Determination of Surfactant Content for Optimum Strength of Multi-Walled Carbon Nanotube Cementitious Composites
Abstract
:1. Introduction
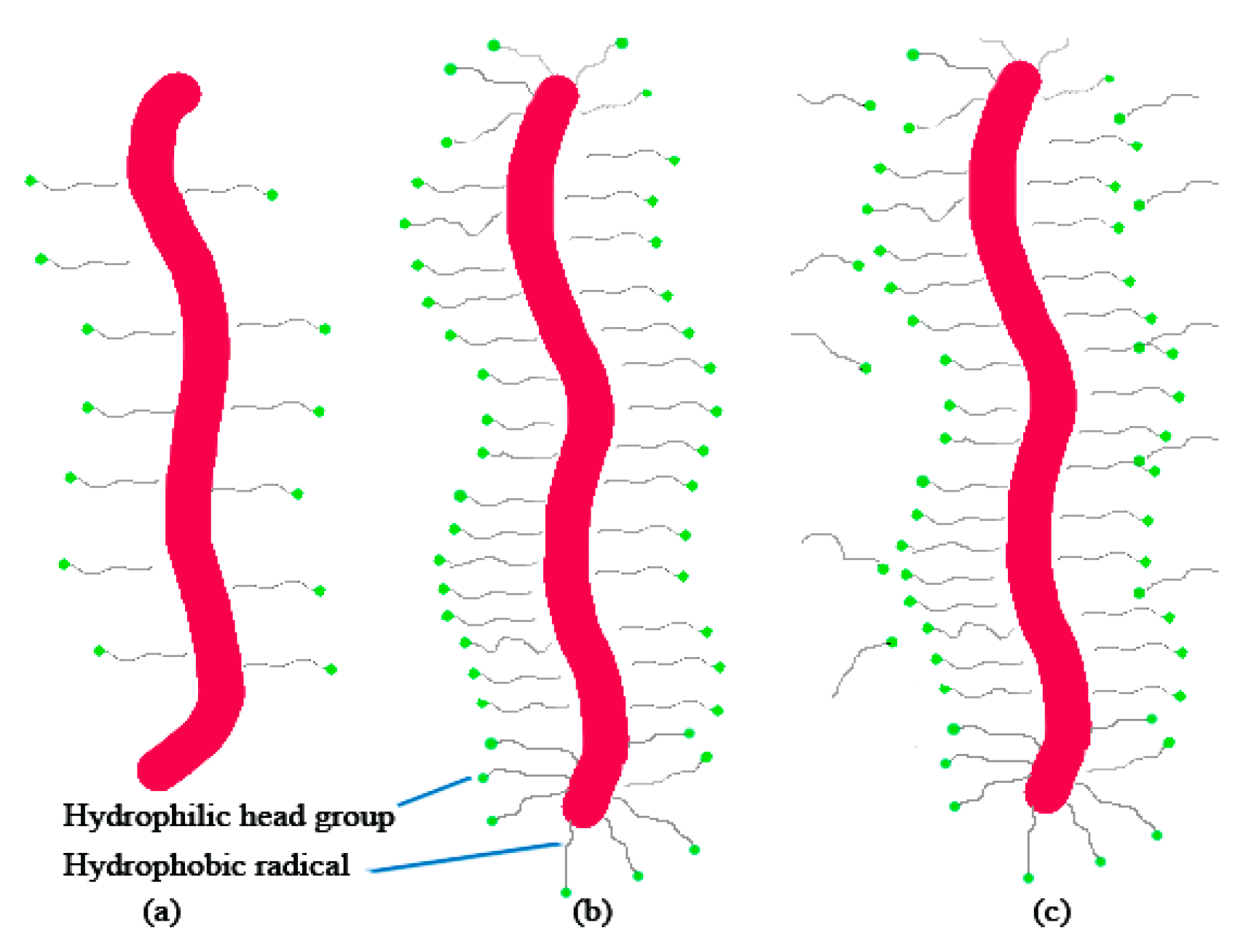
2. Effective Surfactant Factor
3. Experimental Program
3.1. Materials and Equipment
3.2. Cement and Surfactant Mixing
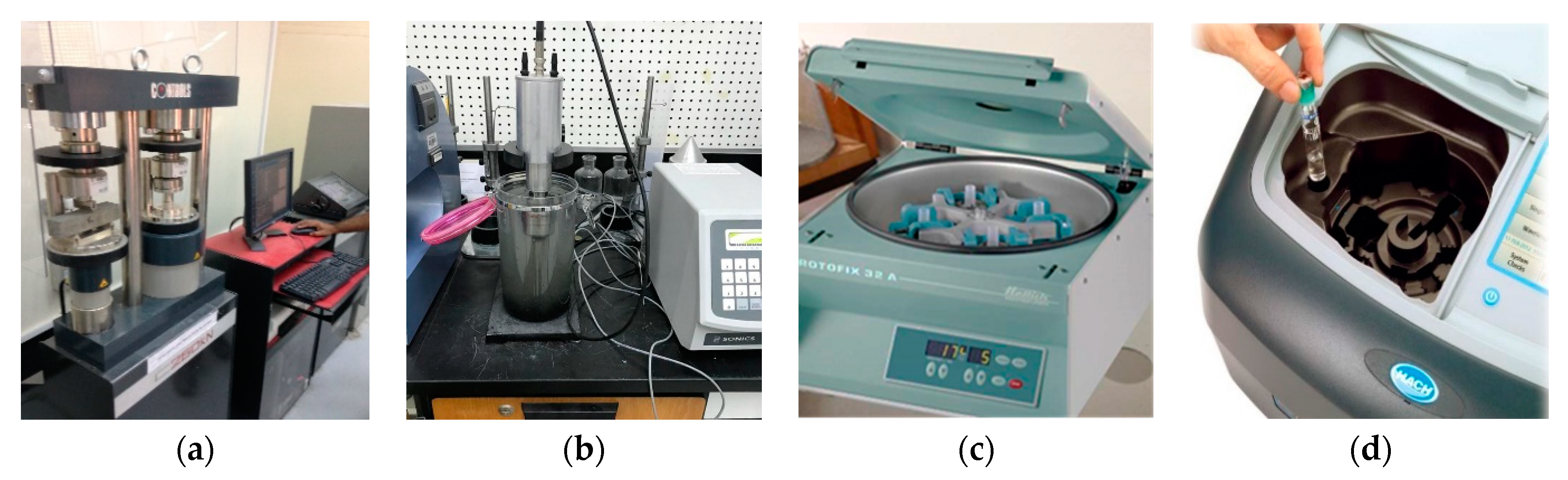
3.3. Mechanical Properties Testing
3.4. Chemical Analysis
3.4.1. Sonication Process
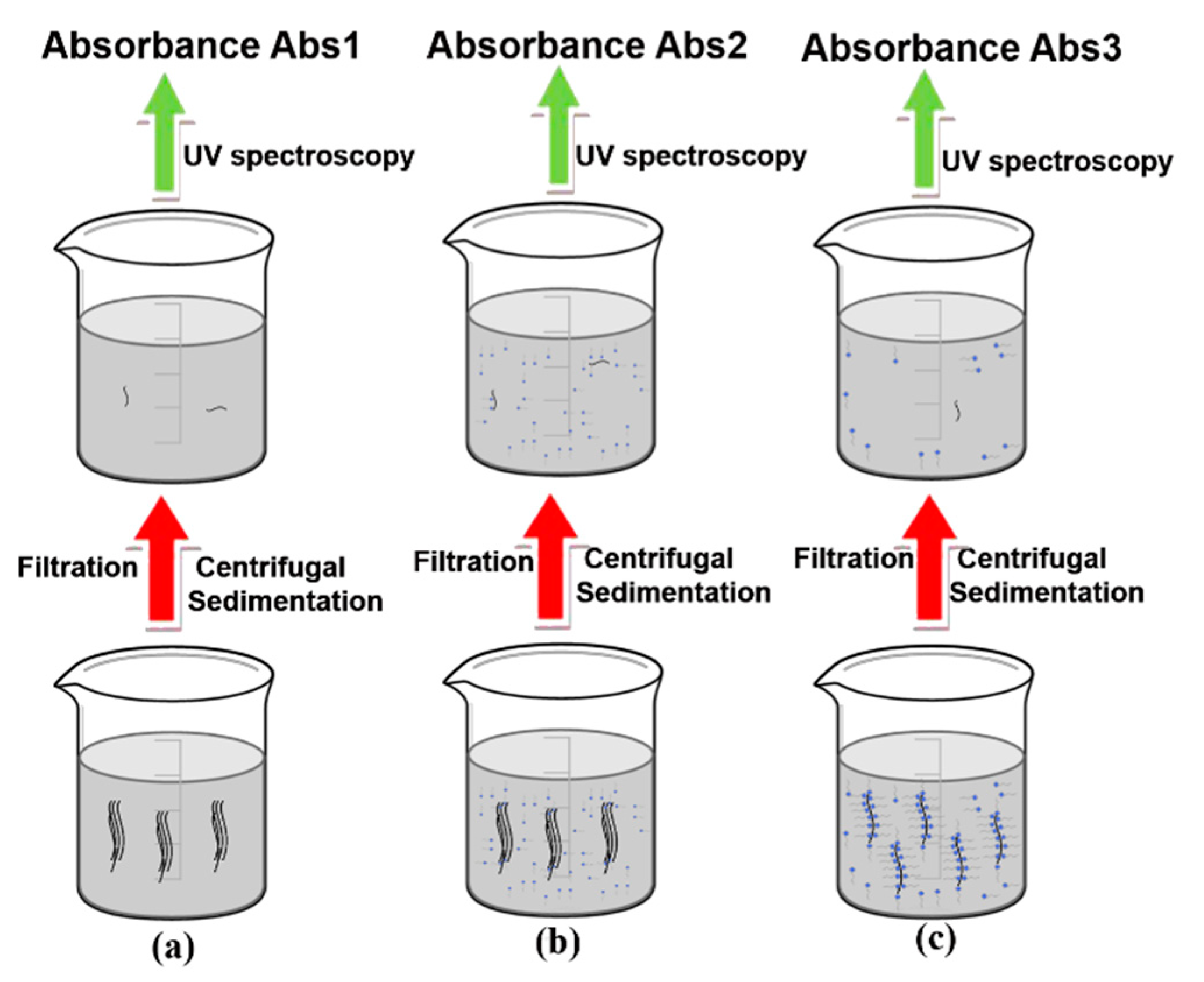
3.4.2. Filtration and Centrifugal Sedimentation Processes
3.4.3. Ultraviolet Spectroscopy
3.5. Microstructural Analysis
4. Results and Discussion
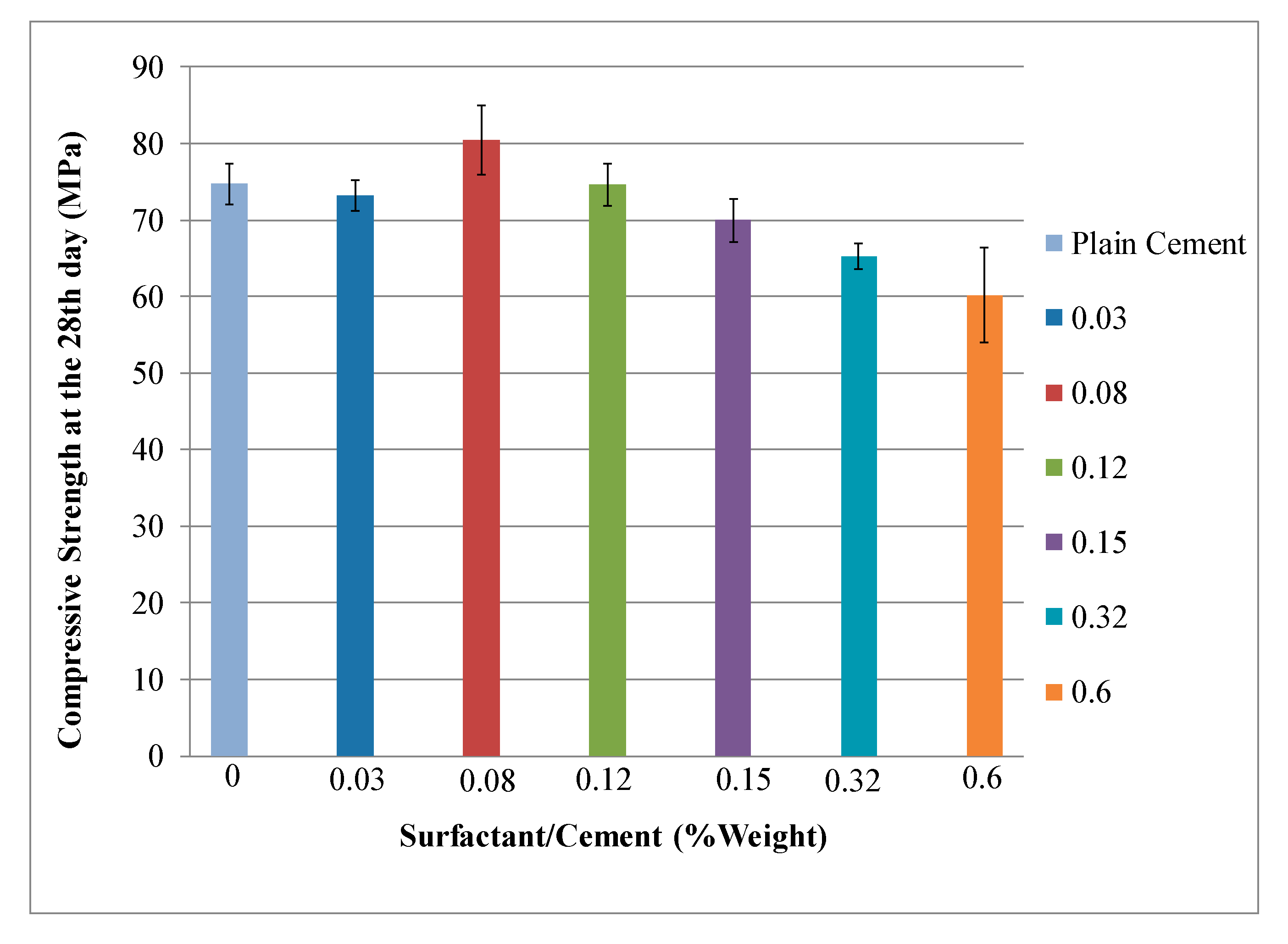
4.1. Mechanical Properties Analysis
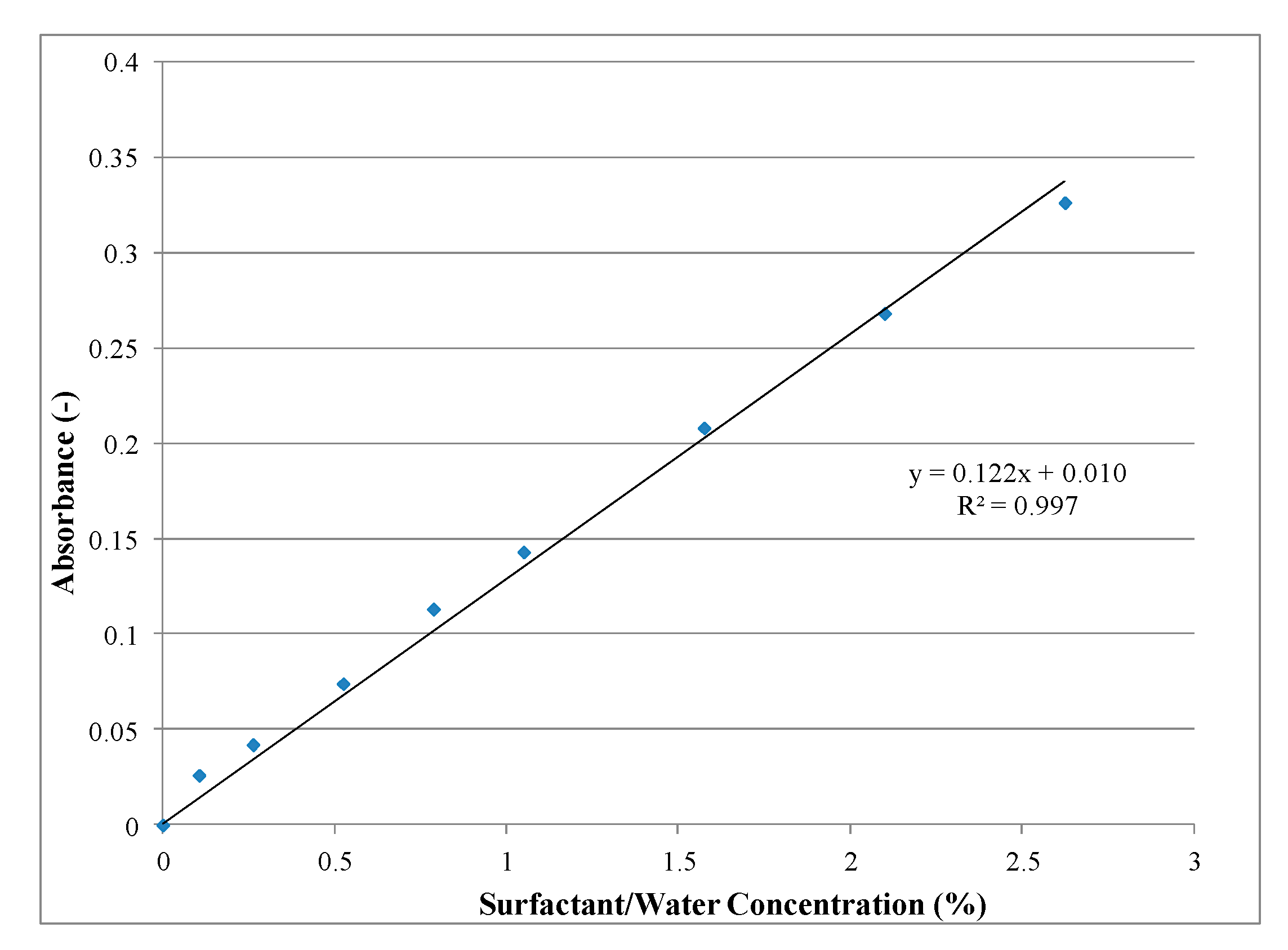
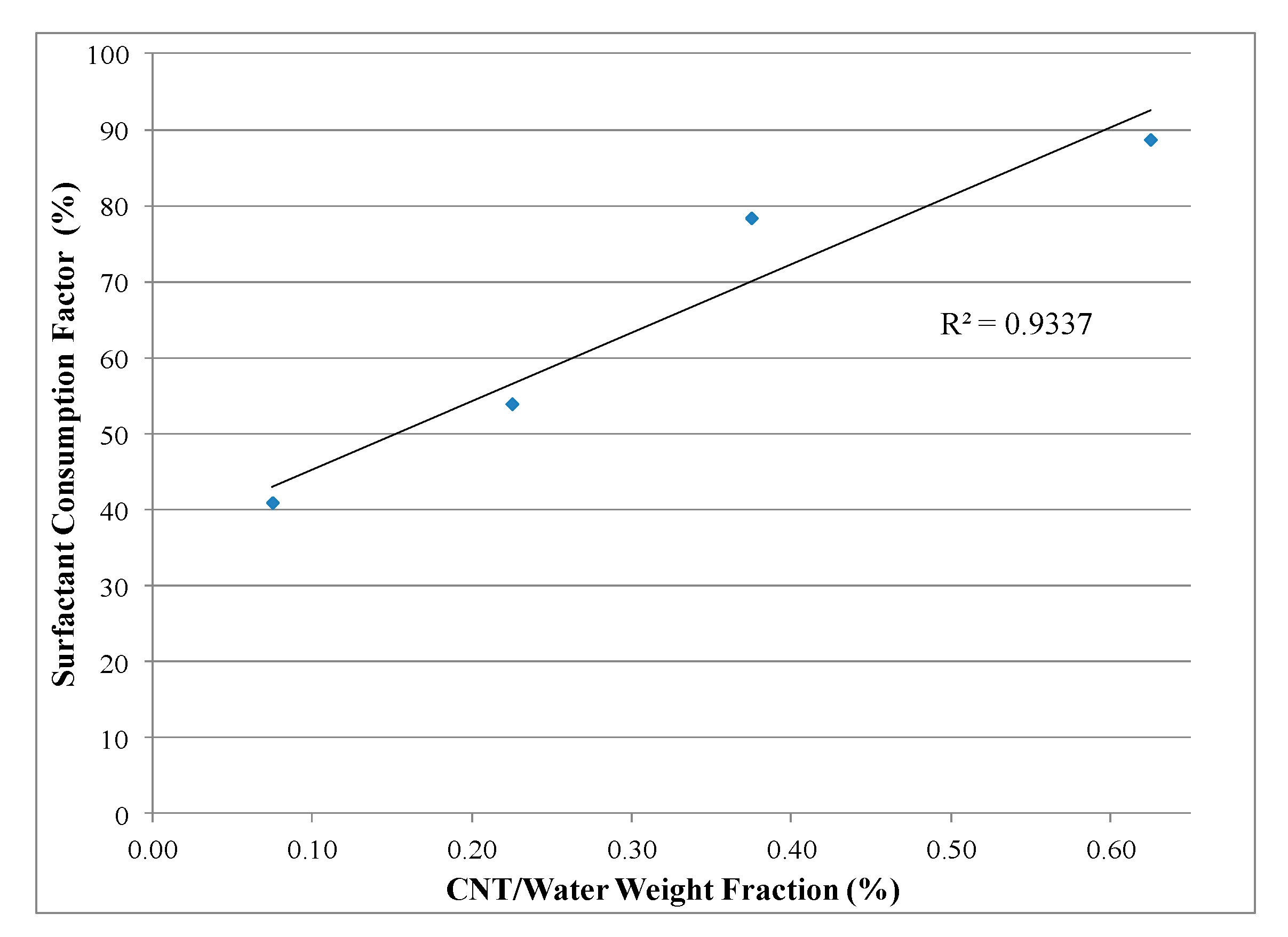
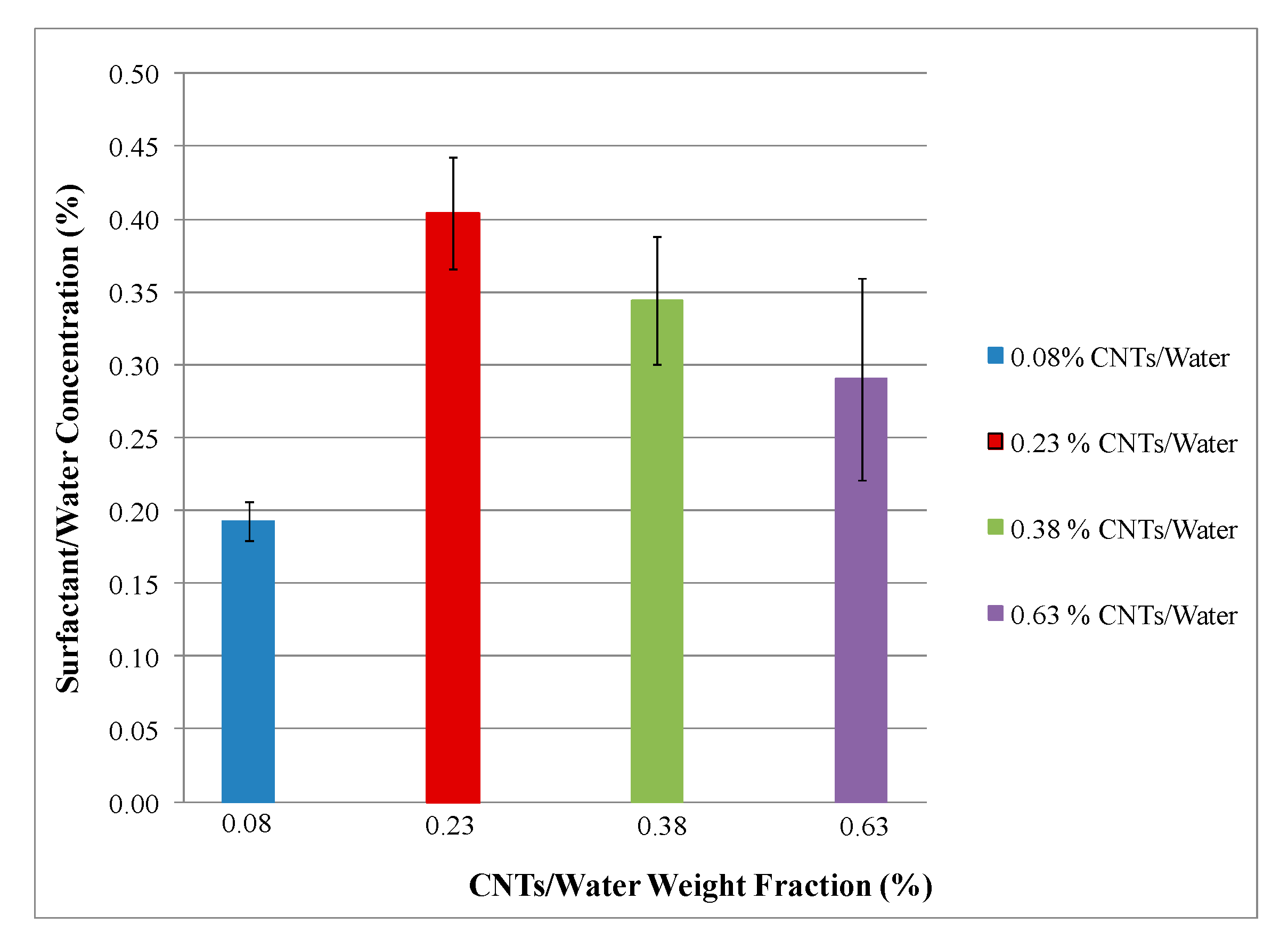
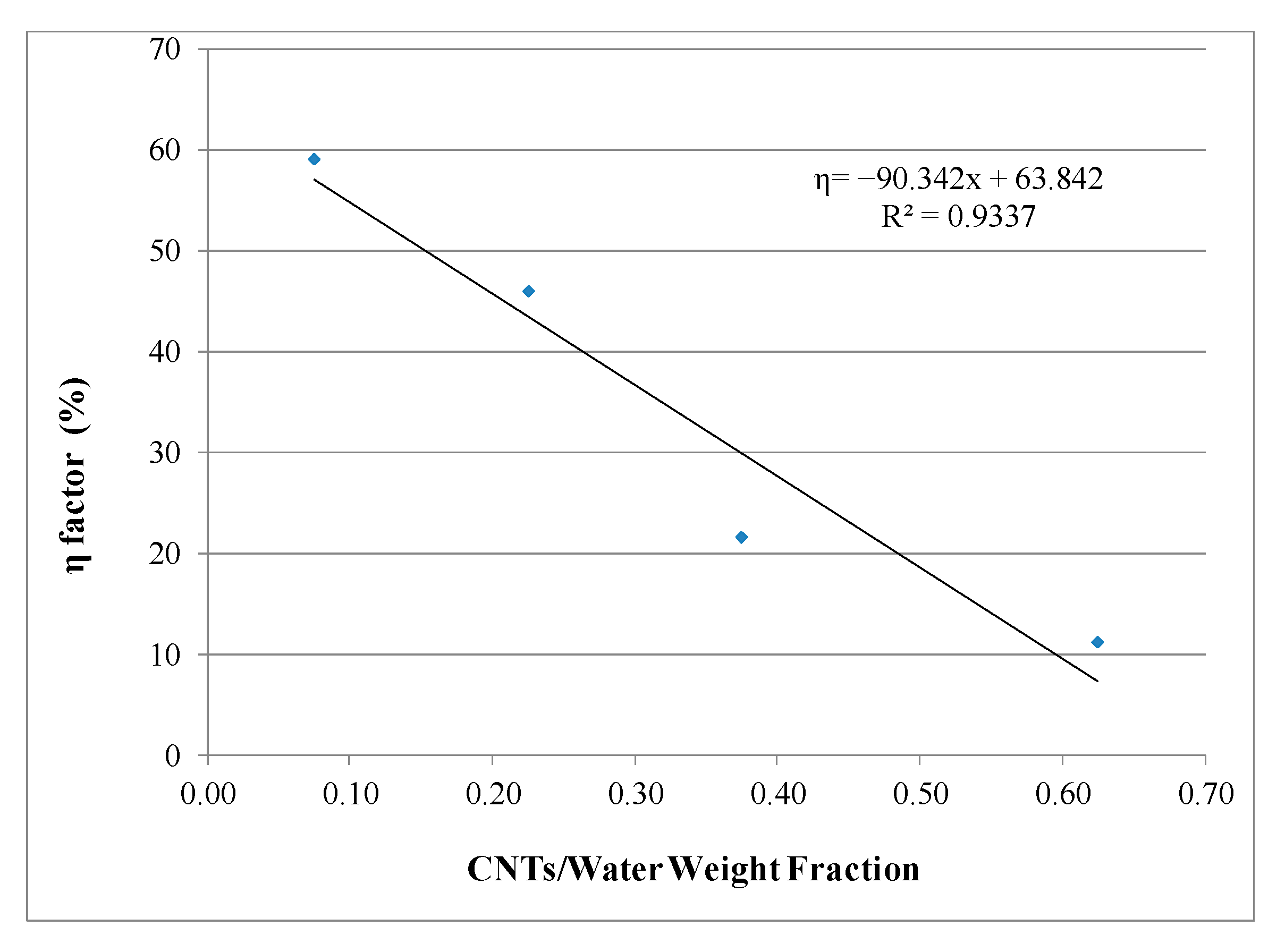
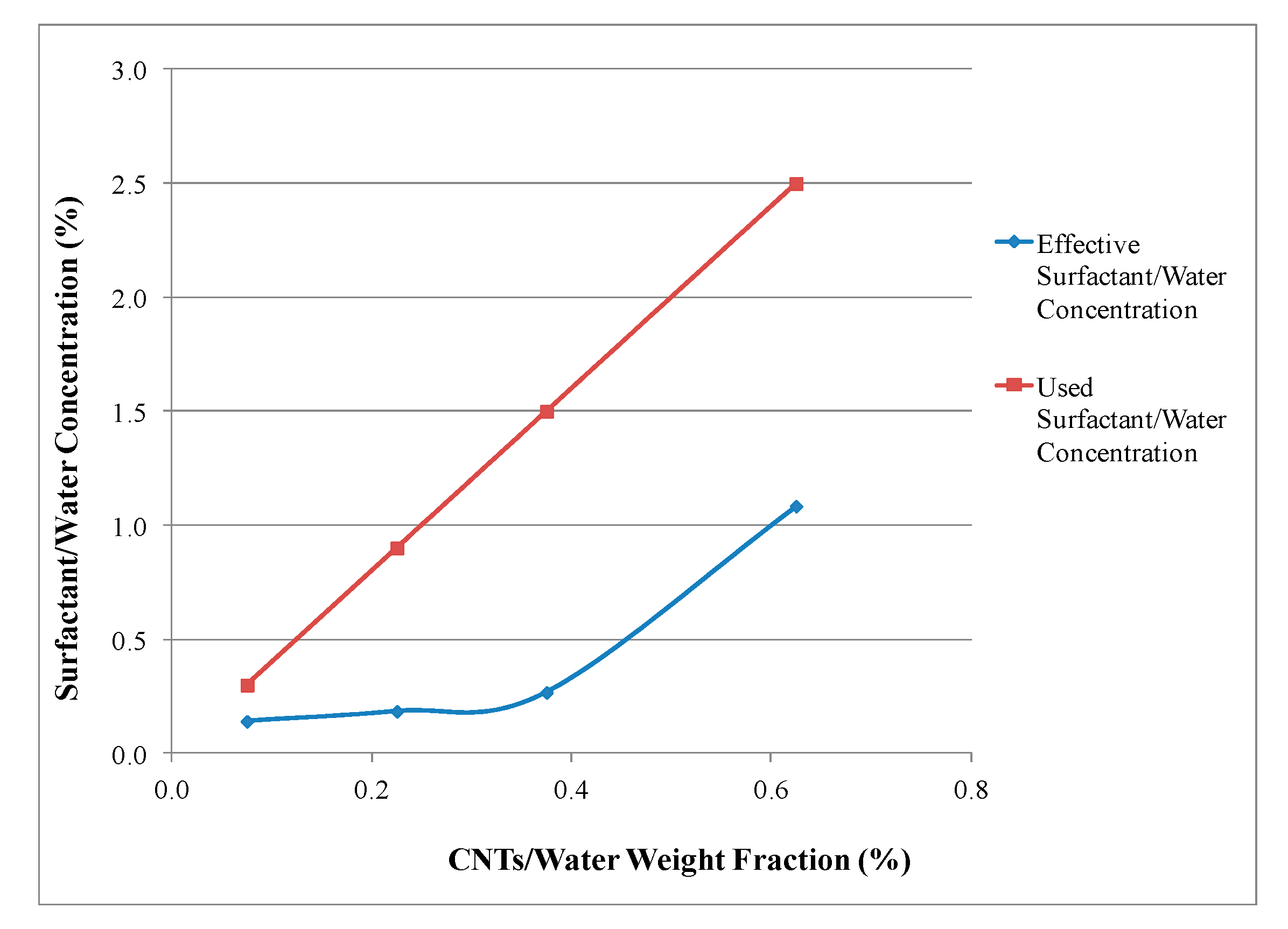
4.2. Chemical Analysis
