Utilization of Selected Nanoparticles (Ag2O and MnO2) for the Production of High-Quality and Environmental-Friendly Gasoline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engine Setup
2.2. Fuel Samples
2.3. Emissions Measurement
2.4. Experimental Steps
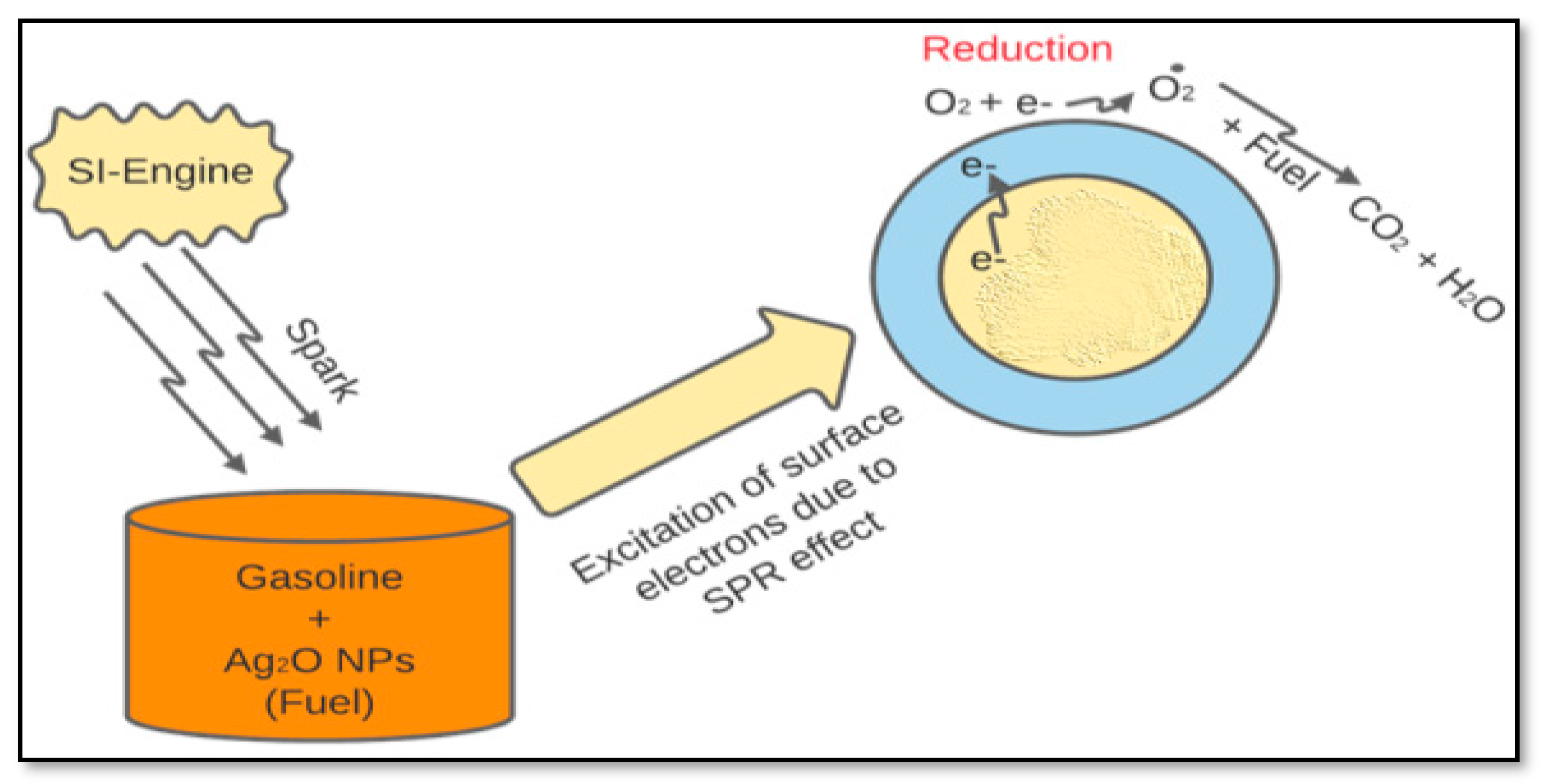
2.5. Nanoparticles Mechanism
3. Results and Discussion
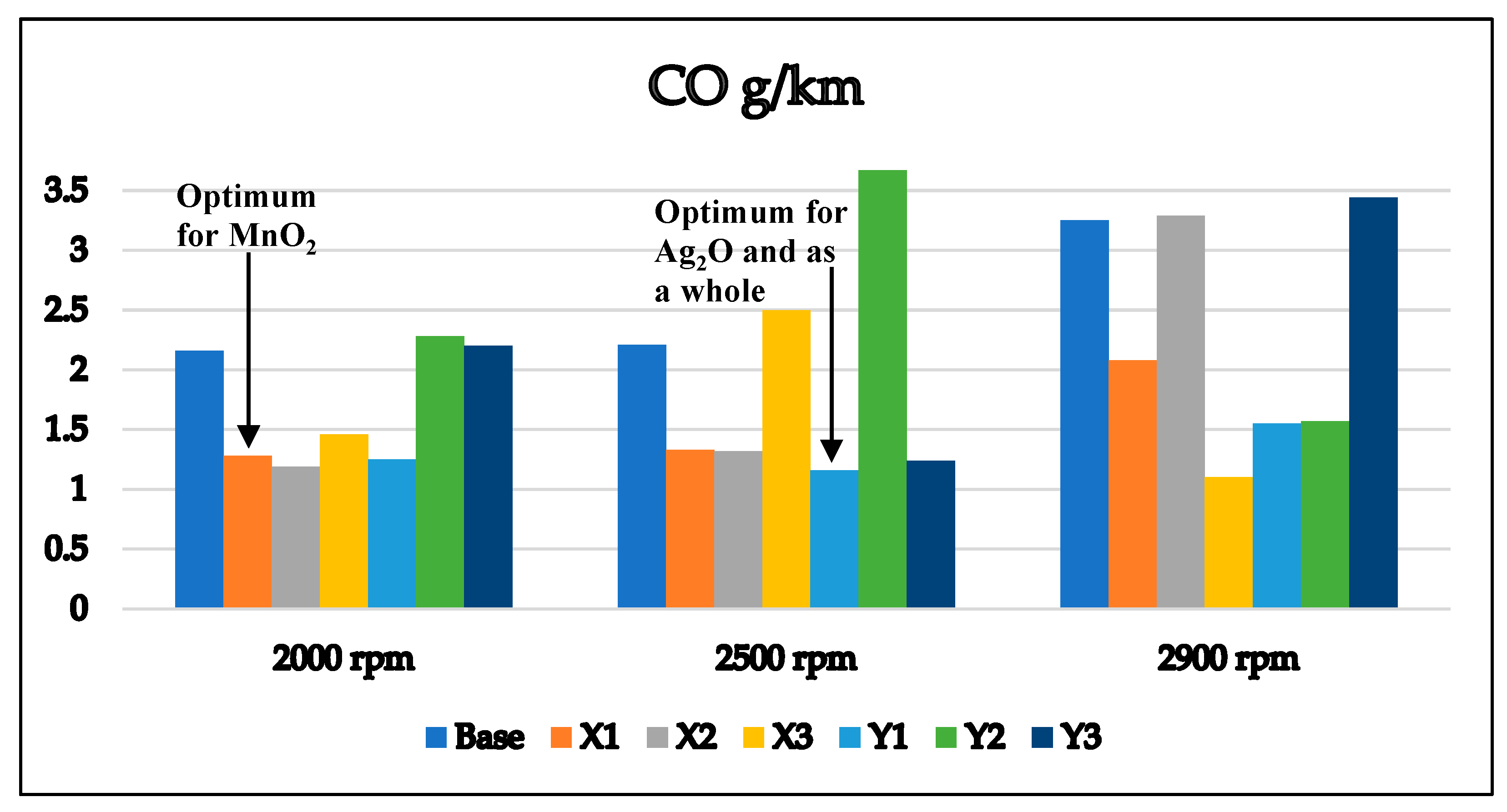
3.1. Exhaust Emissions Analysis
3.1.1. Base Gasoline RON 80 Free Additives
3.1.2. Gasoline RON 80 with MnO2 and Ag2O
