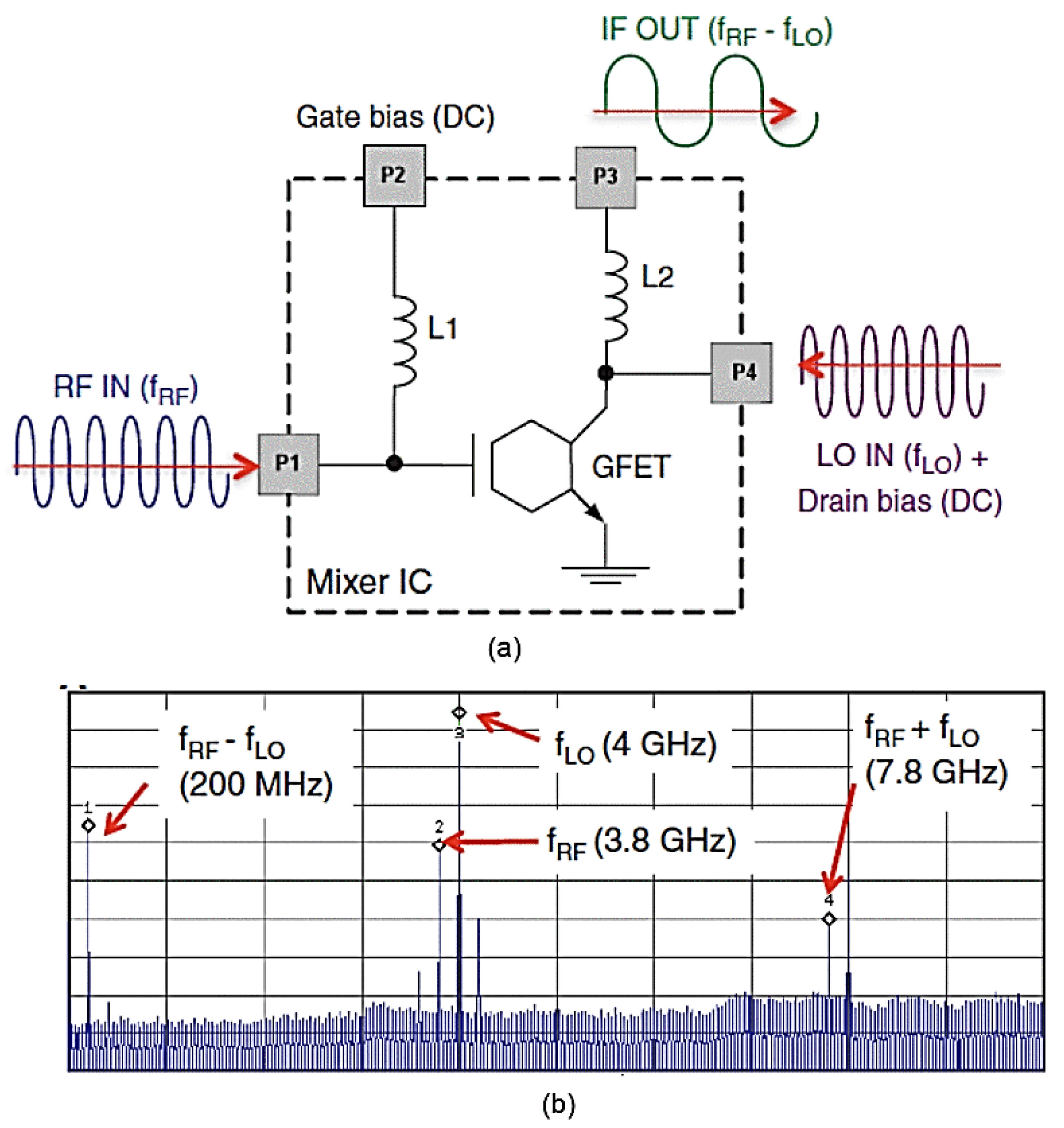
5.3. Graphene’s Applications in Electronics
5.4. Other Applications of Graphene
6. Future Trends
- Graphene can conduct electrons nearly at light speed, making it a superconductive material. Researchers in the US have confirmed graphene to be an excellent conductor of heat as carbon nanotubes act as a good thermal conductor. Researchers are putting efforts into exploiting the thermal property of graphene to improve the efficiency of heat exchangers. Heat exchangers constructed from graphene coating show a better result in satellite cooling technology and, therefore, are highly appreciated by technologists. Hence, graphene nanocoated heat exchangers are certain to be commercialized in the near future;
- Graphene possesses ultra-lightness and great mechanical strength. Researchers are optimistic about building solar sails weighing only a few pounds made from graphene which would help us to explore and learn more about solar systems;
- The ultra-thin, lightweight feature has already accentuated its possibility and utilization in aeronautics and airspace. Airplane wings made from graphene particles resulted in a lower-weight structure which eventually improved thrust and take-off as well as fuel consumption. Researchers at University Central Lancashire successfully installed graphene-based wings on a drone named “Juno”, which is 17% lighter than carbon fiber. Now, there is a fine possibility to build a system integrated into the wings that could produce paints resulting in a reduction in radar footprints, as well as the establishment of invisible aircraft. EU’s white elephant, a zero-G aircraft whose heat exchangers are coated with graphene sheets, completed its parabolic flight in weightless conditions, enabling prospects in space;
- Graphene oxide nanofilms impregnated on composite surfaces exhibit unprecedented aspects based on empirical tests and applications. GO nanofilms can be used as a heat shield capable of equalizing local heat formation, which enables it to be a stable flame retardant. Graphene’s coherent polymer matrix, combined with ceramics and epoxy resin, extinguishes the flame by delaying ignition and releasing exorbitant moisture;
- Though nanomedicine is still in its embryonic stage, graphene is set to be incorporated with a variety of nanoparticles to assist in disease diagnosis and effective modern treatment. A significant amount of EU-backed funding has been disbursed into this material, and perpetual research projects concerning graphene make for a promising future. Antimicrobial applications in tissue engineering, neurological disorders, and genetic diseases are the newest opportunities in the field of medical science to alleviate the suffering of patients. It is believed that graphene nanocoated medical devices and apparels are safer to use and can effectively decelerate the spreading of lethal, antibiotic-resistant bugs. Researchers in Korea are optimistic about the attributes of graphene sheets capable of being used as a potential catalyst to support the growth of neural stem cells;
- The sports and apparel industry is racing ahead towards superior performance and safer and much more comfortable clothing and accessories. Graphene-coated products are available in tennis, skiing, cycling; a lot of initiatives are being taken by sport product manufacturing heads [180]. The world is witnessing the evolution of sports equipment. Graphene’s unique characteristics, such as its higher strength-to-weight ratio and prolonged strength and durability, flexibility, and superior heat exchanging, improve the technical performance of sports accessories and attributes of sports products. Graphene, the game-changer, is ready to redeem the sports community on a large scale with its prominent commercial usage.
