Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Activated Biochar Production
2.3. Preparation of Organomineral Nitrogen Fertilizers (OMF-N) Based on Activated Biochar
2.4. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
2.5. Specific Surface Area (SSA) and Pore Size and Distribution
2.6. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
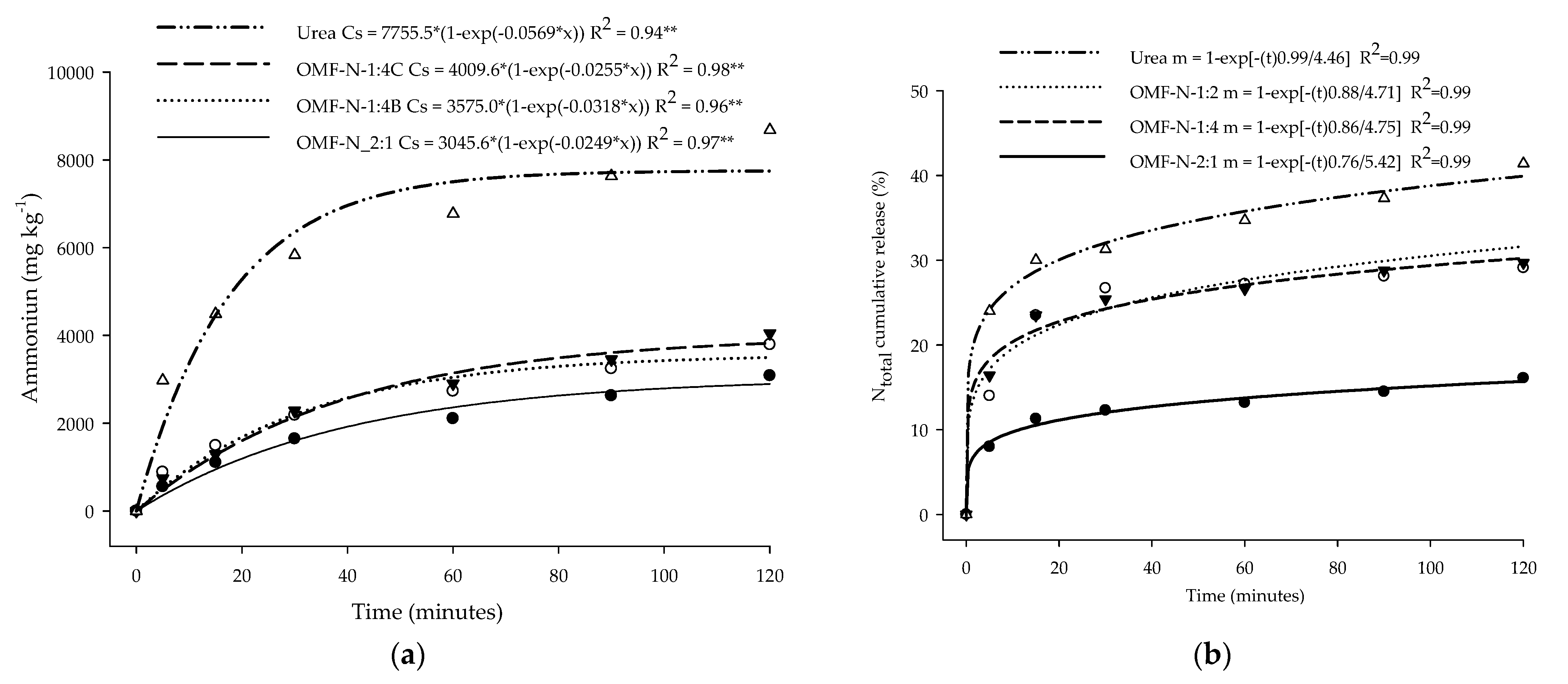
2.7. OMF-N Solubilization Kinetics
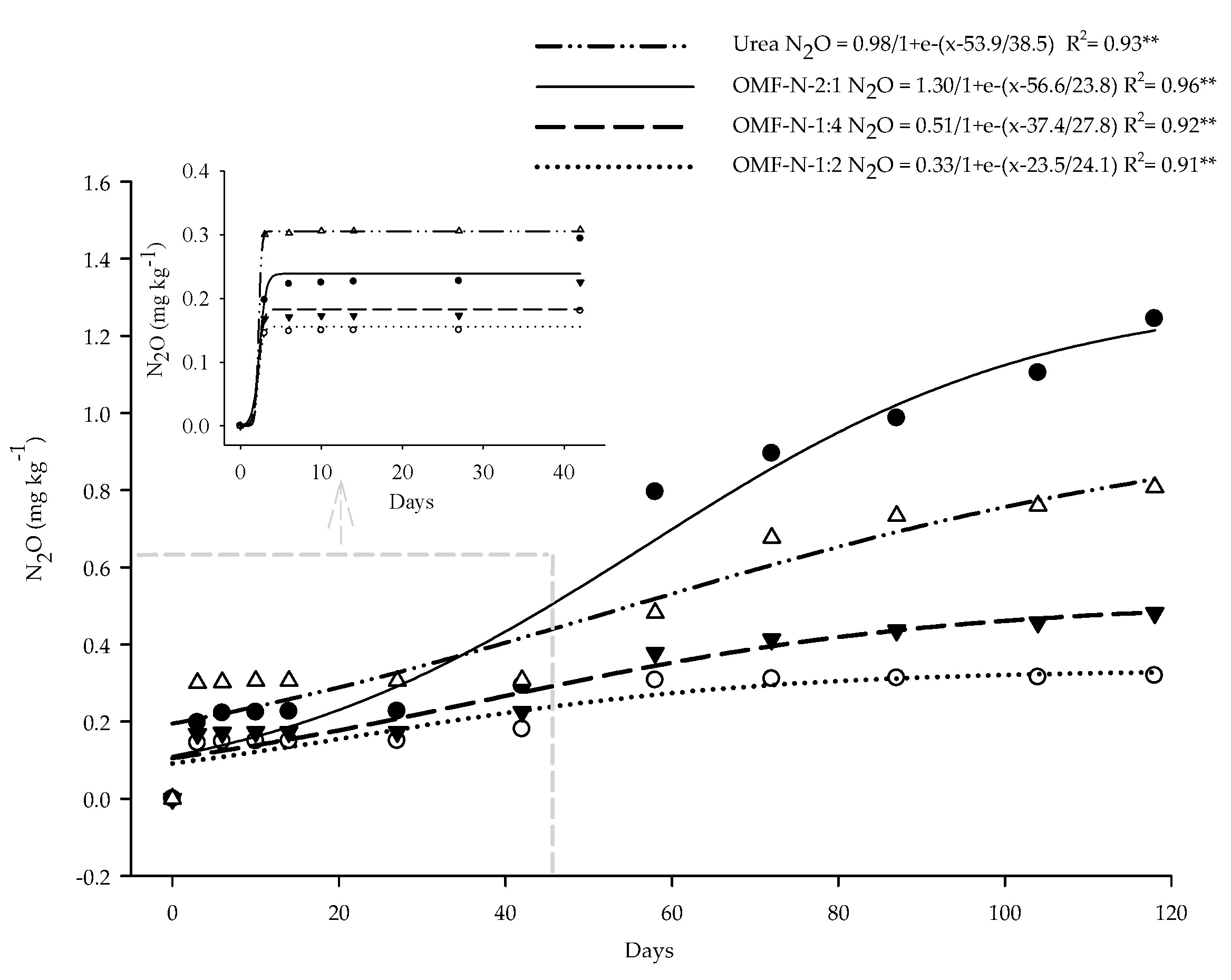
2.8. OMF-N N2O and CO2 Emission Kinetics
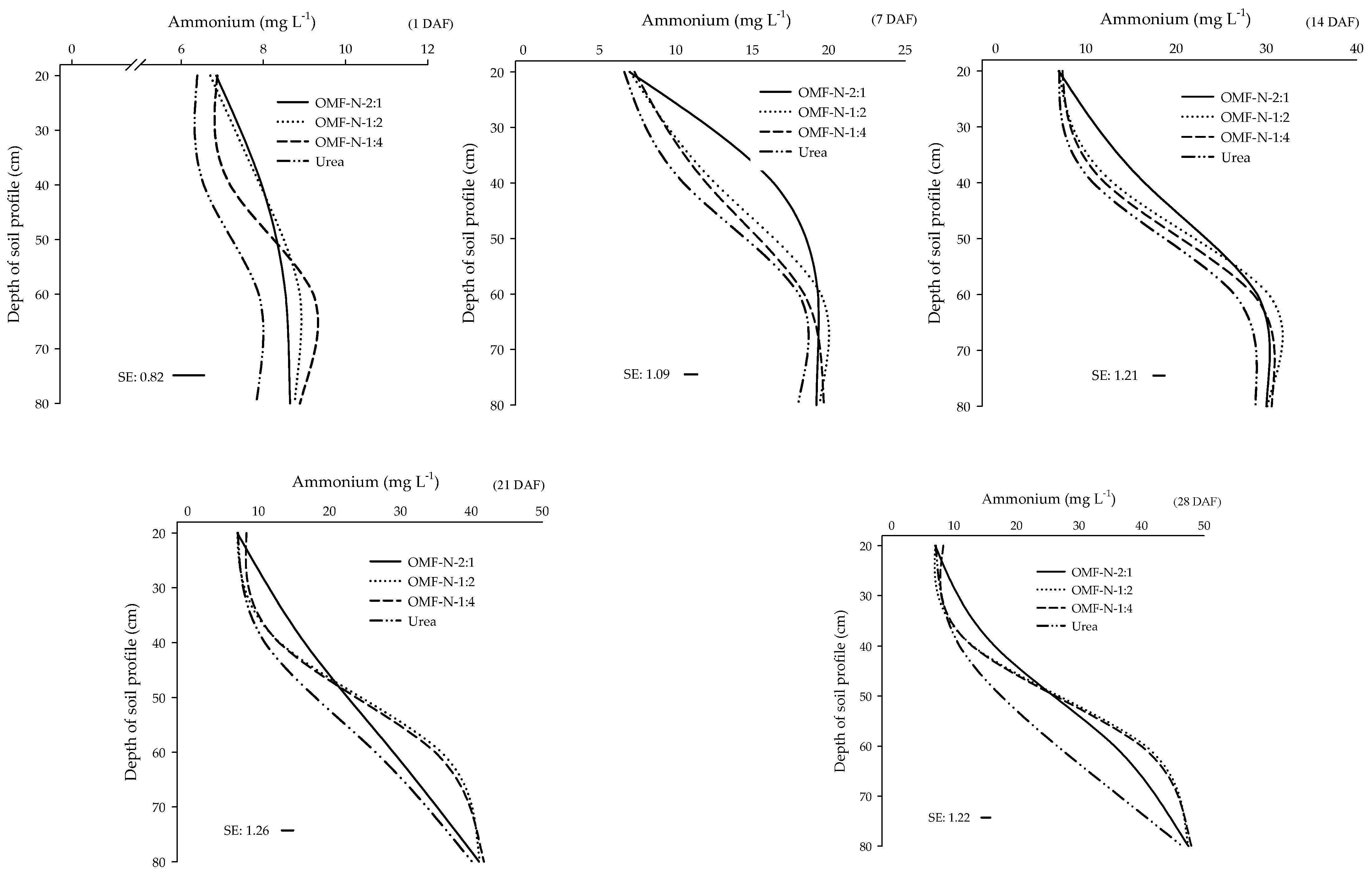
2.9. NH4+ and NO3− Leaching in Soil Fertilized with Urea and OMF-N
2.10. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Interaction of OMF-N
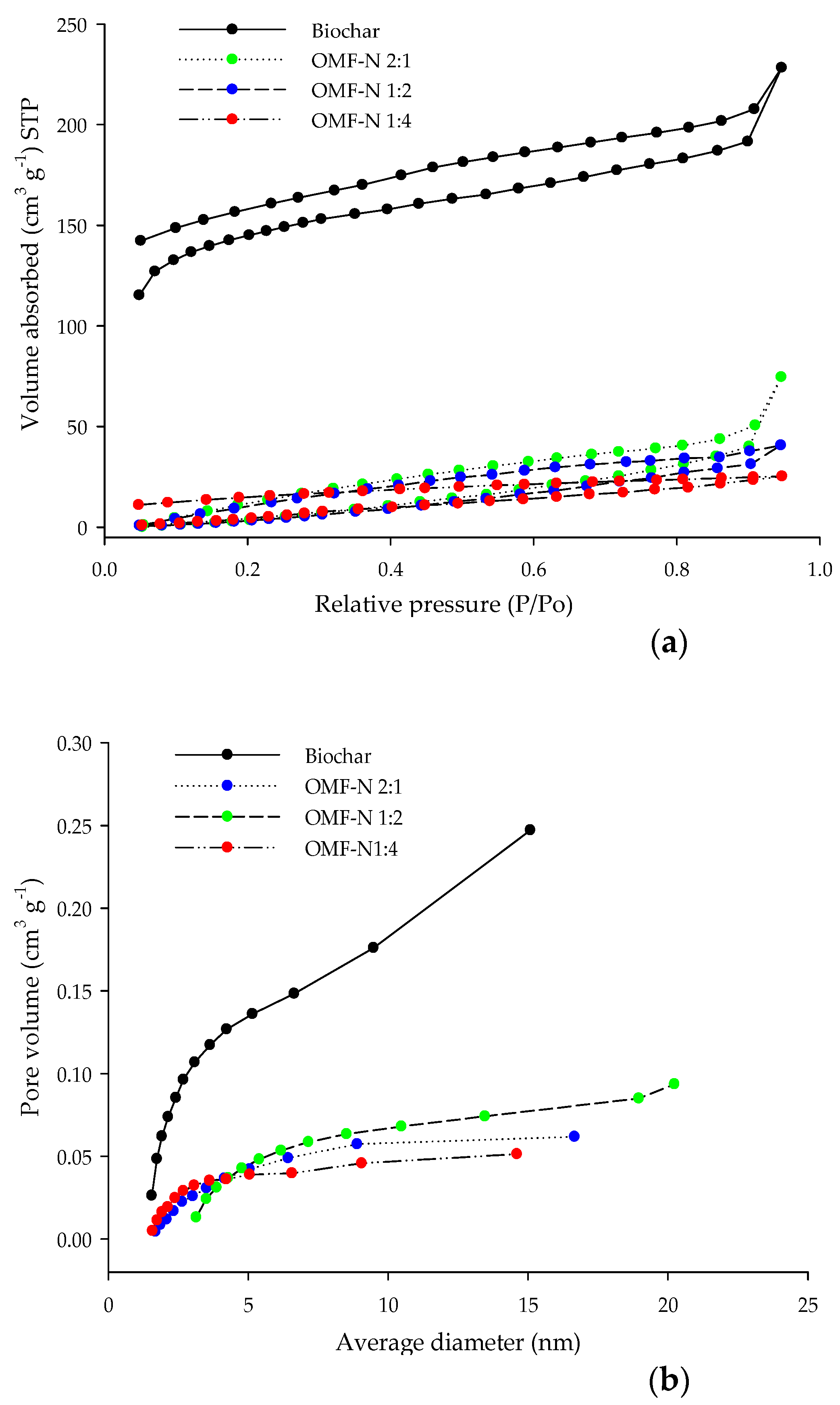
3.2. Physical Properties of the OMF-N
3.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
