Abstract
The Vilariça River was restored in order to improve the fish habitat due to the construction of dams. However, due to the pressure that agriculture exerts on water resources, it is crucial to evaluate its consequences on fish health from a histological perspective. The gonad histopathological changes of two cyprinid species were assessed microscopically and evaluated with semi-quantitative analysis. Histopathological changes in the gonad, gill, and liver were used as biomarkers in the determination of the Integrated Biomarker Response (IBR), as well as to evaluate the histopathological scores between seasons. The observation of the gonad histopathological changes showed that interstitial fibrosis and macrophage aggregates were present exclusively in the Summer and more prevalent in males of large size. In addition, the histopathological scores of the three organs were higher in the Summer. In terms of the severity degree, 98% and 68% of the fish showed pronounced and severe histopathological changes in the gill and liver, respectively, while 28% of the fish showed slight histopathological changes in the gonad. The IBR showed higher values in the Summer and in the middle stream and downstream, which is in agreement with the metal concentrations. Thus, this work showed a relationship between the anomalies present in the fish organs and the quality of the water (classified as polluted). Therefore, minimization measures are presented, such as improving the irrigation methods, preserving the vegetation cover; promoting reforestation in degraded and burned areas; and improving the riparian vegetation.
1. Introduction
Agricultural and industrial activities and urban effluents, as well as natural events, are widely responsible for the increase in the level of metals in the aquatic environment [1,2,3]. Due to their toxicity, high persistence, resistance to degradation, bioaccumulation, and biomagnification, metals make their presence in the aquatic environment a threat to human and animal health [4,5,6,7,8]. In fact, the discharge of untreated effluents (from sources such as agrochemicals, industries, and urban waste) into rivers and open water bodies has reached worrying levels worldwide [2,4,9,10]. In addition, pollution resulting from agricultural activities has the disadvantage of being a diffuse pollution, which makes the treatment process difficult [11]. Moreover, increasing the temperature of water bodies has been shown to increase the toxicity of metals to various species of aquatic animals, including fish [2,7]. Thus, greater toxicity of metals in water bodies is expected in the Summer than in other seasons due to the increase in temperature. Therefore, it is important to assess the concentrations of heavy metals in the water, as well as the health conditions of the fish.
The fact that fish live in water makes them highly vulnerable to aquatic pollutants and susceptible to the development of injuries. Histopathology is a valid tool to describe and quantify the changes that exposure to contaminants may induce in fish organs [12,13]. Because they easily respond to xenobiotic exposure, the gill, liver, and gonad of fish are particularly preferred in studies of histological analysis to determine the effects of water pollution [4,13,14]. The gill is supposed to be the main target organ due to its direct and constant contact with the water, making this organ valuable in assessing the effects of aquatic contamination in fish [15]. Likewise, the liver plays a crucial role in metabolic vital functions, such as storage, biotransformation, detoxification, and excretion of xenobiotics [16,17]. In turn, changes in the gonad may be indicative of reproductive disturbances due to environmental contamination, such as reduced gonadosomatic index, altered sex hormone levels, and intersex [13,18,19].
For the analysis of the histopathological changes, the semi-quantitative methodology described in the protocol by Bernet et al. [15] has been widely used. The reason is that it provides an assessment of the severity of the histopathological changes that can be applied to different organs [20,21,22]. The application of this methodology gives a computation of reaction pattern indices and the organ index. These indices may be further subject to statistical analysis [12,23].
The use of gill, liver, and gonad histopathology as a biomarker may provide an assessment of the fish’s health as well as the aquatic ecosystem. Indeed, the use of histopathological biomarkers as analysis tools of the response of an organism to a stressor is well established in ecotoxicology [4]. The most well-known of the indices proposed to incorporate individual responses from biomarkers is the Integrated Biomarker Response (IBR), which was developed by Beliaeff and Burgeot [24] and then improved by Devin et al. [25]. The IBR is calculated by combining multiple biomarkers into a single value, being used to estimate the environmental pressure on populations from diverse places [24,26,27,28]. This method provides a graphical synthesis (by star plots) for understanding the relationships between the stress of the organism and contamination level [25].