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, P.V.R.; Azeredo, N.F.B.; Garcia, L.M.S.; Zambiazi, P.J.; Morselli, G.R.; Ando, R.A.; Otubo, L.; Lazar, D.R.R.; de Souza, R.F.B.; Rodrigues, D.F.; et al. Layered graphene/hexagonal boron nitride nanosheets (Gr/h-BNNs) applied to the CO2 photoconversion into methanol. Appl. Mater. Today 2022, 29, 101605. [Google Scholar] [CrossRef]
- Alshamkhani, M.T.; Teong, L.K.; Putri, L.K.; Mohamed, A.R.; Lahijani, P.; Mohammadi, M. Effect of graphite exfoliation routes on the properties of exfoliated graphene and its photocatalytic applications. J. Environ. Chem. Eng. 2021, 9, 106506. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nat. Mater. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.; Dubonos, S.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J.J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Jenkins, K.A.; Valdes-Garcia, A.; Small, J.P.; Farmer, D.B.; Avouris, P.J. Operation of graphene transistors at gigahertz frequencies. Nano Lett. 2009, 9, 422–426. [Google Scholar] [CrossRef]
- Bunch, J.S.; Van Der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L.J. Electromechanical resonators from graphene sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef]
- Raju, A.P.A.; Lewis, A.; Derby, B.; Young, R.J.; Kinloch, I.A.; Zan, R.; Novoselov, K.S.J. Wide-area strain sensors based upon graphene-polymer composite coatings probed by Raman spectroscopy. Adv. Funct. Mater. 2014, 24, 2865–2874. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R.J.N.l. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef]
- Patchkovskii, S.; John, S.T.; Yurchenko, S.N.; Zhechkov, L.; Heine, T.; Seifert, G.J. Graphene nanostructures as tunable storage media for molecular hydrogen. Proc. Natl. Acad. Sci. USA 2005, 102, 10439–10444. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Subramonian, S.; Ramasamy, D.; Samykano, M.; Mahamude, A.S.F. Applications of Graphene Nanomaterials in Energy Storage—A State-of-Art Short Review. In Proceedings of the International Conference on Mechanical Engineering Research 2021, 26–27 October 2021; Springer: Singapore, 2023; pp. 595–609. [Google Scholar]
- Wang, X.; Zhi, L.; Müllen, K.J. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Miao, X.; Tongay, S.; Petterson, M.K.; Berke, K.; Rinzler, A.G.; Appleton, B.R.; Hebard, A.F.J. High efficiency graphene solar cells by chemical doping. Nano Lett. 2012, 12, 2745–2750. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Rahman, M.; Ramasamy, D.; Noor, M.; Najafi, G.; Samykano, M.; Mahamude, A.J. Improvement in the performance of solar collectors with nanofluids—A state-of-the-art review. Nano-Struct. Nano-Objects 2019, 18, 100276. [Google Scholar] [CrossRef]
- Jia, X.; Campos-Delgado, J.; Terrones, M.; Meunier, V.; Dresselhaus, M.S.J. Graphene edges: A review of their fabrication and characterization. Nanoscale 2011, 3, 86–95. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C.J. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-doped graphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Sun, Y.; Tan, Y.-Z.; Yang, S.; Feng, X.; Müllen, K. Three-dimensional graphene-based macro-and mesoporous frameworks for high-performance electrochemical capacitive energy storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef]
- Mahamude, A.S.F.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Salih, K.; Yusaf, T. Experimental Study on the Efficiency Improvement of Flat Plate Solar Collectors Using Hybrid Nanofluids Graphene/Waste Cotton. Energies 2022, 15, 2309. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Das, S.; Kim, M.; Lee, J.-W.; Choi, W.J. Synthesis, properties, and applications of 2-D materials: A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2014, 39, 231–252. [Google Scholar] [CrossRef]
- Mahamude, A.S.F.; Harun, W.S.W.; Kadirgama, K.; Farhana, K.; Ramasamy, D.; Samylingam, L.; Aslfattahi, N. Thermal performance of nanomaterial in solar collector: State-of-play for graphene. J. Energy Storage 2021, 42, 103022. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Noor, M.; Rahman, M.; Ramasamy, D.; Mahamude, A. CFD modelling of different properties of nanofluids in header and riser tube of flat plate solar collector. IOP Conf. Series: Mater. Sci. Eng. 2019, 469, 012041. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S.J. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent developments of metallic nanoparticle-graphene nanocatalysts. Prog. Mater. Sci. 2018, 94, 306–383. [Google Scholar] [CrossRef]
- Mahamude, A.S.F.; Harun, W.S.W.; Kadirgama, K.; Farhana, K.; Ramasamy, D. Numerical Studies of Graphene Hybrid Nanofluids in Flat Plate Solar Collector. In Proceedings of the 2021 International Congress of Advanced Technology and Engineering (ICOTEN), Taiz, Yemen, 4–5 July 2021; pp. 1–6. [Google Scholar]
- Kadirgama, G.; Bin Razman, M.I.; Ramasamy, D.; Kadirgama, K.; Farhana, K. Graphene as an Alternative Additive in Automotive Cooling System. In Proceedings of the International Conference on Mechanical Engineering Research, ICMER 2021, 26–27 October 2021; Springer: Singapore, 2023; pp. 13–35. [Google Scholar]
