Polychlorinated Biphenyls Interactions with Water—Characterization Based on the Analysis of Non-Covalent Interactions and Energy Partitioning
Abstract
:1. Introduction
2. Computational Methodology
2.1. Structure Description and Energy Partitioning
- electrostatic energy defined as Coulombic force between the molecular skeletons and electron densities of the non-interacting, unperturbed monomers, without polarization effects,
- Pauli repulsion exchange energy, which is a purely quantum-mechanical effect related to the orbital overlap, calculated using the unperturbed wavefunctions of the monomers,
- induction term gathering the effects of mutual polarization of the monomers on the electron density distribution within them, including corrections to the electrostatic and exchange terms,
- dispersion energy related to the instantaneous multipoles—fluctuations of the electron density—between the monomers.
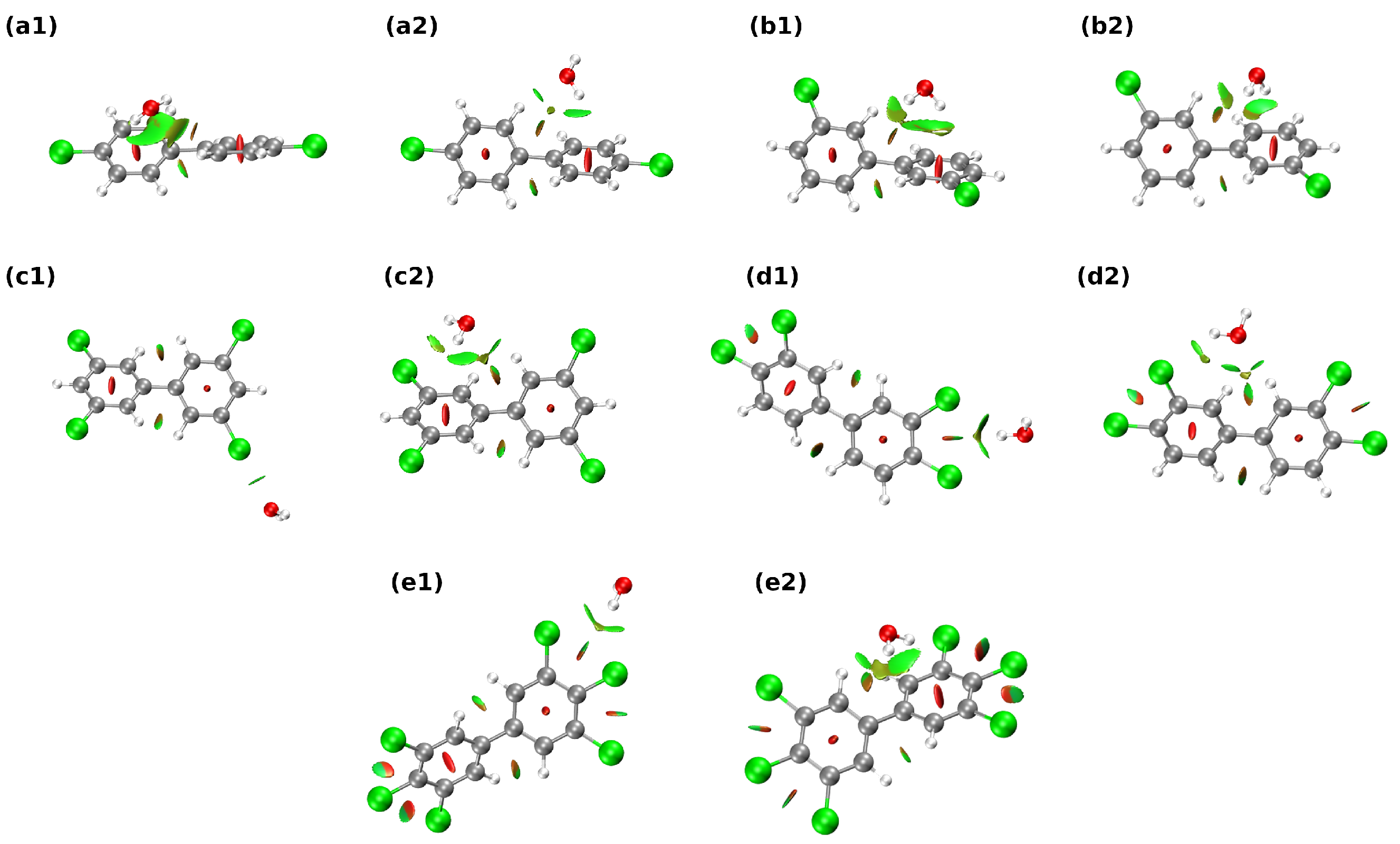
2.2. Non-Covalent Interactions (NCI) Description
3. Results and Discussion
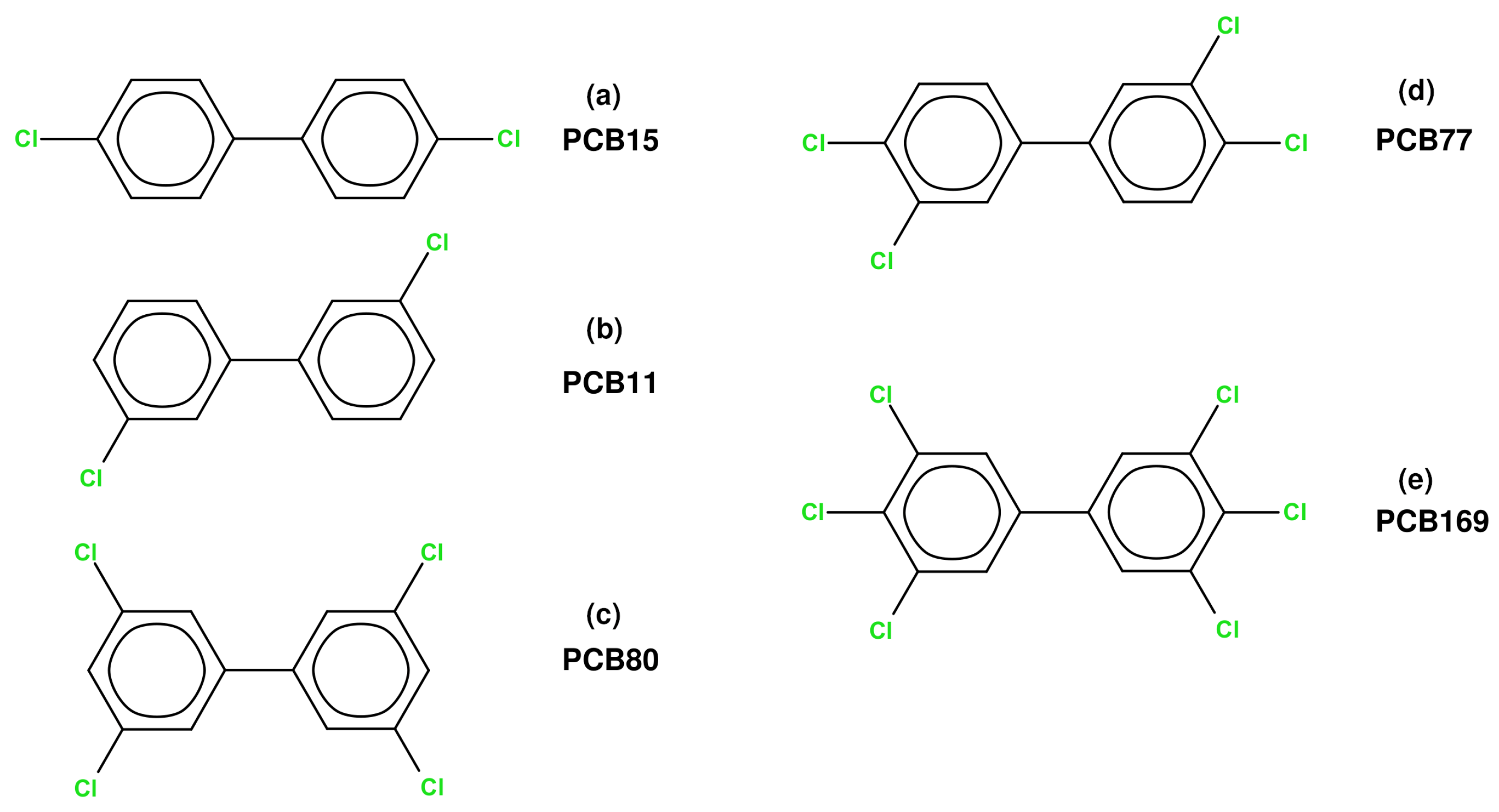
3.1. Non-Covalent Bonding Analysis of the Studied 1:1 PCB Complexes
3.2. Interaction Energy Analysis in 1:1 PCB Complexes with Water, Chlorine, and Chlorine Dioxide
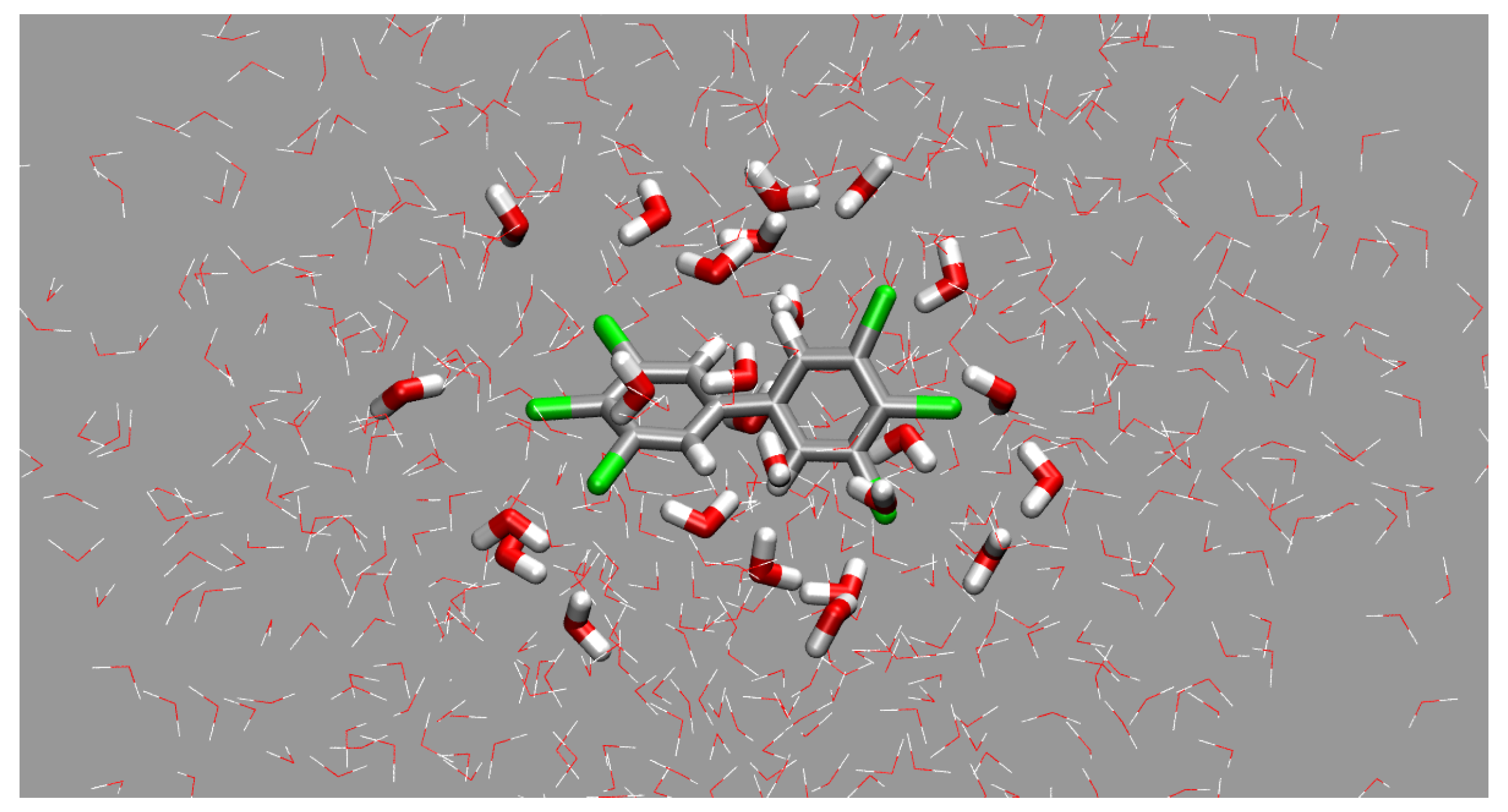
3.3. Interactions between PCBs and Their Solvation Shells
4. Conclusions
- PCBs do not interact with water and accumulate in non-polar environments,
- the relatively inert C–Cl bonds must be activated.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIM | Atoms In Molecules |
| BCP | Bond Critical Point |
| GGA | Generalized Gradient Approximation |
| HB | Hydrogen Bond |
| MD | Molecular Dynamics |
| NCI | Non-Covalent Interactions |
| PCB | Polychlorinated Biphenyl |
| POP | Persistent Organic Pollutant |
| RDG | Reduced Density Gradient |
| RI-MP2 | Second-order Møller-Plesset perturbation theory with Resolution of Identity acceleration |
| SAPT | Symmetry-Adapted Perturbation Theory |
References
- United Nations Stockholm Convention on Persistent Organic Pollutants. 2001. Available online: http://chm.pops.int/Portals/0/Repository/convention_text/UNEP-POPS-COP-CONVTEXT-FULL.English.PDF (accessed on 19 August 2022).
- The 12 Initial POPs under the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs (accessed on 19 August 2022).