The histopathological analysis was applied to two species of endemic cyprinids of the Iberian Peninsula: Iberian barbel (Luciobarbus bocagei) (Steindachner, 1864) and Douro nase (Pseudochondrostoma duriense) (Coelho, 1985). These potamodromous species migrate from the Douro River to its affluents, such as the Sabor River, to spawn during the reproductive period (March–July). However, currently, due to the construction of the two dams on the Sabor River, migration is impeded [29]. Consequently, the Vilariça River has become an important replacement habitat for endemic species to complete their reproductive cycle [30]. However, the inherent factors of this river, which can compromise the quality of the fish life, were not taken into consideration. In fact, the Vilariça River has been experiencing environmental contamination due to pollutants from agricultural runoff, the erosion of soil particles, the instability of river banks, and, to a lesser extent, domestic discharge [31]. Thus, the aims of this work were: (1) to identify the gonad histopathological changes and respective severity degrees; (2) to assess the integrated analysis of histopathological indices of the gill, liver, and gonad; and (3) to assess the relationship between the IBR (using the histopathological changes of the three organs as biomarkers) and the metal concentration and water quality. All analyses were performed in two different seasons, Summer and Winter, to best understand the influence of agricultural activities and natural events (that range over the year) on the water quality and fish health.
2. Materials and Methods
2.1. Sampling Site
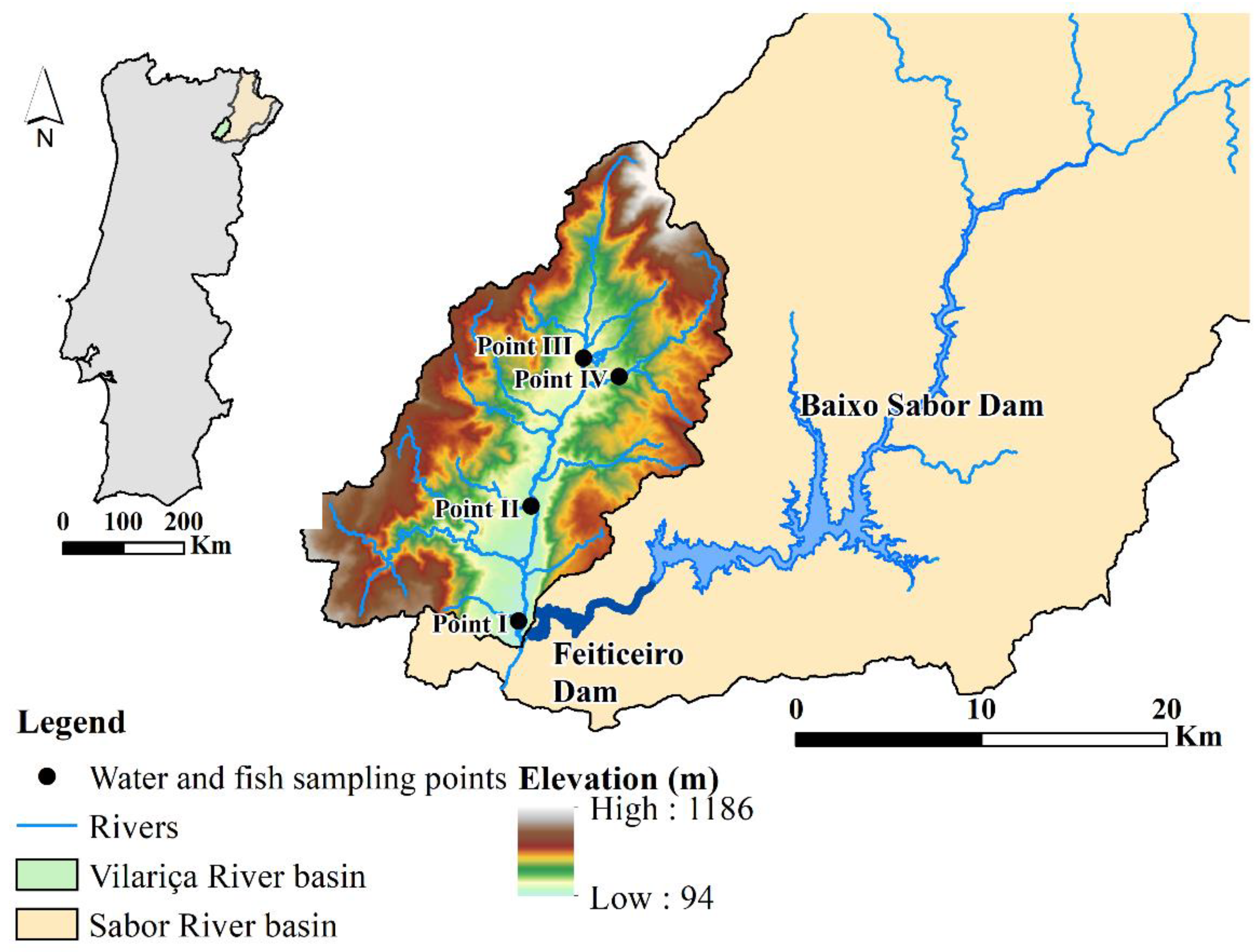
The Vilariça River, situated northeast of Portugal, is an affluent of the Sabor and Douro Rivers (Figure 1). The basin is bounded between the latitudes and longitudes of 41°11′ to 41°26′ N and 6°57′ to 7°14′ W, respectively, with elevations varying from 94 to 1186 m (m.s.l.). The Vilariça River, with an area of 324 km2 [32], is a mountain watershed exposed to intensive agricultural activity. Olive groves, fruit trees, and vineyards are the main crops that are cultivated in areas of lower altitudes and that are close to bodies of water [33]. Thus, the main stressors are excessive fertilization and the use of pesticides in agriculture, as well as wastewater, recurrent wildfires, soil erosion, and habitat disturbance [31,34]. The fieldwork was carried out in the Summer (27 July and 3 August 2016) and in the Winter (7 March 2017). Fish and water samples were collected in four sampling points along the Vilariça River (points I, II, III, and IV), except in point IV, where fish were absent in the Summer and had a short length (less than 50 mm) in the Winter (Figure 1). The fish sampling sites were chosen according to the WFD indications. In rivers less than 30 m wide, the length of the sampling stretch was twenty times the width, while in rivers wider than 30 m, the length considered was ten times the width.
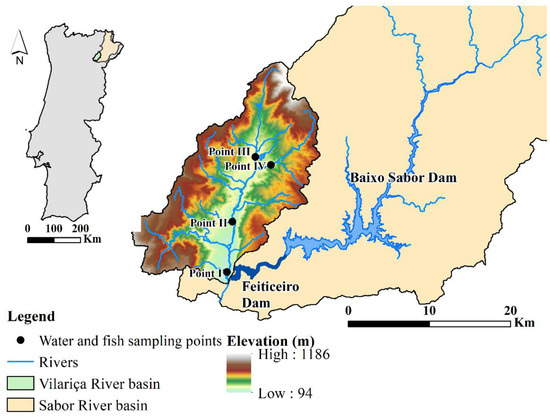
Figure 1.
Location of the water and fish sampling points in the Vilariça River (northeast Portugal).
2.2. Sampling and Analysis of the Water Quality
The physicochemical parameters such as pH, water temperature (T °C), conductivity (µS/cm), dissolved oxygen, NO2–, NO3–, PO43–, TSS, Ca, Mg, K, Na, SO42–, and the metals Fe, Al, As, Cd, Pb, Co, Cu, Mn, Zn, Ni and Cr (expressed in mg/L) were analyzed. In Santos [34], these physicochemical parameters and metals have already been analyzed in order to link them to gill histopathological changes. In this study, the analysis of water quality was used to correlate it with the assessment of the overview of fish health conditions. As a result, all information about water quality is presented in detail in the Supplementary Material, including the steps of collecting water samples and transporting them to a laboratory, the analytical method and the equipment used (Table S1), as well as the methodologies proposed for classifying the water quality (Table S2). In addition, a summary of the water quality analysis is provided in the results section and is related to the histopathological changes in the discussion section.
2.3. Fish Sampling and Histological Processing
The samples of L. bocagei and P. duriense were captured using pulsed direct current electrofishing equipment with a DC-500 V generator. In total, 47 representatives of both species were caught, ranging from one to seven fish per sampling point, with an approximate proportion of females and males. Fish collecting was performed following the Ethical Guidelines of the European Union Council (European Directive 2010/63/EU) [35] and the Portuguese Agricultural Ministry [36] for the protection of animals used for experimental and other scientific purposes.