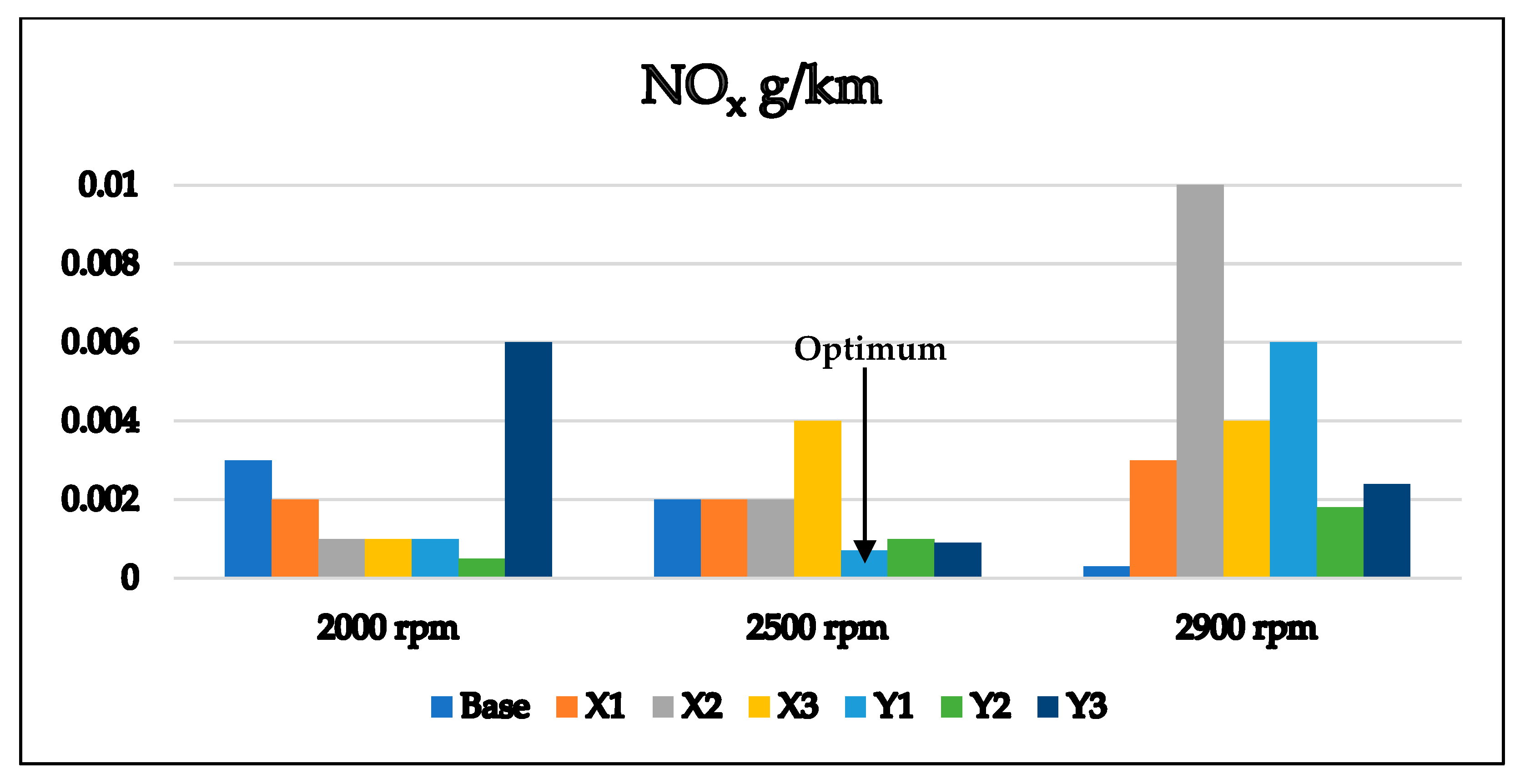
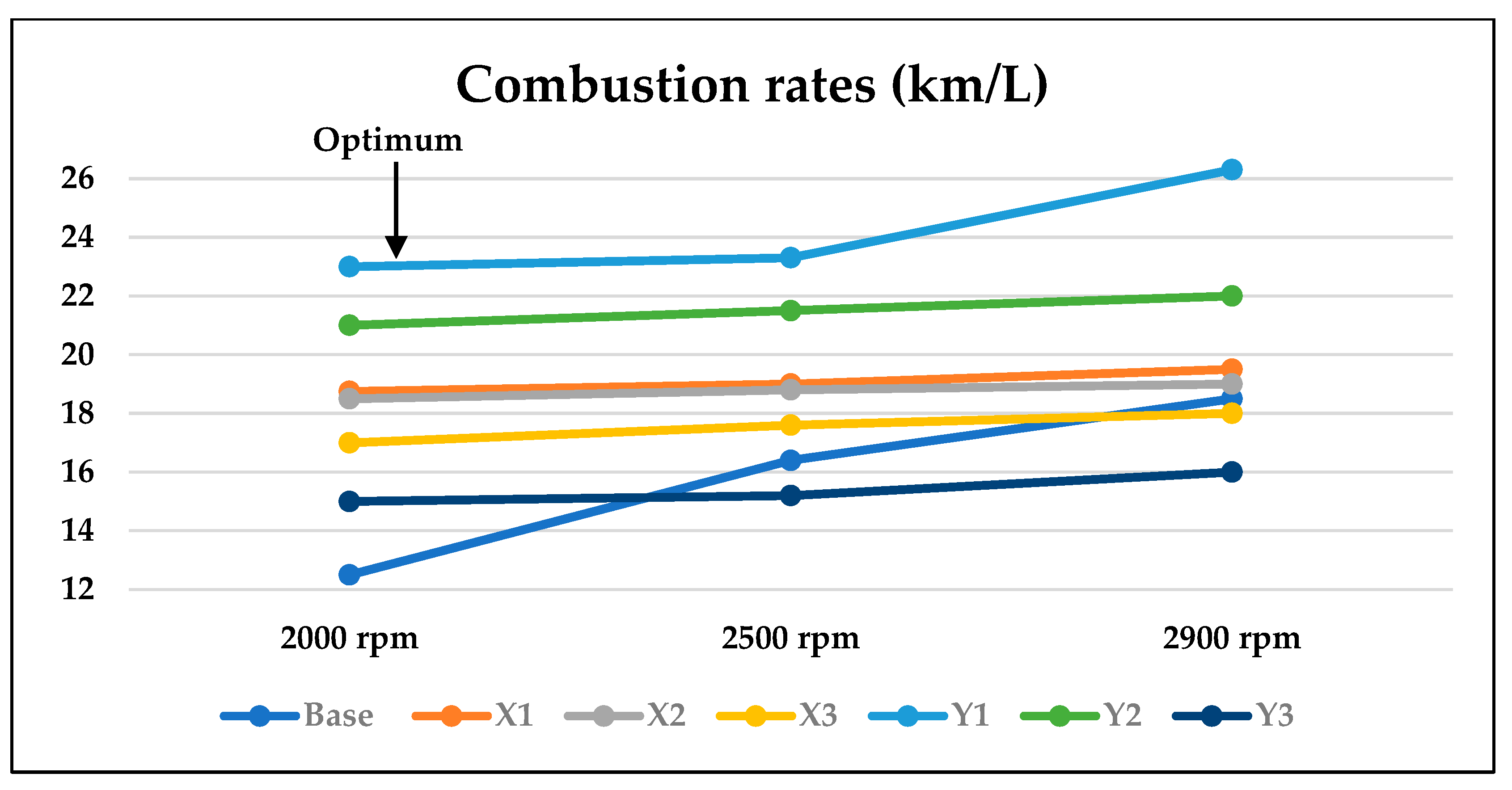
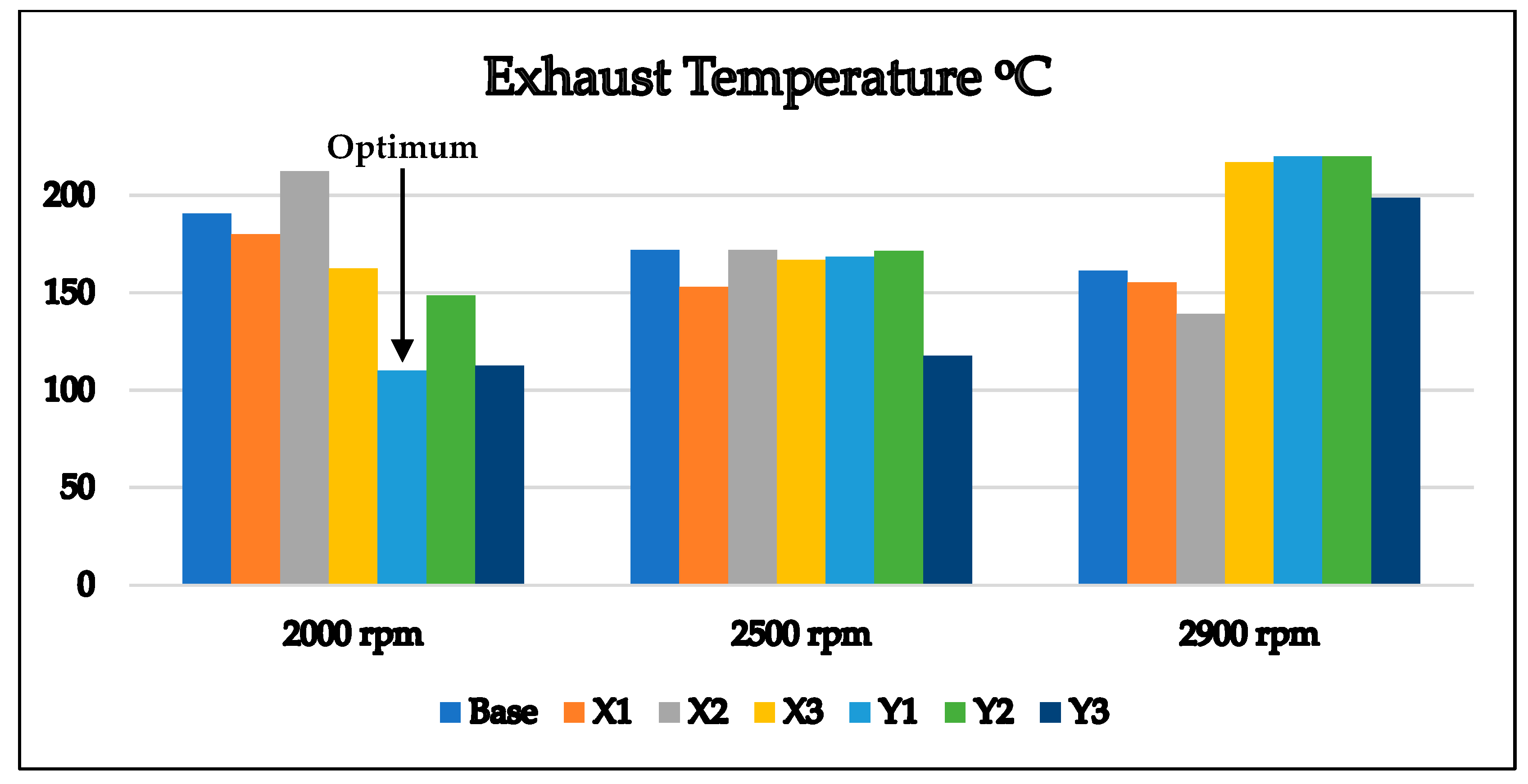
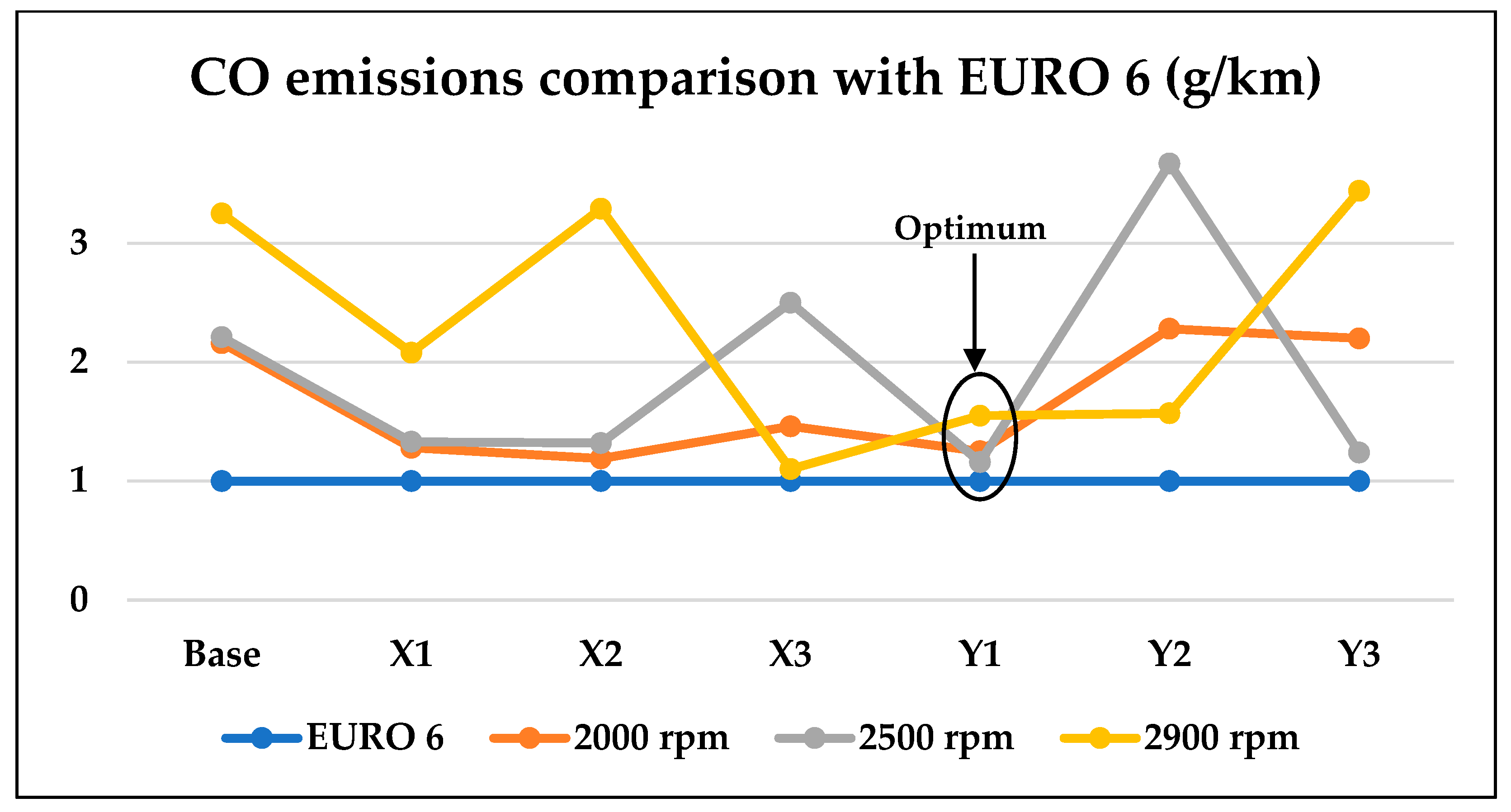
3.2. Optimum Sample and the Comparison with EURO Standard Limits for Gasoline Engine’s Exhaust
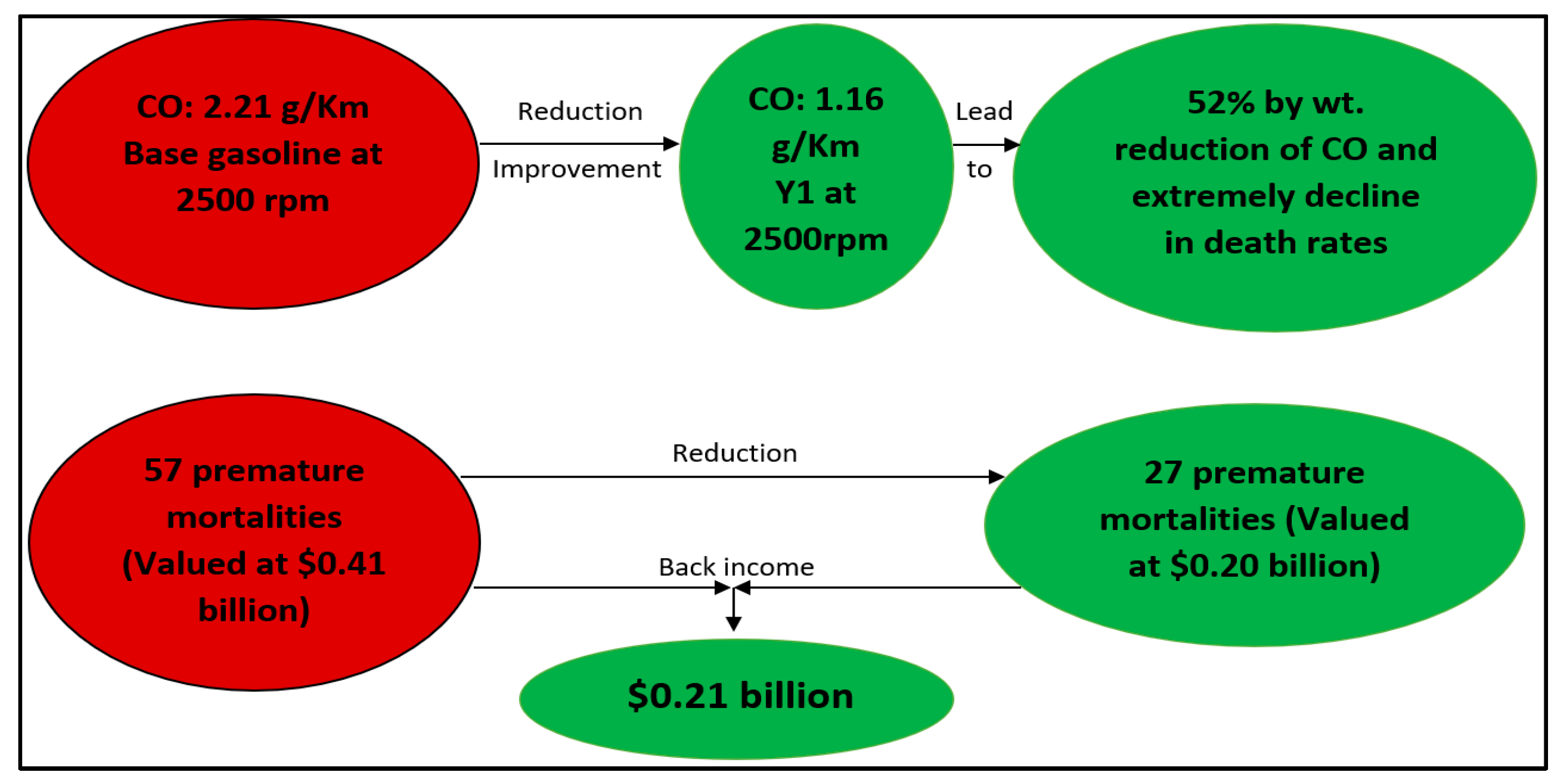
3.3. Effects on Health and Environment
4. Conclusions
- Its CO emissions are lower at all speeds than other samples meeting the EURO 6 requirements, and its CO2 emissions are quite low, in contrast to the X1 sample, which has high CO2 emissions.