- Zhang, X.; Rajaraman, B.R.; Liu, H.; Ramakrishna, S.J. Graphene’s potential in materials science and engineering. Rsc Adv. 2014, 4, 28987–29011. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Gu, P.; Yan, Z.; Tang, C.; Lv, B.; Wang, X.; Yi, Z.; Zhu, M. Broadband, wide-incident-angle, and polarization-insensitive high-efficiency absorption of monolayer graphene with nearly 100% modulation depth at communication wavelength. Results Phys. 2022, 40, 105833. [Google Scholar] [CrossRef]
- Yi, L.; Li, C. Enhanced absorption and electrical modulation of graphene based on the parity-time symmetry optical structure. Chin. Opt. Lett. 2022, 20, 022201. [Google Scholar] [CrossRef]
- Teng, Y.; Tong, S.; Zhang, M. Secondary-Transferring Graphene Electrode for Stable FOLED. Nanoscale Res. Lett. 2018, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tang, C.; Chen, J.; Xie, N.; Zheng, L.; Wang, S. Tri-band absorption enhancement in monolayer graphene in visible spectrum due to multiple plasmon resonances in metal–insulator–metal nanostructure. Appl. Phys. Express 2018, 11, 072201. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.; Chen, J.; Wang, Q.; Pei, M.; Tang, H. Dual-band light absorption enhancement of monolayer graphene from surface plasmon polaritons and magnetic dipole resonances in metamaterials. Opt. Express 2017, 25, 12061–12068. [Google Scholar] [CrossRef] [PubMed]
- Hiura, H.; Ebbesen, T.; Fujita, J.; Tanigaki, K.; Takada, T.J. Role of sp 3 defect structures in graphite and carbon nanotubes. Nature 1994, 367, 148–151. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C.J. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E.J. Experimental review of graphene. Int. Sch. Res. Not. 2012, 2012, 258. [Google Scholar] [CrossRef]
- McCann, E. Electronic properties of monolayer and bilayer graphene. In Graphene Nanoelectronics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–275. [Google Scholar]
- Wallace, P.R.J. The band theory of graphite. Phys. Rev. 1947, 71, 622. [Google Scholar] [CrossRef]
- Eizenberg, M.; Blakely, J.J. Carbon monolayer phase condensation on Ni (111). Surf. Sci. 1979, 82, 228–236. [Google Scholar] [CrossRef]
- Eizenberg, M.; Blakely, J.J. Carbon interaction with nickel surfaces: Monolayer formation and structural stability. J. Chem. Phys. 1979, 71, 3467–3477. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Dhawan, S.J. Polymer-graphene nanocomposites: Preparation, characterization, properties, and applications. Nanocompos.-New Trends Dev. 2012, 37–72. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C.J. Production and processing of graphene and 2d crystals. Mater. Today 2012, 15, 564–589. [Google Scholar] [CrossRef]
- Kitko, K.E.; Zhang, Q.J. Graphene-based nanomaterials: From production to integration with modern tools in neuroscience. Front. Syst. Neurosci. 2019, 13, 26. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B.J. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.J.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S.J. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.; Abdullah, E.; Nizamuddin, S.; Khalid, M.J. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A.J. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Zhang, Y.; Small, J.P.; Pontius, W.V.; Kim, P.J. Fabrication and electric-field-dependent transport measurements of mesoscopic graphite devices. Appl. Phys. Lett. 2005, 86, 073104. [Google Scholar] [CrossRef]
- Rao, C.; Maitra, U.; Matte, H.S.S. Synthesis, characterization, and selected properties of graphene. Acc. Chem. Res. 2013, 46, 149–159. [Google Scholar] [CrossRef]
- Hader, G.G.J. Synthesis, Modelling Characterization of 2D Materials Their Heterostructures; Elsevier: Amsterdam, The Netherlands, 2020; Volume 181. [Google Scholar]
- Casiraghi, C.; Hartschuh, A.; Lidorikis, E.; Qian, H.; Harutyunyan, H.; Gokus, T.; Novoselov, K.S.; Ferrari, A.J. Rayleigh imaging of graphene and graphene layers. Nano Lett. 2007, 7, 2711–2717. [Google Scholar] [CrossRef]
- Teng, C.; Xie, D.; Wang, J.; Yang, Z.; Ren, G.; Zhu, Y.J. Ultrahigh conductive graphene paper based on ball-milling exfoliated graphene. Adv. Funct. Mater. 2017, 27, 1700240. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S.J. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.J. Thermal properties of graphene-based polymer composite materials: A molecular dynamics study. Results Phys. 2020, 16, 102974. [Google Scholar] [CrossRef]
- Xiao, J.; Zhan, H.; Wang, X.; Xu, Z.-Q.; Xiong, Z.; Zhang, K.; Simon, G.P.; Liu, J.Z.; Li, D.J. Electrolyte gating in graphene-based supercapacitors and its use for probing nanoconfined charging dynamics. Nat. Nanotechnol. 2020, 15, 683–689. [Google Scholar] [CrossRef]
- Samantaray, M.R.; Mondal, A.K.; Murugadoss, G.; Pitchaimuthu, S.; Das, S.; Bahru, R.; Mohamed, M.A.J. Synergetic effects of hybrid carbon nanostructured counter electrodes for dye-sensitized solar cells: A review. Materials 2020, 13, 2779. [Google Scholar] [CrossRef] [PubMed]