After capturing, the fish were anaesthetized by immersion in 3-aminobenzoic acid ethyl ester methanesulfonate (MS-222), weighed and measured, and immediately euthanized by decapitation. The gonad was weighed, randomly sampled and preserved in 4 percent buffered formaldehyde (Panreac, Spain) for 24 h [34,37]. Following fixation, the samples were dehydrated through a series of graded ethanol (Fisher Scientific, U.K.) solutions (70–100%), cleared in xylene (Fisher Scientific, U.K.), and embedded in paraffin Histosec® pastilles (Merck, Darmstadt, Germany) in a semi-enclosed tissue processor (Tissue-Tek II model 4634). Next, the gonads were included in paraffin blocks in a modular tissue embedding station (Leica EG 1160) to be sectioned into thin sections (3 μm thick) in a rotary microtome (Leica RM 2135). Three replicates of the sampled sections were mounted on slides and stained with hematoxylin-eosin (H&E stain) before being coverslipped. A qualitative evaluation of the histopathological changes was performed on each fish using a microscope (Olympus, IX 51, Tokyo, Japan) for the gonad.
2.4. Histological Analysis of the Gonad
The gonad’s histological analysis enabled the identification of histopathological changes and their prevalence. The prevalence of each histopathological change was calculated based on the percentage of fish with that change (e.g., [22,38]). The severity of these histopathological changes was assessed using a semi-quantitative methodology modified from the protocol by Bernet et al. [15]. This method classifies histopathological changes into five reaction patterns: circulatory disturbances, regressive changes, progressive changes, inflammation, and tumours. Each reaction pattern includes several histopathological changes. The value of each histopathological change results from the multiplication of the score value by the importance factor. Thus, the score values were defined as follows: 0 (unchanged), 2 (mild occurrence), 4 (moderate occurrence), and 6 (severe/diffuse occurrence). The importance factors 1, 2, and 3 were of minimal, moderate, and marked pathological importance, respectively. The sum of the histopathological changes of each reaction pattern provides the index for the respective reaction pattern. In addition, the sum of all reaction patterns gives the organ index. The equation for the histopathological index of each organ is:
where represents the organ (gonad), the reaction pattern, the alteration, the score value, and w the importance factor.
The values of the organ index are ranked into classes based on the scoring scheme proposed by [39]: Class I (index ≤ 10) – normal tissue structure with slight histological alterations; Class II (index 11–20) – normal tissue structure with moderate histological alterations; Class III (index 21–30) – moderate modifications of normal tissue; Class IV (index 31–40) pronounced histological alterations of the organ; Class V (index > 40)–severe histological alterations of the organ. Additionally, macrophage aggregates (MA) and interstitial fibrosis (IF) were classified as regressive changes (reaction pattern) and were assigned an importance factor of 2 and 3, respectively, according to Agbohessi et al. [22].
2.5. Integrated Biomarker Response
The integrated biomarker response is a simple multivariate graphic method that is represented by star plots. The computation of the star plot area gives a visual integration of the biomarker response to chemicals. The IBR calculation was based on four major steps as described in Beliaeff and Burgeot [24] and improved by Devin et al. [25].
- The mean value for a site () was standardized using the mean value for all sites () and the standard deviation for all sites () to produce a value called .
- For each biomarker, the value or was computed according to the expected biological effect activation or inhibition, respectively.
- The value was computed with , where is the minimal value observed for all sites for each biomarker.
- Star plots were then used to display biomarker results. If a station is to be characterized by a set of biomarkers measured, a star plot radius coordinate represents the score of a given biomarker at a given station. Let and be two consecutive clockwise scores (radius coordinates) of a given star plot, and let be the number of biomarkers used in the survey. This formula can be applied only when four or more biomarkers are measured.
Hence, the total area corresponding to a given station sampled, that is, the integrated biomarker response (IBR), is
This approach was applied to the sampling points I, II, and III of the Vilariça River, and the star plots were used in both species and both seasons. In all cases, IBR values were visually compared to metal concentrations measured in water samples.
2.6. Data Analysis
The prevalence of the gonad histopathological changes in females and males of L. bocagei and P. duriense was calculated for each sampling point. Additionally, the gonad histopathological index was used to compare the severity degree of the histopathological changes observed in each species between seasons and sampling points.
In order to acquire a comprehensive knowledge of the fish’s health status, the previous information from the histopathological changes observed in the gills by Santos et al. [34] and in the liver by Santos et al. [40] was integrated (Table S3). From these previous studies, histopathological changes observed in the gills and the liver and the respective organ index (gill histopathological index – Gill HI, and liver histopathological index – Liver HI) were used. These indices and the gonad histopathological index (Gonad HI) were used to detect statistical differences between seasons, and between fish lengths, while the histopathological changes were used to calculate the IBR.