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.D.; Kaley, R.G. Applications of polychlorinated biphenyls. Environ. Sci. Poll. Res. 2010, 18, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dong, J.; Huang, Z.; Liu, C.; Qiao, X.; Wang, X.; Zhao, X.; Zheng, B.; Shen, J. Polychlorinated biphenyls in the drinking water source of the Yangtze River: Characteristics and risk assessment. Environ. Sci. Eur. 2020, 32, 29. [Google Scholar] [CrossRef] [Green Version]
- Ergül, H.A.; Erdem, T.; Pelin, E.G.; Tonay, A.M.; Aksan, S.; Öztürk, B. Assessment of Pesticide, Dioxin-like Polychlorinated Biphenyl, and Polycyclic Aromatic Hydrocarbon Existence around Galindez Island, and Comparison with the Organic Pollution Bibliography of the Antarctic Peninsula. Sustainability 2022, 14, 3994. [Google Scholar] [CrossRef]
- European Food Safety Authority. Results of the monitoring of dioxin levels in food and feed. EFSA J. 2010, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Marsan, E.S.; Bayse, C.A. Halogen Bonding Interactions of Polychlorinated Biphenyls and the Potential for Thyroid Disruption. Chem. Eur. J. 2020, 26, 5200–5207. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Limpanuparb, T. Quantum Chemical Investigation of Polychlorinated Dibenzodioxins, Dibenzofurans and Biphenyls: Relative Stability and Planarity Analysis. Molecules 2020, 25, 5697. [Google Scholar] [CrossRef] [PubMed]
- Landeros-Rivera, B.; Jancik, V.; Moreno-Esparza, R.; Martínez Otero, D.; Hernández-Trujillo, J. Non-Covalent Interactions in the Biphenyl Crystal: Is the Planar Conformer a Transition State? Chem. Eur. J. 2021, 27, 11912–11918. [Google Scholar] [CrossRef] [PubMed]
- Chana, A.; Concejero, M.A.; de Frutos, M.; González, M.J.; Herradón, B. Computational Studies on Biphenyl Derivatives. Analysis of the Conformational Mobility, Molecular Electrostatic Potential, and Dipole Moment of Chlorinated Biphenyl: Searching for the Rationalization of the Selective Toxicity of Polychlorinated Biphenyls (PCBs). Chem. Res. Toxicol. 2002, 15, 1514–1526. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Zell, M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Zeit. Anal. Chem. 1980, 302, 20–31. [Google Scholar] [CrossRef]
- Gan, W.; Ge, Y.; Zhong, Y.; Yang, X. The reactions of chlorine dioxide with inorganic and organic compounds in water treatment: Kinetics and mechanisms. Environ. Sci.: Water Res. Technol. 2020, 6, 2287–2312. [Google Scholar] [CrossRef]