- Dzyazko, Y.S.; Volfkovich, Y.M.; Chaban, M.O. Composites Containing Inorganic Ion Exchangers and Graphene Oxide: Hydrophilic–Hydrophobic and Sorption Properties. In Nanomaterials and Nanocomposites, Nanostructure Surfaces, and Their Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 93–110. [Google Scholar]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M.J. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Y.; Zhu, Y.J. Towards industrialization of graphene oxide. Sci. China Mater. 2020, 63, 1861–1869. [Google Scholar] [CrossRef]
- Sharotri, N.; Rana, A.K.; Thakur, N.; Dogra, S.; Dhiman, N. Fundamental of Graphene Nanocomposites. In Handbook of Polymer and Ceramic Nanotechnology; Springer: Cham, Switzerland, 2021; pp. 1161–1184. [Google Scholar]
- Guohua, C.J. Exfoliation of graphite flake and its nanocomposites. Carbon 2003, 41, 579. [Google Scholar]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S.J. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.J. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Li, L.-S.; Yan, X.J. Colloidal graphene quantum dots. J. Phys. Chem. Lett. 2010, 1, 2572–2576. [Google Scholar] [CrossRef]
- Zhi, L.; Müllen, K.J. A bottom-up approach from molecular nanographenes to unconventional carbon materials. J. Mater. Chem. 2008, 18, 1472–1484. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N.J. Graphene dispersion and exfoliation in low boiling point solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R.J. High-yield organic dispersions of unfunctionalized graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N.J. Measurement of multicomponent solubility parameters for graphene facilitates solvent discovery. ACS Publ. 2010, 26, 3208–3213. [Google Scholar] [CrossRef]
- Qian, M.; Zhou, Y.S.; Gao, Y.; Park, J.B.; Feng, T.; Huang, S.M.; Sun, Z.; Jiang, L.; Lu, Y.F.J. Formation of graphene sheets through laser exfoliation of highly ordered pyrolytic graphite. Appl. Phys. Lett. 2011, 98, 173108. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Feng, X.; Müllen, K.J. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef]
- William, S.; Hummers, J.; Offeman, R.E.J.J. Preparation of graphitic oxide. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B.J. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.M.; Park, H.K.; Jung, I.S.; Jin, M.H.; Jeong, H.K.; Kim, J.M.; Choi, J.Y.J. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Lellala, K.; Namratha, K.; Byrappa, K.J. Ultrasonication assisted mild solvothermal synthesis and morphology study of few-layered graphene by colloidal suspensions of pristine graphene oxide. Microporous Mesoporous Mater. 2016, 226, 522–529. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M.J. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P.J.M.S.; Reports, E.R. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J.A.J. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Kurasch, S.; Kaiser, U.; Antonietti, M. Synthesis of monolayer-patched graphene from glucose. Wiley Online Libr. 2012, 51, 9689–9692. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xia, M.; Qiu, J.; Xie, Z.J. Biomass Derived Graphene-Like Carbons for Electrocatalytic Oxygen Reduction Reaction. ChemNanoMat 2019, 5, 682–689. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W.J. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, Y.; Zhao, Q.; Pan, F.; Zhang, B.; Zhang, J.J.L. Direct synthesis of nitrogen-doped carbon nanosheets with high surface area and excellent oxygen reduction performance. ACS Publ. 2014, 30, 8238–8245. [Google Scholar] [CrossRef]
- Wen, G.; Gu, Q.; Liu, Y.; Schlögl, R.; Wang, C.; Tian, Z.; Su, D.S.J. Biomass-Derived Graphene-like Carbon: Efficient Metal-Free Carbocatalysts for Epoxidation. Angew. Chem. Int. Ed. 2018, 57, 16898–16902. [Google Scholar] [CrossRef]
- Bhaviripudi, S.; Jia, X.; Dresselhaus, M.S.; Kong, J.J. Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett. 2010, 10, 4128–4133. [Google Scholar] [CrossRef]
- Ren, S.; Rong, P.; Yu, Q.J. Preparations, properties and applications of graphene in functional devices: A concise review. Ceram. Int. 2018, 44, 11940–11955. [Google Scholar]
- Journet, C.; Maser, W.; Bernier, P.; Loiseau, A.; de La Chapelle, M.L.; Lefrant, D.S.; Deniard, P.; Lee, R.; Fischer, J.J. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Shukrullah, S.; Mohamed, N.; Shaharun, M.; Naz, M.J. Mass production of carbon nanotubes using fluidized bed reactor: A short review. Trends Appl. Sci. Res. 2014, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Ruan, M.; Guo, Z.; Dong, R.; Palmer, J.; Hankinson, J.; Berger, C.; De Heer, W.A.J. Structured epitaxial graphene: Growth and properties. J. Phys. D Appl. Phys. 2012, 45, 154010. [Google Scholar]
- Charrier, A.; Coati, A.; Argunova, T.; Thibaudau, F.; Garreau, Y.; Pinchaux, R.; Forbeaux, I.; Debever, J.-M.; Sauvage-Simkin, M.; Themlin, J.-M.J. Solid-state decomposition of silicon carbide for growing ultra-thin heteroepitaxial graphite films. J. Appl. Phys. 2002, 92, 2479–2484. [Google Scholar] [CrossRef]
- Howell, S.W.; Biedermann, L.B.; Ohta, T.; Ross III, A.J.; Beechem, I.; Edwin, T.; Pan, W.; Trotter, D.C. Characterization of Devices Fabricated from Electrostatically Transferred Graphene: Comparison with Epitaxial Based Devices; Sandia National Lab. (SNL-NM): Albuquerque, NM, USA, 2010.