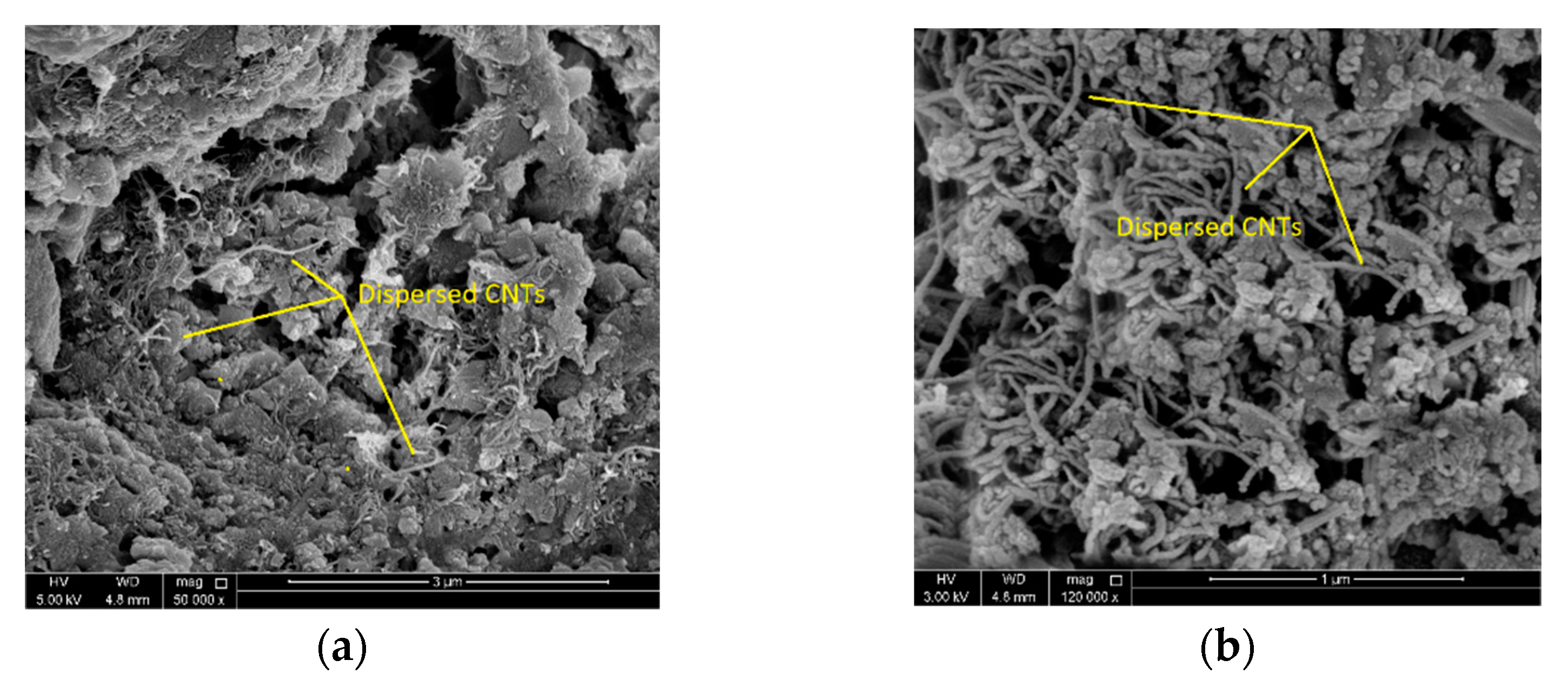
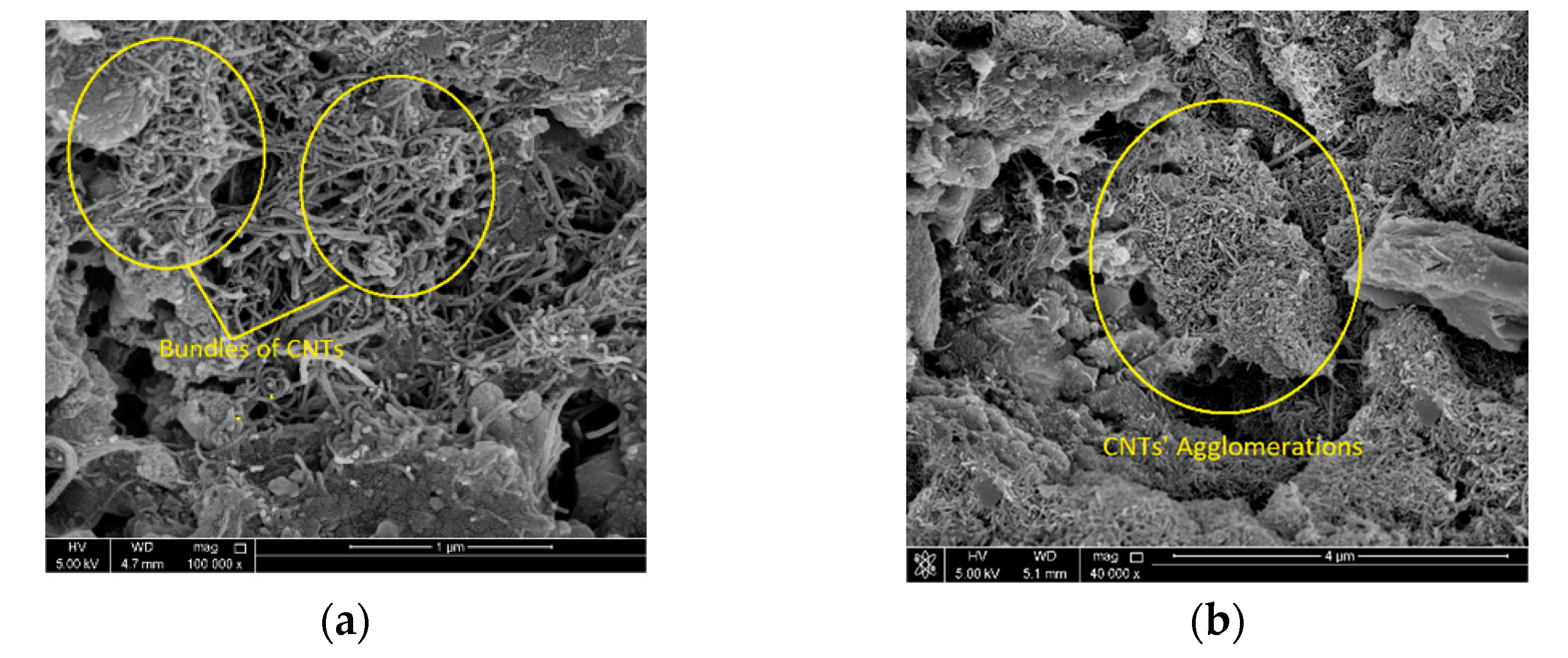
4.3. Microstructure Analysis
5. Conclusions and Recommendations
- The amount of surfactant in the CNT dispersion process should be selected carefully to account for the remaining amount in the solution after the sonication process;
- The performance of UV tests should be done carefully as the absorbance results are very sensitive to the existence of CNTs in the solution at the time of measurement;
- A practical and faster CNT filtration methodology should be used in order to determine the exact amount of surfactant remaining in the solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, H.; Yan, X.; Monasterio, M.; Xing, F. Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution. Nanomaterials 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. Surfactant-aided dispersion of carbon nanomaterials in aqueous solution. Phys. Fluids 2019, 31, 071301. [Google Scholar] [CrossRef]