3.4. NH4+ and Ntotal Release
3.5. N2O and CO2 Emissions
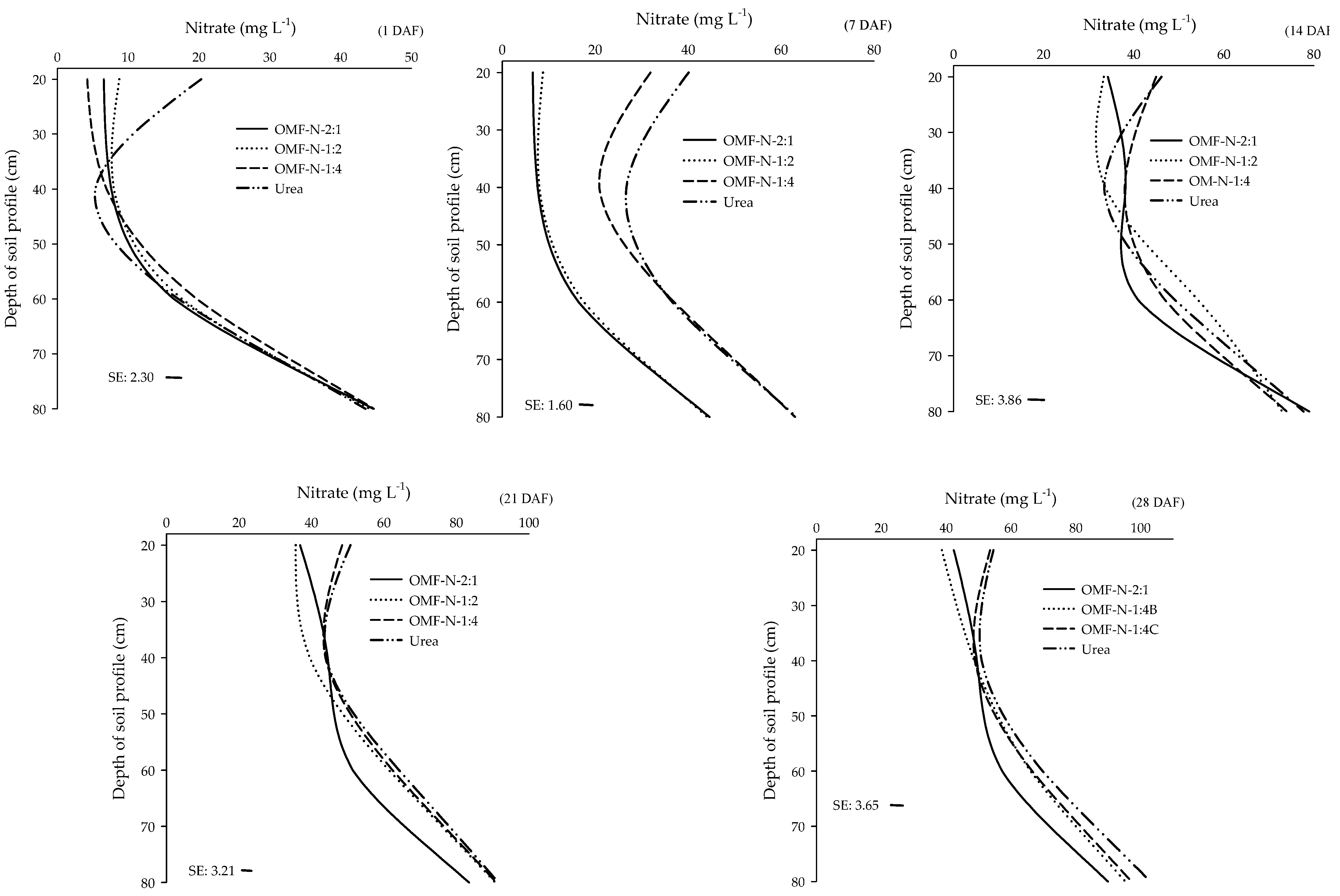
3.6. NH4+ and NO3− Leaching
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology_ the new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 15–23. [Google Scholar] [CrossRef]
- Luo, W.; Qian, L.; Liu, W.; Zhang, X.; Wang, Q.; Jiang, H.; Cheng, B.; Ma, H.; Wu, Z. A potential mg-enriched biochar fertilizer: Excellent slow-release performance and release mechanism of nutrients. Sci. Total Environ. 2021, 768, 144454. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Lu, P.; Zhang, M. Preparation and characterization of environmentally friendly controlled release fertilizers coated by leftovers-based polymer. Processes 2020, 8, 417. [Google Scholar] [CrossRef]