- Yu, X.; Hwang, C.; Jozwiak, C.M.; Köhl, A.; Schmid, A.K.; Lanzara, A.; Phenomena, R. New synthesis method for the growth of epitaxial graphene. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 100–106. [Google Scholar] [CrossRef]
- Tetlow, H.; De Boer, J.P.; Ford, I.; Vvedensky, D.; Coraux, J.; Kantorovich, L.J. Growth of epitaxial graphene: Theory and experiment. Phys. Rep. 2014, 542, 195–295. [Google Scholar] [CrossRef]
- Wang, L.; Meric, I.; Huang, P.; Gao, Q.; Gao, Y.; Tran, H.; Taniguchi, T.; Watanabe, K.; Campos, L.; Muller, D.J. One-dimensional electrical contact to a two-dimensional material. Science 2013, 342, 614–617. [Google Scholar]
- Yan, J.-A.; Ruan, W.; Chou, M.J. Electron-phonon interactions for optical-phonon modes in few-layer graphene: First-principles calculations. Phys. Rev. B 2009, 79, 115443. [Google Scholar]
- Ando, T.J. The electronic properties of graphene and carbon nanotubes. NPG Asia Mater. 2009, 1, 17–21. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K.J. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y.J. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Wijeratne, S.; Zhang, Y.; Coskun, U.; Bao, W.; Lau, C.J. Phase-coherent transport in graphene quantum billiards. Science 2007, 317, 1530–1533. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P.J. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Blees, M.K.; Barnard, A.W.; Rose, P.A.; Roberts, S.P.; McGill, K.L.; Huang, P.Y.; Ruyack, A.R.; Kevek, J.W.; Kobrin, B.; Muller, D.A.J. Graphene kirigami. Nature 2015, 524, 204–207. [Google Scholar] [CrossRef]
- Peres, N.; Guinea, F.; Neto, A.C.J. Electronic properties of disordered two-dimensional carbon. Phys. Rev. B 2006, 73, 125411. [Google Scholar] [CrossRef]
- Gusynin, V.; Sharapov, S.; Carbotte, J.J. Unusual microwave response of Dirac quasiparticles in graphene. Phys. Rev. Lett. 2006, 96, 256802. [Google Scholar] [CrossRef]
- Osváth, Z.; Deák, A.; Kertész, K.; Molnár, G.; Vértesy, G.; Zámbó, D.; Hwang, C.; Biró, L.P.J. The structure and properties of graphene on gold nanoparticles. Nanoscale 2015, 7, 5503–5509. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S.J. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C.J. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Tang, B.; Yuan, B.; Sun, L.; Wang, X.-G.J. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials 2013, 34, 9519–9534. [Google Scholar] [CrossRef] [PubMed]
- Shahil, K.M.; Balandin, A.A.J. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Hone, J.; Stormer, H.; Kim, P.J. Temperature-dependent transport in suspended graphene. Phys. Rev. Lett. 2008, 101, 096802. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, S.; Kargar, F.; Wright, D.; Huang, C.Y.T.; Mohammadzadeh, A.; Barani, Z.; Salgado, R.; Balandin, A.A. Noncuring graphene thermal interface materials for advanced electronics. Adv. Electron. Mater. 2020, 6, 1901303. [Google Scholar] [CrossRef]
- Ranjbartoreh, A.R.; Wang, B.; Shen, X.; Wang, G.J. Advanced mechanical properties of graphene paper. J. Appl. Phys. 2011, 109, 014306. [Google Scholar] [CrossRef]
- Rhee, K.Y. Electronic and Thermal Properties of Graphene. Nanomaterials 2020, 10, 926. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J.J. Electronic and thermal properties of graphene and recent advances in graphene based electronics applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef]
- Wang, Y.; Al-Saaidi, H.A.I.; Kong, M.; Alvarado, J.L.J. Thermophysical performance of graphene based aqueous nanofluids. Int. J. Heat Mass Transf. 2018, 119, 408–417. [Google Scholar] [CrossRef]
- Nagyte, V.; Kelly, D.J.; Felten, A.; Picardi, G.; Shin, Y.; Alieva, A.; Worsley, R.E.; Parvez, K.; Dehm, S.; Krupke, R.J. Raman fingerprints of graphene produced by anodic electrochemical exfoliation. Nano Lett. 2020, 20, 3411–3419. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.J. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.J. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.J. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R.S.J. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.J.E.; Science, E. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- Thiruppathi, A.R.; Sidhureddy, B.; Boateng, E.; Soldatov, D.V.; Chen, A.J. Synthesis and electrochemical study of three-dimensional graphene-based nanomaterials for energy applications. Nanomaterials 2020, 10, 1295. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Shi, G.J. Graphene based catalysts. Energy Environ. Sci. 2012, 5, 8848–8868. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M.J. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z.J. The advancing uses of nano-graphene in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 601–612. [Google Scholar] [CrossRef]