- Aiad, I. Influence of time addition of superplasticizers on the rheological properties of fresh cement pastes. Cem. Concr. Res. 2003, 33, 1229–1234. [Google Scholar] [CrossRef]
- Aiad, I.; El-Aleem, S.A.; El-Didamony, H. Effect of delaying addition of some concrete admixtures on the rheological properties of cement pastes. Cem. Concr. Res. 2002, 32, 1839–1843. [Google Scholar] [CrossRef]
- Shahzad, S.; Toumi, A.; Balayssac, J.-P.; Turatsinze, A.; Mazars, V. Cementitious composites incorporating Multi-Walled Carbon Nanotubes (MWCNTs): Effects of annealing and other dispersion methods on the electrical and mechanical properties. Matériaux Tech. 2022, 110, 104. [Google Scholar] [CrossRef]
- Shao, H.; Chen, B.; Li, B.; Tang, S.; Li, Z. Influence of dispersants on the properties of CNTs reinforced cement-based mate-rials. Constr. Build. Mater. 2017, 131, 186–194. [Google Scholar] [CrossRef]
- Inam, F.; Heaton, A.; Brown, P.; Peijs, T.; Reece, M.J. Effects of dispersion surfactants on the properties of ceramic–carbon nanotube (CNT) nanocomposites. Ceram. Int. 2014, 40, 511–516. [Google Scholar] [CrossRef]
- Sindu, B.; Sasmal, S. Properties of carbon nanotube reinforced cement composite synthesized using different types of surfactants. Constr. Build. Mater. 2017, 155, 389–399. [Google Scholar] [CrossRef]
- Xu, P.; Shi, L.; Huang, J.; Wang, C.; Zhang, Y.; Xu, H. Methods of dispersion and stabilization of several nanomaterials in water. Ferroelectrics 2018, 527, 133–148. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Boutsioukou, S.; Amenta, M.; Favvas, E.P.; Kourkoulis, S.K.; Alexopoulos, N.D. Dispersion of Multi-Walled Carbon Nanotubes into White Cement Mortars: The Effect of Concentration and Surfactants. Nanomaterials 2022, 12, 1031. [Google Scholar] [CrossRef]