- Rozo, G.; Bohorques, L.; Santamaría, J. Controlled release fertilizer encapsulated by a κ-carrageenan hydrogel. Polímeros 2019, 29, 3. [Google Scholar] [CrossRef]
- Sun, H.; Shi, W.; Zhou, M.; Ma, X.; Zhang, H. Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ. 2019, 65, 83–89. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of biochar for the removal of nitrogen and phosphorus. J. Clean. Prod. 2020, 257. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction; Routledge: London, UK, 2015. [Google Scholar]
- Madari, B.E.; Silva, M.A.; Carvalho, M.T.; Maia, A.H.; Petter, F.A.; Santos, J.L.; Tsai, S.M.; Leal, W.G.; Zeviani, W.M. Properties of a sandy clay loam Haplic Ferralsol and soybean grain yield in a five-year field trial as affected by biochar amendment. Geoderma 2017, 305, 100–112. [Google Scholar] [CrossRef]
- Petter, F.A.; Ferreira, T.; Sinhorin, A.; Lima, L.B.; Almeida, F.; Pacheco, L.; Silva, A. Biochar Increases Diuron Sorption and Reduces the Potential Contamination of Subsurface Water with Diuron in a Sandy Soil. Pedosphere 2019, 6, 801–809. [Google Scholar] [CrossRef]
- Peres, F.S.C.; Petter, F.A.; Sinhorin, A.P.; de Lima, L.B.; Tavanti, T.R.; da Silva Freddi, O.; Marimon, B.H., Jr. Influence of biochar on the sorption and leaching of thiamethoxan in soil. J. Environ. Sci. Health B 2022, 57, 153–163. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.; Ramavandi, B. Cadmium ion removal from aqueous media using banana peel biochar/Fe3O4/ZIF-67. Environ. Res. 2022, 211, 113020. [Google Scholar] [CrossRef] [PubMed]