- Combustion rates of the Y1 sample are recording the highest rates among all samples.
- Lowest exhaust temperatures in the most commonly used engine speeds (2000–2500) rpm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J. Air pollution and health. Lancet Public Health 2017, 2, e4–e5. [Google Scholar] [CrossRef]
- Nassar, Y.; Aissa, K.; Alsadi, S. Air pollution sources in Libya. Res. Rev. J Ecol. Environ. Sci. 2017, 6, 63–79. [Google Scholar]
- Seibert, R.; Nikolova, I.; Volná, V.; Krejčí, B.; Hladký, D. Air pollution sources’ contribution to PM2. 5 concentration in the northeastern part of the Czech Republic. Atmosphere 2020, 11, 522. [Google Scholar] [CrossRef]
- Khalife, E.; Tabatabaei, M.; Demirbas, A.; Aghbashlo, M. Impacts of additives on performance and emission characteristics of diesel engines during steady state operation. Prog. Energy Combust. Sci. 2017, 59, 32–78. [Google Scholar] [CrossRef]
- Saxena, V.; Kumar, N.; Saxena, V.K. A comprehensive review on combustion and stability aspects of metal nanoparticles and its additive effect on diesel and biodiesel fuelled CI engine. Renew. Sustain. Energy Rev. 2017, 70, 563–588. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tsuang, B.-J.; Chiang, C.-J.; Ku, K.-C.; Tseng, J.-S.; Yang, T.-Y.; Hsu, K.-H.; Chen, K.-C.; Yu, S.-L.; Lee, W.-C.; et al. The Relationship Between Air Pollution and Lung Cancer in Nonsmokers in Taiwan. J. Thorac. Oncol. 2019, 14, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Sarkar, A.; Chakraborty, J.P. Influence of Alternate Fuels on the Performance and Emission from Internal Combustion Engines and Soot Particle Collection Using Thermophoretic Sampler: A Comprehensive Review. Waste Biomass-Valorization 2018, 10, 2801–2823. [Google Scholar] [CrossRef]
- McLaughlin, S.B. Effects of Air Pollution on Forests: A Critical Review; PB-86-175544/XAB; Oak Ridge National Lab., TN (USA): Oak Ridge, TN, USA, 1985. [Google Scholar]
- Gouveia, N.; Junger, W.L.; Romieu, I.; Cifuentes, L.A.; de Leon, A.P.; Vera, J.; Strappa, V.; Hurtado-Díaz, M.; Miranda-Soberanis, V.; Rojas-Bracho, L.; et al. Effects of air pollution on infant and children respiratory mortality in four large Latin-American cities. Environ. Pollut. 2018, 232, 385–391. [Google Scholar] [CrossRef]
- Wu, I.-P.; Liao, S.-L.; Lai, S.-H.; Wong, K.-S. The respiratory impacts of air pollution in children: Global and domestic (Taiwan) situation. Biomed. J. 2021, 45, 88–94. [Google Scholar] [CrossRef]
- WHO. Air Pollution and Child Health: Prescribing Clean Air: Summary; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Goldizen, F.C.; Sly, P.D.; Knibbs, L.D. Respiratory effects of air pollution on children. Pediatr. Pulmonol. 2015, 51, 94–108. [Google Scholar] [CrossRef]
- Tm, A.F.; A Mazen, O.; Ashour, I. An Experimental Study on the Influence of Ethanol and Automotive Gasoline Blends. J. Pet. Environ. Biotechnol. 2017, 08, 1–6. [Google Scholar] [CrossRef]
- Yu, X.; Guo, Z.; Sun, P.; Wang, S.; Li, A.; Yang, H.; Li, Z.; Liu, Z.; Li, J.; Shang, Z. Investigation of combustion and emissions of an SI engine with ethanol port injection and gasoline direct injection under lean burn conditions. Energy 2019, 189, 116231. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Huang, Y.; Shi, W.; Guo, Z.; Li, Z.; Du, Y.; Jin, Z.; Li, D.; Wang, T.; et al. Experimental study on the effects of EGR on combustion and emission of an SI engine with gasoline port injection plus ethanol direct injection. Fuel 2021, 305, 121412. [Google Scholar] [CrossRef]
- Gadalla, M.A.; Mazen, O.M.; Aboul-Fotouh, T.M.; Ashour, F.H.; ElAzab, H.A. Effect of using Nanoparticle based Diesel Fuel on Enhancement of Performance and Emissions of Diesel Engines. Nanosci. Nanotechnol.-Asia 2020, 10, 1. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altıparmak, D. Influence of metallic based fuel additives on performance and exhaust emissions of diesel engine. Energy Convers Manag. 2011, 52, 60–65. [Google Scholar] [CrossRef]
- Varon, J.; Marik, P.E.; Robert, E.F., Jr.; Gueler, A. Carbon monoxide poisoning: A review for clinicians. J. Emerg. Med. 1999, 17, 87–93. [Google Scholar] [CrossRef]
- Svedberg, U.R.A.; Högberg, H.-E.; Högberg, J.; Galle, B.O. Emission of hexanal and carbon monoxide from storage of wood pellets, a potential occupational and domestic health hazard. Ann. Occup. Hyg. 2004, 48, 339–349. [Google Scholar]
- Svedberg, U.; Samuelsson, J.; Melin, S. Hazardous off-gassing of carbon monoxide and oxygen depletion during ocean transportation of wood pellets. Ann. Occup. Hyg. 2008, 52, 259–266. [Google Scholar] [CrossRef]
- Mirhashemi, F.S.; Sadrnia, H. NOX emissions of compression ignition engines fueled with various biodiesel blends: A review. J. Energy Inst. 2019, 93, 129–151. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A comprehensive review on the environmental impacts of diesel/biodiesel additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Shaafi, T.; Sairam, K.; Gopinath, A.; Kumaresan, G.; Velraj, R. Effect of dispersion of various nanoadditives on the performance and emission characteristics of a CI engine fuelled with diesel, biodiesel and blends—A review. Renew. Sustain. Energy Rev. 2015, 49, 563–573. [Google Scholar] [CrossRef]