- Collins, F.; Lambert, J.; Duan, W.H. The influences of admixtures on the dispersion, workability, and strength of carbon nanotube–OPC paste mixtures. Cem. Concr. Composites 2012, 34, 201–207. [Google Scholar] [CrossRef]
- Abu Al-Rub, R.; Tyson, B.M.; Yazdanbakhsh, A.; Grasley, Z. Mechanical Properties of Nanocomposite Cement Incorporating Surface-Treated and Untreated Carbon Nanotubes and Carbon Nanofibers. J. Nanomech. Micromech. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Tyson, B.M.; Abu Al-Rub, R.K.; Yazdanbakhsh, A.; Grasley, Z. Carbon Nanotubes and Carbon Nanofibers for Enhancing the Mechanical Properties of Nanocomposite Cementitious Materials. J. Mater. Civ. Eng. 2011, 23, 1028–1035. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Al Ansari, M.S.; Taha, R.; Al Nuaimi, N.; Abu Taqa, A. Carbon Nanotube Effect on the Ductility, Flexural Strength, and Permeability of Concrete. J. Nanomater. 2019, 2019, 6490984. [Google Scholar] [CrossRef]
- Mohsen, M.; Al Ansari, M.; Taha, R.; Senouci, A.; AbuTaqa, A. Optimum Dispersion Parameters of Carbon Nanotubes: Concrete Strength by Response Surface Methodologies. In Proceedings of the International Conference on Civil Infrastructure and Construction, Doha, Qatar, 2–5 February 2020; Volume 1, pp. 298–307. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Al-Nuaimi, N.; Al-Rub, R.K.A.; Senouci, A.; Bani-Hani, K.A. Effect of mixing duration on flexural strength of multi walled carbon nanotubes cementitious composites. Constr. Build. Mater. 2016, 126, 586–598. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Taha, R.; Abu Taqa, A.; Al-Nuaimi, N.; Abu Al-Rub, R.; Bani-Hani, K.A. Effect of Nanotube Geometry on the Strength and Dispersion of CNT-Cement Composites. J. Nanomater. 2017, 2017, 6927416. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Alansari, M.; Taha, R.; Senouci, A.; Abutaqa, A. Impact of CNTs’ treatment, length and weight fraction on ordinary concrete mechanical properties. Constr. Build. Mater. 2020, 264, 120698. [Google Scholar] [CrossRef]
- Mohsen, M.; Taha, R.; Abu Taqa, A.; Shaat, A. Optimum carbon nanotubes’ content for improving flexural and compressive strength of cement paste. Constr. Build. Mater. 2017, 150, 395–403. [Google Scholar] [CrossRef]
- Sobolkina, A.; Mechtcherine, V.; Khavrus, V.; Maier, D.; Mende, M.; Ritschel, M.; Leonhardt, A. Dispersion of carbon nanotubes and its influence on the mechanical properties of the cement matrix. Cem. Concr. Compos. 2012, 34, 1104–1113. [Google Scholar] [CrossRef]
- Swinehart, D.F. The beer-lambert law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- ASTM C348-14; Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2014; 6p.
- ASTM C109/C109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2020; 10p.