- Petter, F.A.; de Lima, L.B.; Marimon Júnior, B.H.; Alves de Morais, L.; Marimon, B.S. Impact of biochar on nitrous oxide emissions from upland rice. J. Environ. Manage 2016, 169, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Informação Tecnológica: Brasília, Brazil, 2009. [Google Scholar]
- Rutland, D.; Frederick, E.; Roth, E. Manual for Determining Physical Properties of Fertilizer; International Fertilizer Development Center: Muscle Shoals, AL, USA, 1986. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Campos, D.V.B.; Alves, B.J.R.; Teixeira, P.C.; Jantalia, C.P.; Mattos, B.B. Nitrato e Amônio. In Manual de Métodos de Análise de Solo, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2017; Volume 3, pp. 377–392. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- dos Santos, A.; Matos, E.S.; Freddi, O.S.; Galbieri, R.; Lal, R. Cotton production systems in the Brazilian Cerrado: The impact of soil attributes on field-scale yield. Eur. J. Agron. 2020, 118, 126090. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Singh, R.; Jana, S. An impact of biofield treatment on spectroscopic characterization of pharmaceutical compounds. Mod. Chem. Appl. 2015, 3, 3. [Google Scholar] [CrossRef]
- Liu, X.; Liao, J.; Song, H.; Yang, Y.; Guan, C.; Zhang, Z. A biochar-based route for environmentally friendly controlled release of nitrogen: Urea-loaded biochar and bentonite composite. Sci. Rep. 2019, 9, 9548. [Google Scholar] [CrossRef]
- Piasek, Z.; Urbanski, T. The infra-red absorption spectrum and structure of urea. Bulletin de L’ Academie Polonaise des Ciences 1962, 10, 113–120. Available online: https://cdnsciencepub.com/doi/pdf/10.1139/v60-025 (accessed on 10 July 2021).