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G.J. Graphene as cancer theranostic tool: Progress and future challenges. Theranostics 2015, 5, 710. [Google Scholar] [CrossRef]
- Eskiizmir, G.; Baskın, Y.; Yapıcı, K. Graphene-based nanomaterials in cancer treatment and diagnosis. In Fullerens, Graphenes and Nanotubes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 331–374. [Google Scholar]
- Chen, Y.-W.; Su, Y.-L.; Hu, S.-H.; Chen, S.-Y.J. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lyv, Z.; Li, Y.; Liu, H.; Wang, J.; Zhan, W.; Chen, H.; Chen, H.; Li, X.J. A theranostic prodrug delivery system based on Pt (IV) conjugated nano-graphene oxide with synergistic effect to enhance the therapeutic efficacy of Pt drug. Biomaterials 2015, 51, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F.J. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf. B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, W.; Xiao, N.; Ye, L.; Xu, Q.J. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int. J. Nanomed. 2014, 9, 1433. [Google Scholar]
- Wang, Y.; Polavarapu, L.; Liz-Marzán, L.M.J. Reduced graphene oxide-supported gold nanostars for improved SERS sensing and drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 21798–21805. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, B.; Zou, M.; Cheng, G.J. Cyclic RGD-modified chitosan/graphene oxide polymers for drug delivery and cellular imaging. Colloids Surf. B Biointerfaces 2014, 122, 332–340. [Google Scholar] [CrossRef]
- Song, E.; Han, W.; Li, C.; Cheng, D.; Li, L.; Liu, L.; Zhu, G.; Song, Y.; Tan, W.J. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef]
- Ou, J.; Wang, F.; Huang, Y.; Li, D.; Jiang, Y.; Qin, Q.-H.; Stachurski, Z.; Tricoli, A.; Zhang, T.J.C.; Biointerfaces, S.B. Fabrication and cyto-compatibility of Fe3O4/SiO2/graphene–CdTe QDs/CS nanocomposites for drug delivery. Colloids Surf. B Biointerface 2014, 117, 466–472. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Tang, H.; Tan, L.; Xie, Q.; Zhang, Y.; Ma, M.; Yao, S.J. Polyamidoamine dendrimer and oleic acid-functionalized graphene as biocompatible and efficient gene delivery vectors. ACS Appl. Mater. Interfaces 2014, 6, 8173–8183. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.J.J. Photothermally controlled gene delivery by reduced graphene oxide–polyethylenimine nanocomposite. Small 2014, 10, 117–126. [Google Scholar] [CrossRef]
- You, P.; Yang, Y.; Wang, M.; Huang, X.; Huang, X.J. Graphene oxide-based nanocarriers for cancer imaging and drug delivery. Curr. Pharm. Des. 2015, 21, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Hamishehkar, H.; Dolatabadi, J.E.N.; Arsalani, N. Nano graphene oxide: A novel carrier for oral delivery of flavonoids. Biointerfaces 2014, 123, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, Y.; Li, H.; Duan, Y. A facile one-pot synthesis of starch functionalized graphene as nano-carrier for pH sensitive and starch-mediated drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, H.; Wang, Y.; Shi, B.; Guo, L.; Wu, D.; Yang, S.; Wu, H.J. Graphene oxide/manganese ferrite nanohybrids for magnetic resonance imaging, photothermal therapy and drug delivery. J. Biomater. Appl. 2016, 30, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W.J.N. Hierarchical construction of a mechanically stable peptide–graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, H.; Zhang, M.G.; Song, J.; Nie, L.; Wang, S.; Niu, G.; Huang, P.; Lu, G.; Chen, X.J.N. An aptamer-targeting photoresponsive drug delivery system using “off–on” graphene oxide wrapped mesoporous silica nanoparticles. Nanoscale 2015, 7, 6304–6310. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.J. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Kafi, M.A.; Paul, A.; Vilouras, A.; Hosseini, E.S.; Dahiya, R.S.J. Chitosan-graphene oxide-based ultra-thin and flexible sensor for diabetic wound monitoring. IEEE Sens. J. 2019, 20, 6794–6801. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Jang, D.; Idrobo, J.C.; Boutilier, M.S.; Wang, L.; Kong, J.; Karnik, R.J. Nanoporous atomically thin graphene membranes for desalting and dialysis applications. Adv. Mater. 2017, 29, 1700277. [Google Scholar] [CrossRef]
- Nishida, E.; Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M.J. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265. [Google Scholar]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lim, Y.; Lee, H.; Lee, G.; Park, H.; Hong, S.Y.; Jin, S.W.; Lee, Y.H.; Lee, S.S.; Ha, J.S.J. A Patterned Graphene/ZnO UV Sensor Driven by Integrated Asymmetric Micro-Supercapacitors on a Liquid Metal Patterned Foldable Paper. Adv. Funct. Mater. 2017, 27, 1700135. [Google Scholar] [CrossRef]