- Sorlini, S.; Collivignarelli, C. Trihalomethane formation during chemical oxidation with chlorine, chlorine dioxide and ozone of ten Italian natural waters. Desalination 2005, 176, 103–111. [Google Scholar] [CrossRef]
- Hofmann, R.; Andrews, R.C.; Ranger, G. Water quality and disinfection impact of ClO2 contamination by free chlorine: A case study. J. Environ. Eng. Sci. 2004, 3, 75–80. [Google Scholar] [CrossRef]
- Butcher, J.; Garvey, E.; Bierman, V. Equilibrium partitioning of PCB congeners in the water column: Field measurements from the Hudson River. Chemosphere 1998, 36, 3149–3166. [Google Scholar] [CrossRef]
- Axelman, J.; Broman, D.; Näf, C. Field Measurements of PCB Partitioning between Water and Planktonic Organisms: Influence of Growth, Particle Size, and Solute-Solvent Interactions. Environ. Sci. Technol. 1997, 31, 665–669. [Google Scholar] [CrossRef]
- Urbaniak, M.; Kiedrzyńska, E.; Wyrwicka, A.; Zieliński, M.; Mierzejewska, E.; Kiedrzyński, M.; Kannan, K.; Zalewski, M. An ecohydrological approach to the river contamination by PCDDs, PCDFs and dl-PCBs—concentrations, distribution and removal using phytoremediation techniques. Sci. Rep. 2019, 9, 19310. [Google Scholar] [CrossRef] [Green Version]
- Hawthorne, S.B.; Grabanski, C.B.; Miller, D.J.; Arp, H.P.H. Improving Predictability of Sediment-Porewater Partitioning Models using Trends Observed with PCB-Contaminated Field Sediments. Environ. Sci. Technol. 2011, 45, 7365–7371. [Google Scholar] [CrossRef]
- Di, P.; Chang, D.P.Y.; Dwyer, H.A. Modeling of Polychlorinated Biphenyl Removal from Contaminated Soil Using Steam. Environ. Sci. Technol. 2002, 36, 1845–1850. [Google Scholar] [CrossRef]
- Fang, F.; Chu, S.; Hong, C.S. Air-Water Henry’s Law Constants for PCB Congeners: Experimental Determination and Modeling of Structure-Property Relationship. Anal. Chem. 2006, 78, 5412–5418. [Google Scholar] [CrossRef]
- Chen, J.; Harner, T.; Schramm, K.W.; Quan, X.; Xue, X.; Kettrup, A. Quantitative relationships between molecular structures, environmental temperatures and octanol—Air partition coefficients of polychlorinated biphenyls. Comput. Biol. Chem. 2003, 27, 405–421. [Google Scholar] [CrossRef]
- Phillips, K.L.; Sandler, S.I.; Greene, R.W.; Di Toro, D.M. Quantum Mechanical Predictions of the Henry’s Law Constants and Their Temperature Dependence for the 209 Polychlorinated Biphenyl Congeners. Environ. Sci. Technol. 2008, 42, 8412–8418. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Q.; Wang, H.; Wang, W. Quantum chemical investigation on the mechanism and kinetics of OH radical-initiated atmospheric oxidation of PCB-47. Chemosphere 2015, 133, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Weng, J.; Stromberg, A.J.; Hilt, J.Z.; Dziubla, T.D. Fluorescence based detection of polychlorinated biphenyls (PCBs) in water using hydrophobic interactions. Analyst 2019, 144, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Li, X.; Flor, S.; Ruiz, P.; Kruve, A.; Ludewig, G.; Lehmler, H.J. Metabolism of 3-Chlorobiphenyl (PCB 2) in a Human-Relevant Cell Line: Evidence of Dechlorinated Metabolites. Environ. Sci. Technol. 2022, 56, 12460–12472. [Google Scholar] [CrossRef] [PubMed]
- Bader, R. Atoms in Molecules: A Quantum Theory; International Series of Monographs on Chemistry; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Pastorczak, E.; Corminboeuf, C. Perspective: Found in translation: Quantum chemical tools for grasping non-covalent interactions. J. Chem. Phys. 2017, 146, 120901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Jeziorski, B.; Moszynski, R.; Szalewicz, K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analys is platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Case, D.; Aktulga, H.; Belfon, K.; Ben-Shalom, I.; Brozell, S.; Cerutti, D.; Cerutti, T.; Cisneros, G.A., III; Cruzeiro, V.; Darden, T.; et al. Amber 2021; University of California: San Francisco, CA, USA, 2021. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Boys, S.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Density fitting of intramonomer correlation effects in symmetry-adapted perturbation theory. J. Chem. Phys. 2010, 133, 014101. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. PSI4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Molec. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Boto, R.A.; Contreras-García, J.; Tierny, J.; Piquemal, J.P. Interpretation of the reduced density gradient. Mol. Phys. 2015, 114, 1406–1414. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Feyereisen, M.W.; Feller, D.; Dixon, D.A. Hydrogen Bond Energy of the Water Dimer. J. Phys. Chem. 1996, 100, 2993–2997. [Google Scholar] [CrossRef]
- Ghosh, S.R.; Debnath, B.; Jana, A.D. Water dimer isomers: Interaction energies and electronic structure. J. Mol. Model. 2020, 26, 20. [Google Scholar] [CrossRef]

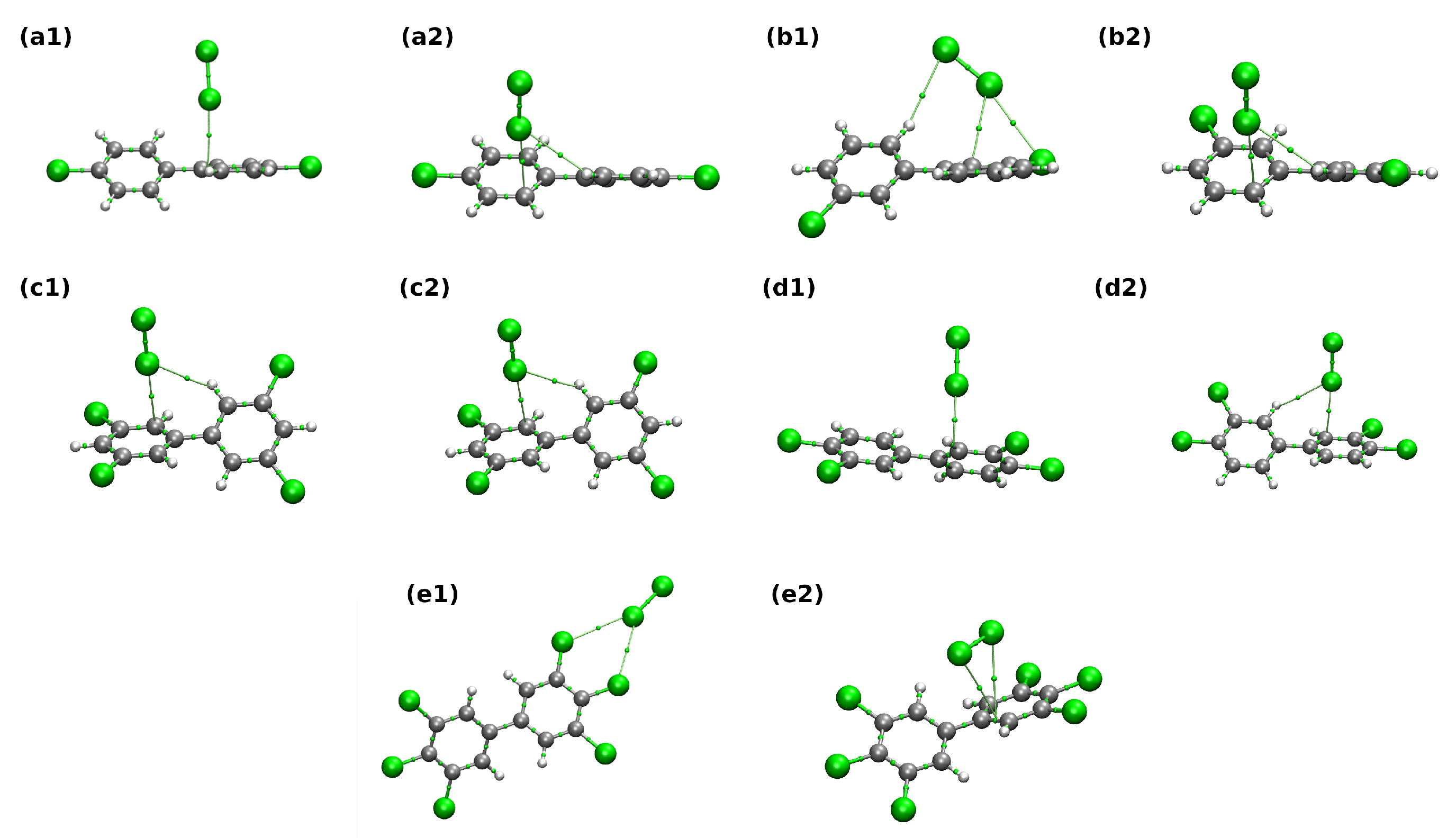
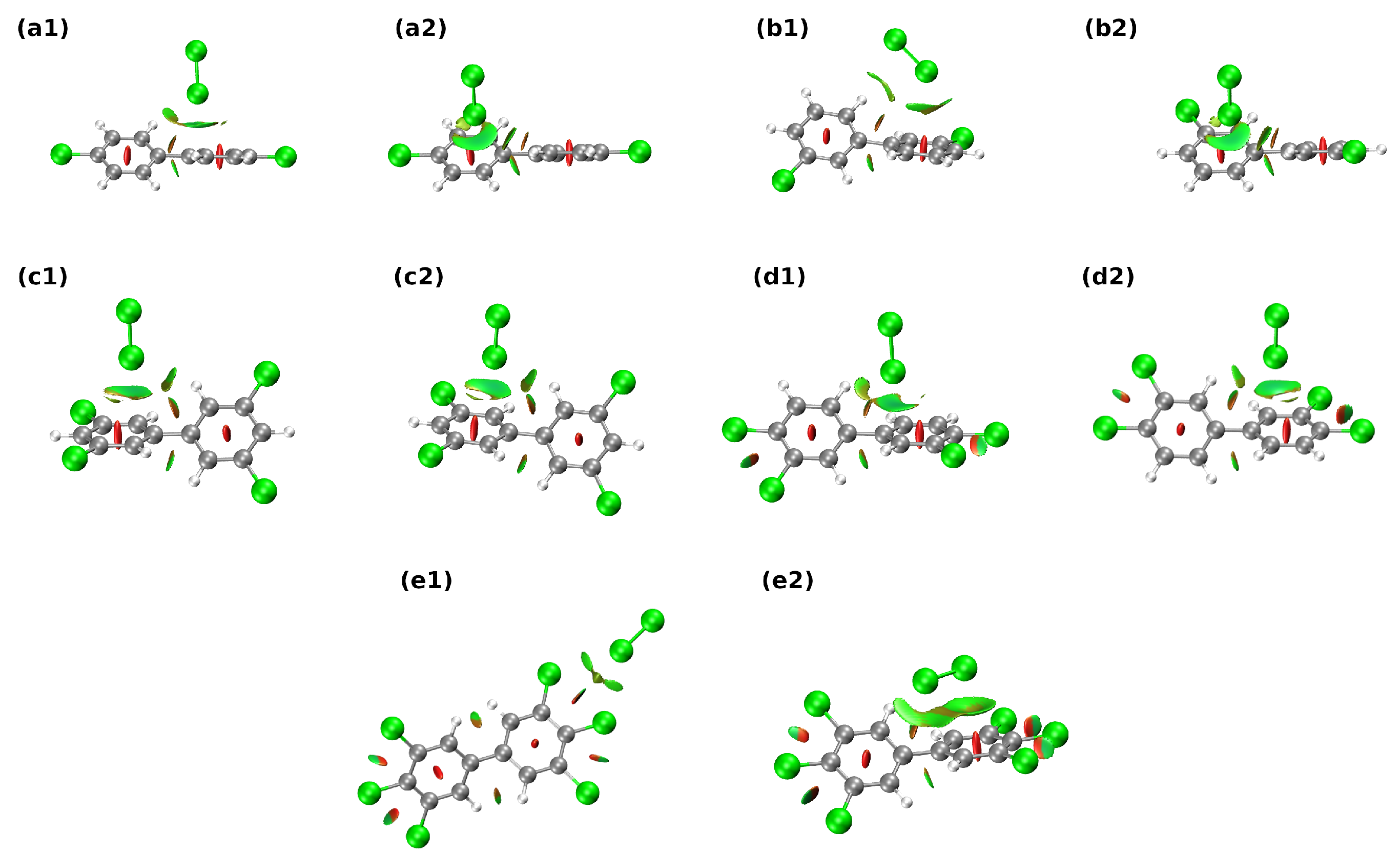
| H2O Complexes | BCP | VCP | E1 | ||
|---|---|---|---|---|---|
| a1 | C–HH–O | 0.008 | −0.004 | 0.026 | 1.278 |
| C–HO-H | 0.005 | −0.003 | 0.019 | 0.853 | |
| a2 | C–HO–H | 0.008 | −0.005 | 0.031 | 1.476 |
| C–HH–O | 0.010 | −0.005 | 0.034 | 1.656 | |
| b1 | C–HH–O | 0.004 | −0.002 | 0.015 | 0.661 |