- e Brito, L.R.; da Silva, M.P.; Rohwedder, J.J.; Pasquini, C.; Honorato, F.A.; Pimentel, M.F. Determination of detergent and dispensant additives in gasoline by ring-oven and near infrared hypespectral imaging. Anal. Chim. Acta 2015, 863, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mda Silva, P.F.; Brito, L.R.e.; Honorato, F.A.; Paim, A.P.S.; Pasquini, C.; Pimentel, M.F. Classification of gasoline as with or without dispersant and detergent additives using infrared spectroscopy and multivariate classification. Fuel 2014, 116, 151–157. [Google Scholar] [CrossRef]
- Amirabedia, M.; Jafarmadar, S.; Khalilarya, S.; Kheyrollahi, J. Experimental comparison the effect of Mn2O3 and Co3O4 nano additives on the performance and emission of SI gasoline fueled with mixture of ethanol and gasoline. Int. J. Eng. 2019, 32, 769–776. [Google Scholar]
- Khond, V.W.; Kriplani, V. Effect of nanofluid additives on performances and emissions of emulsified diesel and biodiesel fueled stationary CI engine: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 59, 1338–1348. [Google Scholar] [CrossRef]
- Konur, O. Nanotechnology Applications in Diesel Fuels and Related Research Fields: A Review of the Research. Biodiesel Fuels 2021, 1, 89–110. [Google Scholar]
- Taghavifar, H.; Kaleji, B.K.; Kheyrollahi, J. Application of composite TNA nanoparticle with bio-ethanol blend on gasoline fueled SI engine at different lambda ratios. Fuel 2020, 277, 118218. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Moghaddam, H.; Pirouzfar, V.; Fayyazbakhsh, A.; Su, C.-H. Improving the gasoline properties by blending butanol-Al2O3 to optimize the engine performance and reduce air pollution. Energy 2020, 218, 119442. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kaviti, A.K.; Mehra, R.; Mer, K. Progress in performance analysis of ethanol-gasoline blends on SI engine. Renew. Sustain. Energy Rev. 2017, 69, 324–340. [Google Scholar] [CrossRef]
- Tibaquirá, J.E.; Huertas, J.I.; Ospina, S.; Quirama, L.F.; Niño, J.E. The Effect of Using Ethanol-Gasoline Blends on the Mechanical, Energy and Environmental Performance of In-Use Vehicles. Energies 2018, 11, 221. [Google Scholar] [CrossRef]
- Iodice, P.; Senatore, A.; Langella, G.; Amoresano, A. Effect of ethanol–gasoline blends on CO and HC emissions in last generation SI engines within the cold-start transient: An experimental investigation. Appl. Energy 2016, 179, 182–190. [Google Scholar] [CrossRef]
- Doğan, B.; EROL, D.; Yaman, H.; Kodanli, E. The effect of ethanol-gasoline blends on performance and exhaust emissions of a spark ignition engine through exergy analysis. Appl. Therm. Eng. 2017, 120, 433–443. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Pirouzfar, V. Comprehensive overview on diesel additives to reduce emissions, enhance fuel properties and improve engine performance. Renew. Sustain. Energy Rev. 2017, 74, 891–901. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2019, 120, 1350–1396. [Google Scholar] [CrossRef]
- Akbari, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano-Structures Nano-Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- Iqbal, S.; Fakhar-E-Alam, M.; Akbar, F.; Shafiq, M.; Atif, M.; Amin, N.; Ismail, M.; Hanif, A.; Farooq, W.A. Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019, 1189, 203–209. [Google Scholar] [CrossRef]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.-U.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Nasir, J.A.; Zahra, S.A.; Shahbaz, A.; Uddin, S.; Hameed, S.; Gul, F.; Kanwal, S.; Mahmood, T. Environmentally friendly green approach for the fabrication of silver oxide nanoparticles: Characterization and diverse biomedical applications. Microsc. Res. Tech. 2020, 83, 1308–1320. [Google Scholar] [CrossRef]
- Fiat. Available online: https://www.ultimatespecs.com/car-specs/Fiat/21091/Fiat-128-Saloon.html (accessed on 1 June 2022).
- Jia, L.; Yu, Y.; Li, Z.-P.; Qin, S.-N.; Guo, J.-R.; Zhang, Y.-Q.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahed, M.S.; Hefny, M.M.; Abd-Elmaksoud, S.; El-Liethy, M.A.; Kamel, M.A.; El-Kalliny, A.S.; Hamza, I.A. Removal of chemical and microbial water pollutants by cold plasma combined with Ag/TiO2-rGO nanoparticles. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Hajjari, M.; Ardjmand, M.; Tabatabaei, M. Experimental investigation of the effect of cerium oxide nanoparticles as a combustion-improving additive on biodiesel oxidative stability: Mechanism. RSC Adv. 2014, 4, 14352–14356. [Google Scholar] [CrossRef]
- Kumar, M.V.; Babu, A.V.; Kumar, P.R. Influence of metal-based cerium oxide nanoparticle additive on performance, combustion, and emissions with biodiesel in diesel engine. Environ. Sci. Pollut. Res. 2019, 26, 7651–7664. [Google Scholar] [CrossRef]
- Mei, D.; Li, X.; Wu, Q.; Sun, P. Role of Cerium Oxide Nanoparticles as Diesel Additives in Combustion Efficiency Improvements and Emission Reduction. J. Energy Eng. 2016, 142, 04015050. [Google Scholar] [CrossRef]
- Chemical Reactions. Available online: Chemequations.com (accessed on 25 July 2022).
- Emission Standards. Available online: https://dieselnet.com/standards/eu/ld.php#stds (accessed on 20 May 2022).