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Sorrenti, G.-B.; Masiello, C.A.; Dugan, B.; Toselli, M. Biochar physico-chemical properties as affected by environmental exposure. Sci. Total Environ. 2016, 563–564, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Petter, F.A.; Leite, L.F.C.; Machado, D.M.; Marimon-Junior, B.H.; Lima, L.B.; Freddi, O.S.; Araújo, A.S.F. Microbial biomass and organic matter in an oxisol under application of biochar. Bragantia 2019, 78, 109–118. [Google Scholar] [CrossRef]
- Mattson, J.S.; Mark, H.B. Activated Carbon: Surface Chemistry and Adsorption from Solution; Marcel Dekker: New York, NY, USA, 1971. [Google Scholar]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresource Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Rochette, P.; Macdonald, J.D.; Angers, D.A.; Chantigny, M.H.; Gasser, M.-O.; Bertrand, N. Banding urea increased ammonia volatilization in a dry acidic soil. J. Environ. Qual. 2009, 38, 1383–1390. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Y.; Hu, C.; Oenema, O.; Li, X.; Zhang, Y.; Dong, W. Yield-scaled N2O emissions in a winter wheat-summer corn double-cropping system. Atmos. Environ. 2012, 55, 240–244. [Google Scholar] [CrossRef]
- Dawar, K.; Fahad, S.; Jahangir, M.M.R.; Munir, I.; Alam, S.S.; Khan, S.A.; Mian, I.A.; Datta, R.; Saud, S.; Banout, J.; et al. Biochar and urease inhibitor mitigate NH3 and N2O emissions and improve wheat yield in a urea fertilized alkaline soil. Sci. Rep. 2021, 11, 17413. [Google Scholar] [CrossRef]
- Jange, C.G.; Wassgren, C.R.; Kingsly Ambrose, R.P. Disintegration and release kinetics of dry compacted urea composites: A formulation and process design study. EFB Bioeconomy J. 2021, 1, 100020. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Pradela Filho, L.A.; Santos, A.L.; Takeuchi, R.M.; Assunção, R.M.N. Evaluation of the ibuprofen delyvery profile in symmetric and asymmetric membranes of cellulose acetate: Effect of morphology. Química Nova 2019, 42, 823–830. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Mathematical and Physiochemical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Lawrence, N.C.; Tenesaca, C.G.; Vanloocke, A.; Hall, S.J. Nitrous oxide emissions from agricultural soils challenge climate sustainability and in the US Corn Belt. Proc. Natl. Acad. USA 2021, 118, e2112108118. [Google Scholar] [CrossRef] [PubMed]
- Maclean, A.M.; Smith, N.R.; Ying, L.; Yuanzhou, H.; Hettiyadura, A.P.S.; Crescenzo, G.C.; Shiraiwa, M.; Laskin, A.; Nizkorodov, S.A.; Bertram, A.K. Humidity-dependent viscosity of secondary organic aerosol from ozonolysis of β-caryophyllene: Measurements, predictions, and implications. ACS Earth Space Chem. 2021, 5, 302–318. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agr. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Bian, Z.; Tian, H.; Yang, Q.; Xu, R.; Pan, S.; Zhang, B. Production and application of manure nitrogen and phosphorus in the United States since 1860. Earth Syst. Sci. Data 2021, 13, 515–527. [Google Scholar] [CrossRef]
- Kim, G.W.; Alam, M.A.; Lee, J.J.; Kim, G.Y.; Kim, P.J.; Khan, M.I. Assessment of direct carbon dioxide emission factor from urea fertilizer in temperate upland soil during warm and cold cropping season. Eur. J. Soil Biol. 2017, 83, 76–83. [Google Scholar] [CrossRef]
- Puga, A.P.; Queiroz, M.C.A.; Ligo, M.A.V.; Carvalho, C.S.; Pires, A.M.M.; Marcatto, J.O.S.; Andrade, C.A. Nitrogen availability and ammonia volatilization in biochar based fertilizers. Arch. Agron. Soil Sci. 2020, 66, 9921004. [Google Scholar] [CrossRef]