| H–CH–O | 0.008 | −0.004 | 0.028 | 1.363 | |
| b2 | C–HO–H | 0.004 | −0.002 | 0.014 | 0.617 |
| H–CH–O | 0.010 | −0.005 | 0.033 | 1.719 | |
| c1 | ClO–H | 0.008 | −0.005 | 0.037 | 1.655 |
| c2 | H–CH–O | 0.008 | −0.004 | 0.027 | 1.348 |
| C–HO–H | 0.008 | −0.004 | 0.027 | 1.274 | |
| d1 | ClH–O | 0.006 | −0.003 | 0.023 | 0.978 |
| ClH–O | 0.006 | −0.003 | 0.022 | 0.952 | |
| d2 | C–HO–H | 0.009 | −0.005 | 0.036 | 1.674 |
| C–HO–H | 0.009 | −0.006 | 0.040 | 1.937 | |
| ClH–O | 0.005 | −0.002 | 0.017 | 0.726 | |
| e1 | ClH–O | 0.005 | −0.003 | 0.020 | 0.846 |
| ClH–O | 0.005 | −0.003 | 0.020 | 0.845 | |
| e2 | H–CH–O | 0.008 | −0.004 | 0.028 | 1.390 |
| C–HO–H | 0.007 | −0.004 | 0.026 | 1.255 |
| Cl2 Complexes | BCP | VCP | E1 | ||
|---|---|---|---|---|---|
| a1 | CCl | 0.013 | −0.008 | 0.045 | 2.503 |
| a2 | C–HCl | 0.005 | −0.002 | 0.018 | 0.765 |
| H–CCl | 0.013 | −0.008 | 0.044 | 2.406 | |
| b1 | C–HCl | 0.004 | −0.002 | 0.012 | 0.554 |
| ClCl | 0.008 | −0.004 | 0.031 | 1.306 | |
| H–CCl | 0.007 | −0.004 | 0.025 | 1.152 | |
| b2 | C–HCl | 0.005 | −0.003 | 0.020 | 0.873 |
| H–CCl | 0.013 | −0.008 | 0.044 | 2.493 | |
| c1 | C–HCl | 0.005 | −0.003 | 0.019 | 0.853 |
| H–CCl | 0.014 | −0.008 | 0.046 | 2.631 | |
| c2 | H–CCl | 0.014 | −0.008 | 0.046 | 2.631 |
| C–HCl | 0.005 | −0.003 | 0.019 | 0.828 | |
| d1 | H–CCl | 0.014 | −0.009 | 0.048 | 2.775 |
| d2 | H–CCl | 0.014 | −0.009 | 0.047 | 2.688 |
| C–HCl | 0.005 | −0.003 | 0.018 | 0.785 | |
| e1 | ClCl | 0.005 | −0.003 | 0.021 | 0.809 |
| ClCl | 0.006 | −0.003 | 0.023 | 0.929 | |
| e2 | H–CCl | 0.006 | −0.003 | 0.023 | 1.073 |
| H–CCl | 0.007 | −0.004 | 0.025 | 1.190 |
| Complex | Elst | Exch | Ind | Disp | SAPT2+/aDZ | RI-MP2/def2-TZVP |
|---|---|---|---|---|---|---|
| Water complexes | ||||||
| a1 | −3.918 | 5.836 | −1.392 | −3.879 | −3.354 | 2.602 |
| a2 | −4.643 | 6.193 | −1.754 | −3.519 | −3.723 | 3.283 |
| b1 | −3.684 | 5.819 | −1.451 | −3.955 | −3.272 | 2.485 |
| b2 | −3.810 | 6.290 | −1.803 | −3.962 | −3.286 | 2.526 |
| c1 | −1.676 | 2.507 | −0.688 | −1.241 | −1.097 | 0.913 |
| c2 | −4.372 | 5.895 | −1.542 | −3.432 | −3.452 | 2.567 |
| d1 | −2.201 | 2.720 | −0.714 | −1.743 | −1.937 | 1.542 |
| d2 | −5.163 | 5.942 | −1.940 | −3.381 | −4.542 | 3.847 |
| e1 | −2.023 | 2.713 | −0.596 | −1.732 | −1.637 | 1.047 |
| e2 | −3.599 | 6.003 | −1.635 | −3.846 | −3.077 | 2.124 |
| Chlorine complexes | ||||||
| a1 | −4.850 | 10.971 | −2.885 | −6.627 | −3.392 | 4.004 |
| a2 | −5.170 | 11.882 | −2.596 | −7.104 | −2.989 | 3.858 |
| b1 | −3.085 | 6.379 | −0.996 | −5.084 | −2.786 | 2.617 |