- Charman, N.; Edmonds, N.; Egyed, M.; Eng, L.; Ling, B.; Matz, C.; Rouleau, M. Human Health Risk Assessment for Gasoline Exhaust; Health Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
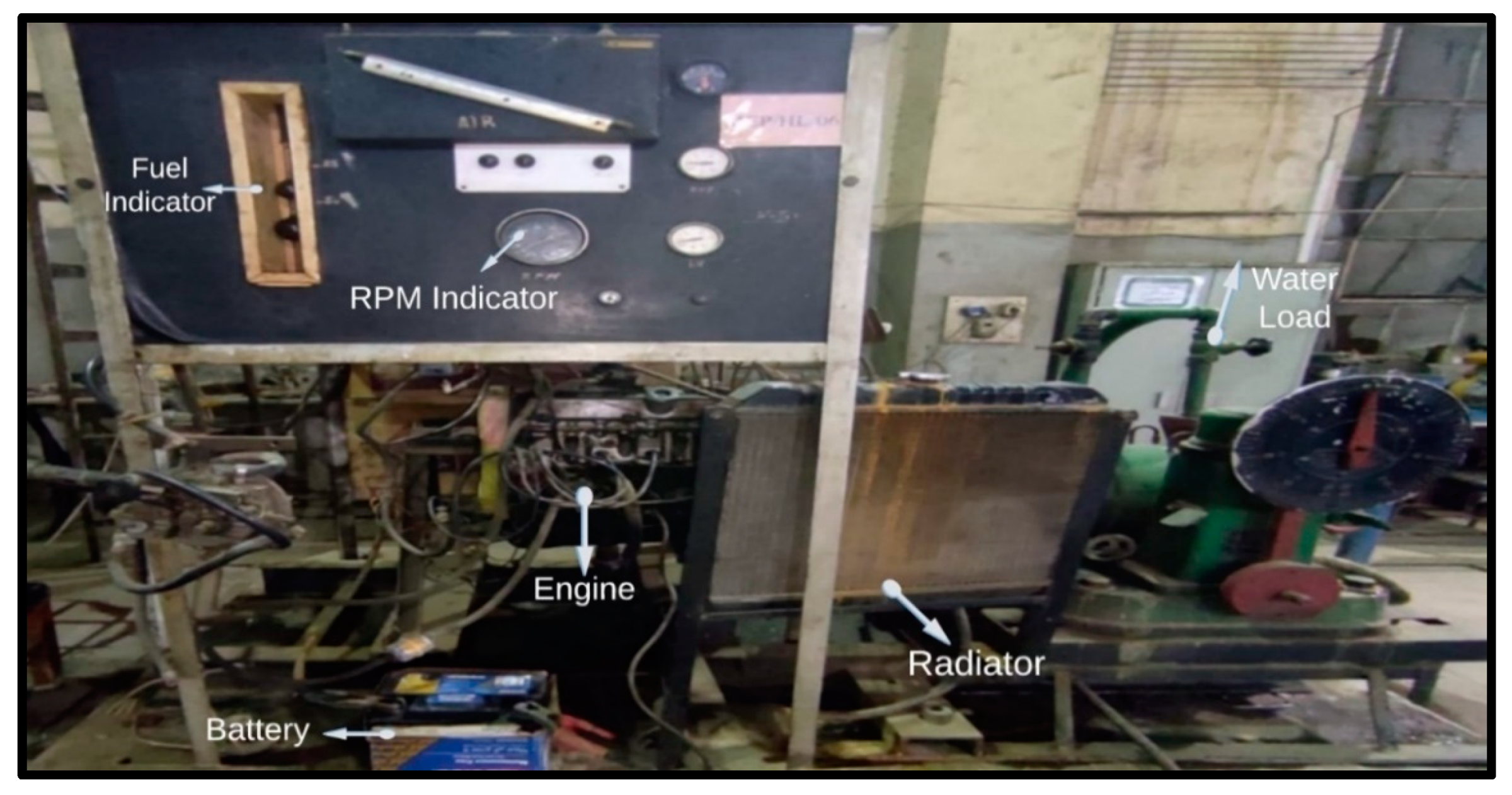

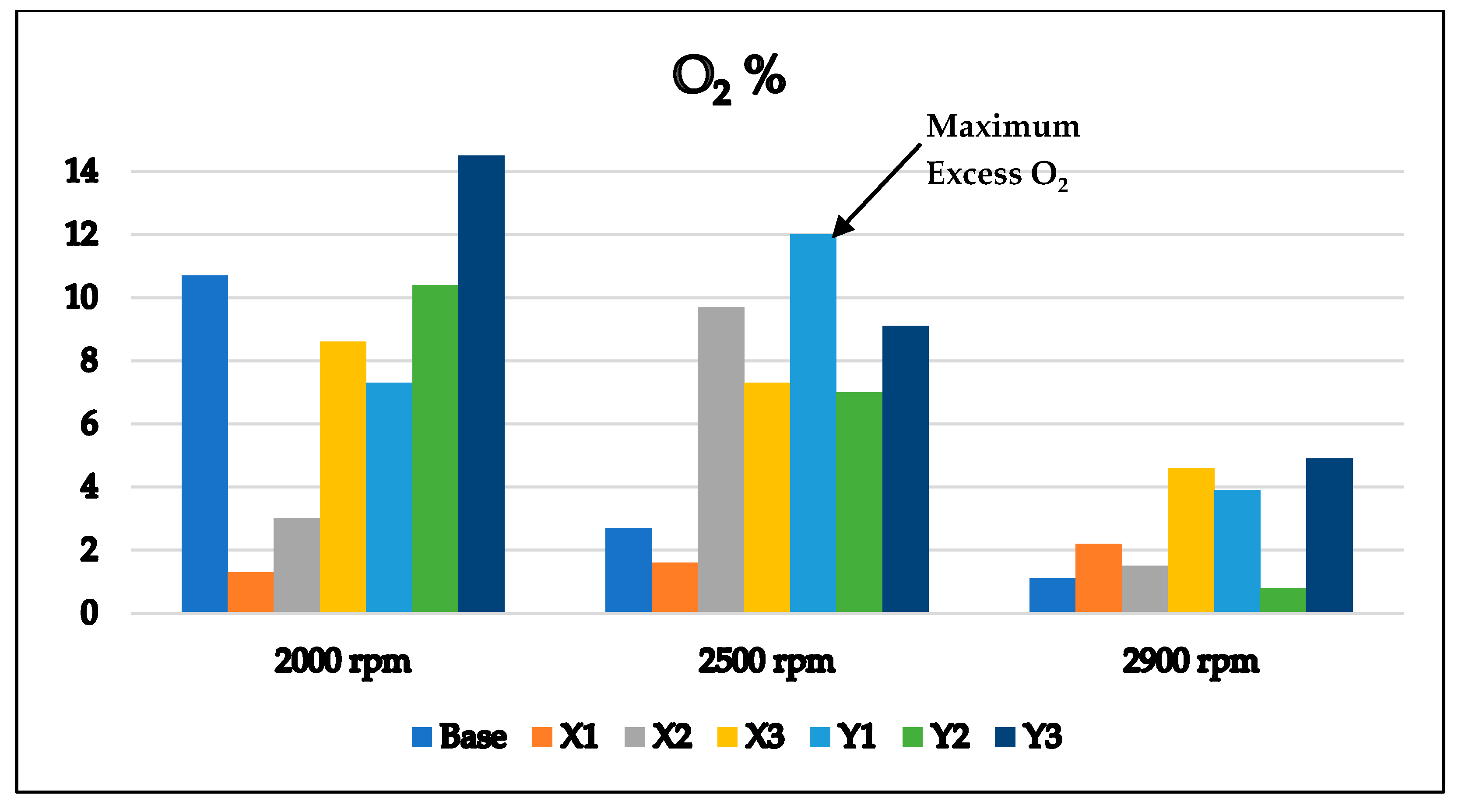
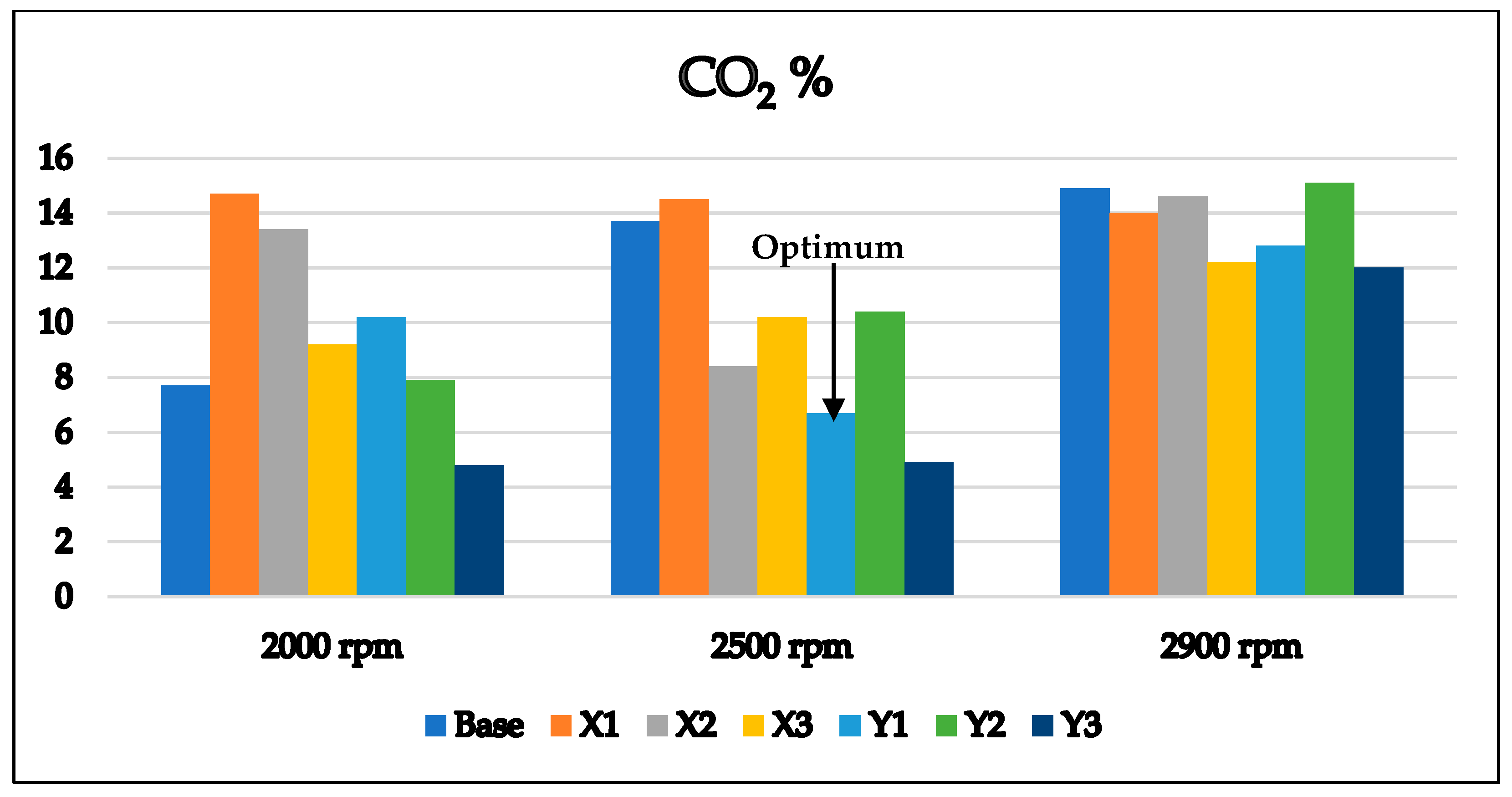
| Engine | Fiat 128 |
|---|---|
| Engine type—number of cylinders | Inline—4 |
| Fuel type | Gasoline |
| Coolant | Water |
| Engine alignment | Transverse |
| Engine capacity (cc) | 1116 cc |
| No of valves | 8 |
| Compression ratio | 8.88 |
| Horsepower—maximum power | 65 hp—6000 rpm |
| No of speeds (transmissions) | Four speeds manual |
| Nano-Additive | MnO2 | Ag2O |
|---|---|---|
| Appearance (color) | Brown | Deep brown to black |
| Appearance (form) | Powder | Powder |
| Approximate Avg. Size | 70 nm | 70 nm |
| Shape | Quasi-Spherical-like shape | Spherical-like shape |
| Gasoline RON 80 (1 L Equivalent to 738 Gram) | |||
|---|---|---|---|
| Nano-Additives Percentage by Mass | |||
| Percentage | 0.05% | 0.10% | 0.15% |
| Mass in gram | 0.37 | 0.74 | 1.10 |
| Gasoline RON 80 Exhaust Emissions | |||
|---|---|---|---|
| 2000 rpm | 2500 rpm | 2900 rpm | |
| O2 (%) | 10.70 | 2.70 | 1.10 |
| CO (g/km) | 2.16 | 2.21 | 3.25 |