- Masvidal-Codina, E.; Illa, X.; Dasilva, M.; Calia, A.B.; Dragojević, T.; Vidal-Rosas, E.E.; Prats-Alfonso, E.; Martínez-Aguilar, J.; Jose, M.; Garcia-Cortadella, R.J. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 2019, 18, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, O.; Kurasch, S.; Krasheninnikov, A.; Kaiser, U.J. Atomic scale study of the life cycle of a dislocation in graphene from birth to annihilation. Nat. Commun. 2013, 4, 2098. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, G.; Balandin, A.A.; Mohanram, K.J. Triple-mode single-transistor graphene amplifier and its applications. ACS Nano 2010, 4, 5532–5538. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Valdes-Garcia, A.; Han, S.-J.; Farmer, D.B.; Meric, I.; Sun, Y.; Wu, Y.; Dimitrakopoulos, C.; Grill, A.; Avouris, P.J.S. Wafer-scale graphene integrated circuit. Science 2011, 332, 1294–1297. [Google Scholar] [CrossRef]
- DiFrancesco, M.L.; Colombo, E.; Papaleo, E.D.; Maya-Vetencourt, J.F.; Manfredi, G.; Lanzani, G.; Benfenati, F.J. A hybrid P3HT-graphene interface for efficient photostimulation of neurons. Carbon 2020, 162, 308–317. [Google Scholar] [CrossRef]
- Tang, H.; Hessel, C.M.; Wang, J.; Yang, N.; Yu, R.; Zhao, H.; Wang, D.J. Two-dimensional carbon leading to new photoconversion processes. Chem. Soc. Rev. 2014, 43, 4281–4299. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Kim, J.; Jang, B.; Chae, Y.; Kim, J.-H.; Ahn, J.-H.J. Graphene-based three-dimensional capacitive touch sensor for wearable electronics. ACS Nano 2017, 11, 7950–7957. [Google Scholar] [CrossRef]
- Secor, E.B.; Prabhumirashi, P.L.; Puntambekar, K.; Geier, M.L.; Hersam, M.C.J. Inkjet printing of high conductivity, flexible graphene patterns. J. Phys. Chem. Lett. 2013, 4, 1347–1351. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; Novoselov, K.S.; Yeates, S.G.J. All inkjet-printed graphene-silver composite ink on textiles for highly conductive wearable electronics applications. Sci. Rep. 2019, 9, 8035. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Han, Y.; Shuang, S.; Dong, C.J. Rational synthesis of graphene–metal coordination polymer composite nanosheet as enhanced materials for electrochemical biosensing. J. Mater. Chem. 2012, 22, 13166–13173. [Google Scholar] [CrossRef]
- Kempegowda, R.; Antony, D.; Malingappa, P.J. Graphene–platinum nanocomposite as a sensitive and selective voltammetric sensor for trace level arsenic quantification. Int. J. Smart Nano Mater. 2014, 5, 17–32. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, H.; Wu, Z.; Li, X.; He, Y.; Yuan, Z.J. The comparison of different gold nanoparticles/graphene nanosheets hybrid nanocomposites in electrochemical performance and the construction of a sensitive uric acid electrochemical sensor with novel hybrid nanocomposites. Biosens. Bioelectron. 2011, 29, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Sinha, A.; Saha, A.; Jana, N.R.J. Tunable Catalytic Performance and Selectivity of a Nanoparticle–Graphene Composite through Finely Controlled Nanoparticle Loading. Chem. Asian J. 2012, 7, 2931–2936. [Google Scholar] [CrossRef]
- Yin, H.; Tang, H.; Wang, D.; Gao, Y.; Tang, Z.J. Facile synthesis of surfactant-free Au cluster/graphene hybrids for high-performance oxygen reduction reaction. ACS Nano 2012, 6, 8288–8297. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Three-dimensional Pt-on-Pd bimetallic Nanodendrites Supported on Graphene Nanosheet: Facile Synthesis and Used as an Advanced Nanoelectrocatalyst for Methanol Oxidation. ACS Nano 2009, 4, 547–555. [Google Scholar] [CrossRef]
- Mondal, A.; Jana, N.R.J. Surfactant-free, stable noble metal–graphene nanocomposite as high performance electrocatalyst. ACS Catal. 2014, 4, 593–599. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G.J. Facile synthesis of silicon nanoparticles inserted into graphene sheets as improved anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 2198–2200. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.-M.J. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-S.; Jiang, L.-Y.; Yan, H.-J.; Wang, W.D.; Wang, W.; Song, W.-G.; Guo, Y.-G.; Wan, L.-J. Mono dispersed SnO2 nanoparticles on both sides of single layer graphene sheets as anode materials in Li-ion batteries. J. Mater. Chem. 2010, 20, 5462–5467. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R.; Hu, D.; Wang, C.; Saraf, L.V.; Zhang, J.J. Self-assembled TiO2–graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef]
- Romanchuk, A.Y.; Slesarev, A.S.; Kalmykov, S.N.; Kosynkin, D.V.; Tour, J.M.J. Graphene oxide for effective radionuclide removal. Phys. Chem. Chem. Phys. 2013, 15, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H.J. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.P.A.; Junior, C.A.C. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Qiao, M.; Ai, L.; Sun, K.; Mi, Q.; Zang, S.; Zuo, Y.; Yuan, X.; Wang, Q.J.S.; et al. Human motion monitoring in sports using wearable graphene-coated fiber sensors. Physical 2018, 274, 132–140. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Peng, Y.; Xu, Z.; Liu, W.; Pang, K.; Wang, J.; Zhong, L.; Yang, Q.; Meng, J.J. A graphene-coated silk-spandex fabric strain sensor for human movement monitoring and recognition. Nanotechnology 2021, 32, 215501. [Google Scholar] [CrossRef]