| b2 | −5.380 | 12.354 | −2.732 | −7.204 | −2.961 | 3.883 |
| c1 | −5.139 | 12.630 | −2.720 | −7.784 | −3.013 | 3.987 |
| c2 | −5.117 | 12.559 | −2.713 | −7.755 | −3.024 | 3.994 |
| d1 | −4.949 | 11.647 | −3.275 | −6.945 | −3.522 | 3.166 |
| d2 | −4.836 | 11.426 | −3.211 | −6.969 | −3.590 | 4.000 |
| e1 | −1.522 | 2.596 | −0.383 | −2.423 | −1.732 | 1.550 |
| e2 | −3.573 | 7.183 | −0.600 | −7.592 | −4.581 | 4.278 |
| Chlorine dioxide complexes | ||||||
| a1 | – | – | – | – | – | −28.14 |
| b2 | – | – | – | – | – | −27.18 |
| c1 | – | – | – | – | – | −29.76 |
| c2 | – | – | – | – | – | −33.62 |
| e2 | – | – | – | – | – | −34.56 |
| PCB | 0.5 ns | 1.5 ns | 2.5 ns | 3.5 ns | 4.5 ns | |
|---|---|---|---|---|---|---|
| E | −12.99 | −17.76 | −10.01 | −12.30 | −10.34 | |
| PCB15 (a) | n | 26 | 29 | 31 | 26 | 31 |
| E/HO | −0.50 | −0.61 | −0.32 | −0.47 | −0.33 | |
| E | −13.08 | −12.88 | −15.70 | −17.56 | −18.34 | |
| PCB11 (b) | n | 30 | 33 | 29 | 26 | 30 |
| E/HO | −0.44 | −0.39 | −0.54 | −0.68 | −0.61 | |
| E | −17.71 | −0.52 | −11.84 | −8.71 | −12.72 | |
| PCB80 (c) | n | 29 | 21 | 27 | 35 | 30 |
| E/HO | −0.61 | −0.02 | −0.44 | −0.25 | −0.42 | |
| E | −11.62 | −14.37 | −10.14 | −13.09 | −18.32 | |
| PCB77 (d) | n | 32 | 30 | 26 | 28 | 30 |
| E/HO | −0.36 | −0.48 | −0.39 | −0.47 | −0.63 | |
| E | −13.68 | −8.36 | −7.86 | −8.64 | −11.69 | |
| PCB169 (e) | n | 31 | 25 | 27 | 28 | 30 |
| E/HO | −0.44 | −0.33 | −0.29 | −0.31 | −0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtkowiak, K.; Panek, J.J.; Jezierska, A. Polychlorinated Biphenyls Interactions with Water—Characterization Based on the Analysis of Non-Covalent Interactions and Energy Partitioning. Sustainability 2022, 14, 12529. https://doi.org/10.3390/su141912529
Wojtkowiak K, Panek JJ, Jezierska A. Polychlorinated Biphenyls Interactions with Water—Characterization Based on the Analysis of Non-Covalent Interactions and Energy Partitioning. Sustainability. 2022; 14(19):12529. https://doi.org/10.3390/su141912529
Chicago/Turabian StyleWojtkowiak, Kamil, Jarosław J. Panek, and Aneta Jezierska. 2022. "Polychlorinated Biphenyls Interactions with Water—Characterization Based on the Analysis of Non-Covalent Interactions and Energy Partitioning" Sustainability 14, no. 19: 12529. https://doi.org/10.3390/su141912529
APA StyleWojtkowiak, K., Panek, J. J., & Jezierska, A. (2022). Polychlorinated Biphenyls Interactions with Water—Characterization Based on the Analysis of Non-Covalent Interactions and Energy Partitioning. Sustainability, 14(19), 12529. https://doi.org/10.3390/su141912529