| CO2 (%) | 7.70 | 13.70 | 14.90 |
| NOx (g/km) | 0.00300 | 0.00200 | 0.00028 |
| Exhaust Temperature (°C) | 190.60 | 171.90 | 161.30 |
| Combustion rate (km/L) | 12.5 | 16.4 | 18.5 |
| Emissions | EURO 1 (1992) | EURO 2 (1996) | EURO 3 (2000) | EURO 4 (2005) | EURO 5&6 (2009–2014) |
|---|---|---|---|---|---|
| CO g/km | 2.73 | 2.20 | 2.30 | 1.14 | 1.00 |
| NOx g/km | 0.30 | 0.30 | 0.15 | 0.09 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattah, A.A.; Aboul-Fotouh, T.M.; Fattah, K.A.; Mohammed, A.H. Utilization of Selected Nanoparticles (Ag2O and MnO2) for the Production of High-Quality and Environmental-Friendly Gasoline. Sustainability 2022, 14, 12513. https://doi.org/10.3390/su141912513
Fattah AA, Aboul-Fotouh TM, Fattah KA, Mohammed AH. Utilization of Selected Nanoparticles (Ag2O and MnO2) for the Production of High-Quality and Environmental-Friendly Gasoline. Sustainability. 2022; 14(19):12513. https://doi.org/10.3390/su141912513
Chicago/Turabian StyleFattah, Ahmed A., Tarek M. Aboul-Fotouh, Khaled A. Fattah, and Aya H. Mohammed. 2022. "Utilization of Selected Nanoparticles (Ag2O and MnO2) for the Production of High-Quality and Environmental-Friendly Gasoline" Sustainability 14, no. 19: 12513. https://doi.org/10.3390/su141912513