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Multi-scale mechanical and fracture characteristics and early-age strain capacity of high performance carbon nanotube/cement nanocomposites. Cem. Concr. Compos. 2010, 32, 110–115. [Google Scholar] [CrossRef]
- Chandra, S.; Björnström, J. Influence of cement and superplasticizers type and dosage on the fluidity of cement mortars—Part I. Cem. Concr. Res. 2002, 32, 1605–1611. [Google Scholar] [CrossRef]
- Metaxa, Z.; Konsta-Gdoutos, M.; Shah, S. Carbon Nanotubes Reinforced Concrete. Nanotechnology of Concrete: The Next Big Thing Is Small; ACI Special Publications: Farmington Hills, MI, USA, 2009; Volume 267, pp. 11–20. [Google Scholar]
- Al-Rub, R.K.A.; Ashour, A.I.; Tyson, B.M. On the aspect ratio effect of multi-walled carbon nanotube reinforcements on the mechanical properties of cementitious nanocomposites. Constr. Build. Mater. 2012, 35, 647–655. [Google Scholar] [CrossRef]

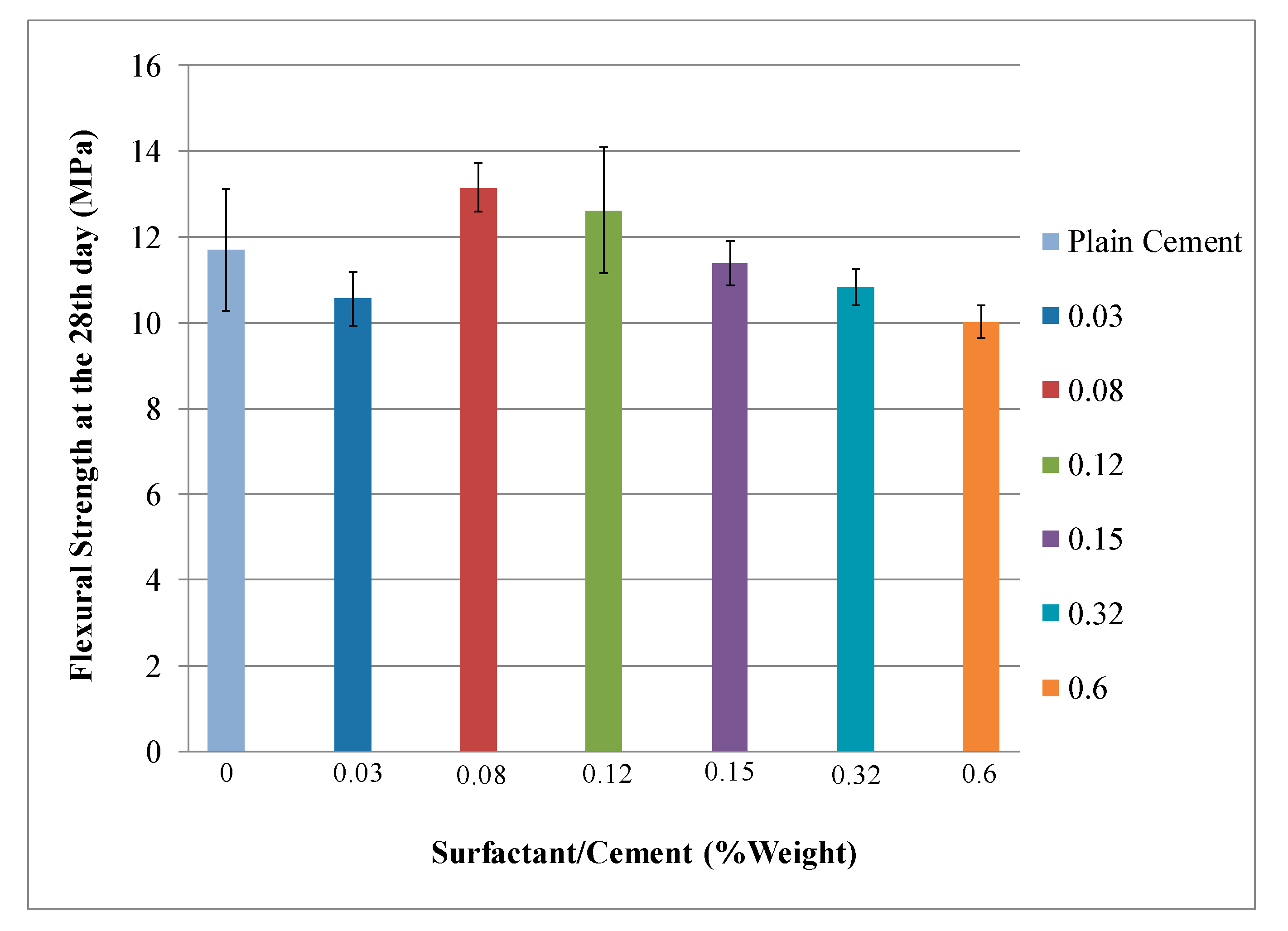


| Aspect Ratio | Purity (wt. %) | Outside Diameter (nm) | Inside Diameter (nm) | Length (μm) | SSA m2/g | Ash (wt. %) | Color | Electrical Conductivity (s/cm) | True Density (g/cm3) | Manufacturing Method |
|---|---|---|---|---|---|---|---|---|---|---|
| 1333 | >95 | 10–20 nm | 5–10 nm | 10–30 um | >200 | <1.5 | Black | >100 | ~2.1 | CVD |
| Batch | Surfactant/Cement Weight Fraction (%) | Specimen No. | w/c | Flexural Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| 0 | 0 (Conrol) | Sa1-Sa5 | 0.4 | Fs = 0.0028 P | Cs = P/A |
| 1 | 0.03 | S1-S6 | |||
| 2 | 0.08 | S7-S12 | |||
| 3 | 0.12 | S13-S18 | |||
| 4 | 0.15 | S19-S24 | |||
| 5 | 0.32 | S25-S30 | |||
| 6 | 0.60 | S31-S36 |
| Solution | CNT/Water (%) | Surfactant/Water (%) | Water Weight (gm) |
|---|---|---|---|
| 1 | 0.075 | 0.3 | 1000 |
| 2 | 0.225 | 0.9 | 1000 |
| 3 | 0.375 | 1.5 | 1000 |
| 4 | 0.625 | 2.5 | 1000 |
| Solution | Stage 1 | Stage 2 | Stage 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Blank (Water + CNTs) | Before Sonication (Water + CNTs + S) | After Sonication (Water + CNTs + S) | ||||||
| Abs1 | Conc. (%) | Abs. 2 | Abs. 2–Abs. 1 | Conc. (%) | Abs. 3 | Abs. 3–Abs. 1 | Conc. (%) | |
| 1 | 0.0082 | 0.0110 | 0.0580 | 0.0498 | 0.3262 | 0.0417 | 0.0335 | 0.1926 |
| 2 | 0.0089 | 0.0111 | 0.1260 | 0.1171 | 0.8779 | 0.0682 | 0.0593 | 0.4041 |
| 3 | 0.0094 | 0.0111 | 0.2140 | 0.2046 | 1.5951 | 0.0614 | 0.0520 | 0.3443 |
| 4 | 0.0097 | 0.0112 | 0.3340 | 0.3243 | 2.5762 | 0.0551 | 0.0454 | 0.2902 |
| Solution | CNT/Water (wt. %) | Original Concentration (wt. %) | Predicted Concentration Using Chemical Analysis Method (wt. %) | Error (%) |
|---|---|---|---|---|
| 1 | 0.075 | 0.30 | 0.33 | 8.74 |
| 2 | 0.225 | 0.90 | 0.88 | 2.46 |
| 3 | 0.375 | 1.50 | 1.60 | 6.34 |
| 4 | 0.625 | 2.50 | 2.58 | 3.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsen, M.O.; Abdel-Jaber, M.; Al-Nuaimi, N.A.; Senouci, A.; Taha, R.A. Determination of Surfactant Content for Optimum Strength of Multi-Walled Carbon Nanotube Cementitious Composites. Sustainability 2022, 14, 12433. https://doi.org/10.3390/su141912433
Mohsen MO, Abdel-Jaber M, Al-Nuaimi NA, Senouci A, Taha RA. Determination of Surfactant Content for Optimum Strength of Multi-Walled Carbon Nanotube Cementitious Composites. Sustainability. 2022; 14(19):12433. https://doi.org/10.3390/su141912433
Chicago/Turabian StyleMohsen, Mohd O., Mu’tasim Abdel-Jaber, Nasser A. Al-Nuaimi, Ahmed Senouci, and Ramzi A. Taha. 2022. "Determination of Surfactant Content for Optimum Strength of Multi-Walled Carbon Nanotube Cementitious Composites" Sustainability 14, no. 19: 12433. https://doi.org/10.3390/su141912433