- Pramono, A.; Adriany, T.A.; Susilawati, H.L. Mitigation scenario for reducing greenhouse gas emission from rice field by water management and rice cultivars. J. Trop. Soils 2020, 25, 53–60. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.-O.; MacDonald, J.D.; Pelster, D.E.; Bertrand, N. Ammonia Volatilization and Nitrogen Retention: How Deep to Incorporate Urea? J. Environ. Qual. 2013, 42, 1635–1642. [Google Scholar] [CrossRef]
- Selvarajh, G.; Chng, H.Y.; Md Zain, N. Effects of rice husk biochar in minimizing ammonia volatilization from urea fertilizer applied under waterlogged condition. AIMS Agric. Food 2021, 6, 159–171. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ahmed, O.H.; Wahid, S.A.; Jalloh, M.B.; Muzah, A.A. Nutrient release and ammonia volatilization from biochar-blended fertilizer with and without densification. Agronomy 2021, 11, 2082. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Yang, Y.; Chen, Y.; Jeyakumar, P.; Shao, Q.; Zhou, Y.; Ma, M.; Zhu, R.; Qian, Q.; et al. Effect of biofertilizer and wheat straw biochar application on nitrous oxide emission and ammonia volatilization from paddy soil. Environ. Pollut. 2021, 275, 116640. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Shangguan, Z. Combined biochar and nitrogen fertilization at appropriate rates could balance the leaching and availability of soil inorganic nitrogen. Agr. Ecosyst. Environ. 2019, 276, 21–30. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Z.; Ba, Y.; Qi, W. Application of biochar-coated urea controlled loss of fertilizer nitrogen and increased nitrogen use efficiency. Chem. Biol. Technol. Agric. 2021, 8, 3. [Google Scholar] [CrossRef]





| Elements | Unit | Concentration |
|---|---|---|
| Total Nitrogen (N) | g kg−1 | 3.30 |
| Phosphorus (P2O5 citric acid) | 0.44 | |
| Phosphorus (P2O5 total) | - | |
| K2O | 0.60 | |
| CaO | 3.78 | |
| MgO | 1.32 | |
| Sulfur (S) | 0.14 | |
| Copper (Cu) | mg kg−1 | 10.0 |
| Zinc (Zn) | 9.0 | |
| Molybdenum (Mo) | 1.35 | |
| Cobalt (Co) | 1.93 | |
| Boron (B) | 12.0 | |
| Total carbon (C) | g kg−1 | 760.0 |
| Moisture | 50.0 | |
| C:N ratio | 230 | |
| Specific surface area (BET) | m2 g−1 | 537 |
| Actual density | g cm−3 | 1.27 |
| Apparent density | g cm−3 | 0.31 |
| Porosity | % | 75 |
| Average pore diameter | Nm | 2.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, V.V.; Morales, M.M.; Pereira, D.H.; de Rezende, F.A.; de Souza Magalhães, C.A.; de Lima, L.B.; Marimon-Junior, B.H.; Petter, F.A. Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission. Sustainability 2022, 14, 12388. https://doi.org/10.3390/su141912388
Pereira VV, Morales MM, Pereira DH, de Rezende FA, de Souza Magalhães CA, de Lima LB, Marimon-Junior BH, Petter FA. Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission. Sustainability. 2022; 14(19):12388. https://doi.org/10.3390/su141912388
Chicago/Turabian StylePereira, Valéria Viana, Marina Moura Morales, Dalton Henrique Pereira, Fabiana Abreu de Rezende, Ciro Augusto de Souza Magalhães, Larissa Borges de Lima, Ben Hur Marimon-Junior, and Fabiano André Petter. 2022. "Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission" Sustainability 14, no. 19: 12388. https://doi.org/10.3390/su141912388
APA StylePereira, V. V., Morales, M. M., Pereira, D. H., de Rezende, F. A., de Souza Magalhães, C. A., de Lima, L. B., Marimon-Junior, B. H., & Petter, F. A. (2022). Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission. Sustainability, 14(19), 12388. https://doi.org/10.3390/su141912388