- Ali, I.; Zakharchenko, E.; Myasoedova, G.; Molochnikova, N.; Rodionova, A.; Baulin, V.; Burakov, A.; Burakova, I.; Babkin, A.; Neskoromnaya, E.J. Preparation and characterization of oxidized graphene for actinides and rare earth elements removal in nitric acid solutions from nuclear wastes. J. Mol. Liq. 2021, 335, 116260. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]










| No | References | Nanocomposites | Advantages |
|---|---|---|---|
| 1 | Li et al. [132] | Pt(IV)-conjugated nano-GO | To upgrade the restorative proficiency of Pt drug |
| 2 | Wang et al. [133] | GQDs | Synchronous focused on cellular imaging and drug conveyance |
| 3 | Wang et al. [134] | Chlorotoxin-conjugated GO | Focused on the conveyance of an anticancer sedate doxorubicin |
| 4 | Wang et al. [135] | GO–gold nanostars | Progressed surface upgraded Raman diffusion detecting and drug conveyance |
| 5 | Wang et al. [136] | RGD-modified chitosan/GO polymers | Drug conveyance and biological imaging |
| 6 | Song et al. [137] | Hyaluronic-acid-decorated GO nanohybrids | Focused on pH-responsive anticancer drug conveyance |
| 7 | Ou et al. [138] | Fe3O4/SiO2/graphene–CdTe QDs/chitosan nanocomposites | To-the-point drug conveyance |
| 8 | Liu et al. [139] | Polyamide dendrimer and oleic-acid-functionalized graphene | Biocompatible and productive quality conveyance vectors |
| 9 | Kim and Kim [140] | rGO–polyethylenimide nanocomposite | Photothermally active DNA delivery |
| 10 | You et al. [141] | Nano-GO | Cancer detection and medicine delivery |
| 11 | Rahmanian et al. [142] | Nano-GO | Oral conveyance of flavonoids |
| 12 | Liu et al. [143] | Starch-functionalized graphene | pH detective and starch-oriented sedate conveyance |
| 13 | Yang et al. [144] | Go/manganese ferrite nanohybrids | MRI, photothermal therapy, and drug conveyance |
| 14 | Wu et al. [145] | Peptide–GO hybrid hydrogel | Vibrationally triggered drug conveyance |
| 15 | Tang et al. [146] | GO-wrapped mesoporous silica nanoparticles |
| No | References | Nanocomposites | Advantages |
|---|---|---|---|
| 1 | Guo et al. [162] | G–Pt–PVP | Highly sensitive property |
| 2 | Kempegowda et al. [163] | G–Pt | Highly sensitive in abruptly finding out analyses in compound solution |
| 3 | Xue et al. [164] | G–Au–chitosan | Highly sensitive in abruptly finding out analyses in compound solution |
| 4 | Mondal et al. [165] | G–Au | Highly sensitive in abruptly finding out analyses in compound solution |
| 5 | Yin et al. [166] | RGO–Au | Aggressive onset potential and high catalyst stability in fuel cell |
| 6 | Guo et al. [167] | G–PtPd | Low CO poisoning in fuel cell |
| 7 | Mondal and Jana [168] | G–noble metal | Stable catalytic current in fuel cell |
| 8 | Liang et al. [169] | G–CO3O4 | High catalyst stability in fuel cell |
| 9 | Zhou et al. [170] | G–Si, G–porous Si | Highly effective and stable storage capacity in Li-ion batteries |
| 10 | Wu et al. [171] | G–Co3O4 | Stable and vital performance in continuous cycles in Li-ion batteries |
| 11 | Zhang et al. [172] | G–SnO2 | Highly effective performance in Li-ion batteries |
| 13 | Wang et al. [173] | G–TiO2 | High charging and discharging capability and flexibility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusaf, T.; Mahamude, A.S.F.; Farhana, K.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Kamarulzaman, M.K.; Subramonian, S.; Hall, S.; Dhahad, H.A. A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications. Sustainability 2022, 14, 12336. https://doi.org/10.3390/su141912336
Yusaf T, Mahamude ASF, Farhana K, Harun WSW, Kadirgama K, Ramasamy D, Kamarulzaman MK, Subramonian S, Hall S, Dhahad HA. A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications. Sustainability. 2022; 14(19):12336. https://doi.org/10.3390/su141912336
Chicago/Turabian StyleYusaf, Talal, Abu Shadate Faisal Mahamude, Kaniz Farhana, Wan Sharuzi Wan Harun, Kumaran Kadirgama, Devarajan Ramasamy, Mohd Kamal Kamarulzaman, Sivarao Subramonian, Steve Hall, and Hayder Abed Dhahad. 2022. "A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications" Sustainability 14, no. 19: 12336. https://doi.org/10.3390/su141912336
APA StyleYusaf, T., Mahamude, A. S. F., Farhana, K., Harun, W. S. W., Kadirgama, K., Ramasamy, D., Kamarulzaman, M. K., Subramonian, S., Hall, S., & Dhahad, H. A. (2022). A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications. Sustainability, 14(19), 12336. https://doi.org/10.3390/su141912336