The gill, liver, and gonad histopathological indices of L. bocagei and P. duriense did not show a normal distribution, according to the normality (Shapiro-Wilk test) and homoscedasticity (Levene’s test). Thus, the Mann-Whitney rank-sum test was performed to detect statistical differences between seasons and between fish lengths. To detect statistical differences between fish lengths, the fish were divided into two size classes (small and large) based on an average total length. Thus, small and large size classes correspond to less and more than 21 cm for L. bocagei and less and more than 14 cm for P. duriense, respectively. The small and large size classes corresponded, respectively, to 16 and nine individuals of L. bocagei and eight and 14 individuals of P. duriense. When p < 0.05, statistical significance was accepted. The IBM SPSS statistics software (version 26) was used in both statistical analyses [41].
3. Results
3.1. Gonad Histopathological Changes and Severity Degree Index
In terms of length and weight for each species, discriminated by sexes, P. duriense presented the same length for both genders (14 cm) and an approximate weight of 26 and 31 cm for females and males, respectively; whereas, L. bocagei showed higher biometric parameters in females (24 cm and 215 g) than males (19 cm and 95 g) (Table S4).
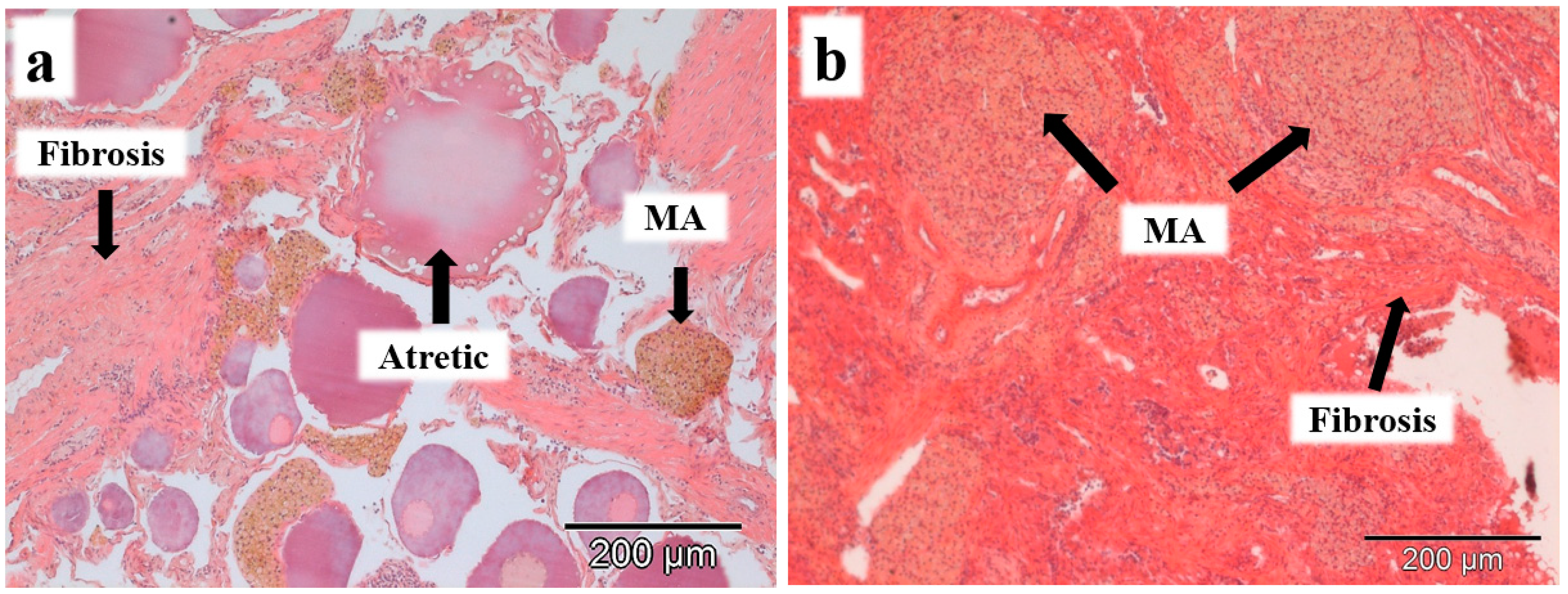
The histopathological changes observed in the gonads of both species were macrophage aggregates and interstitial fibrosis (Table 1). Macrophage aggregates were observed in 27% of the males and 22% of the females of L. bocagei, and 50% of the males and 21% of the females of P. duriense. Interstitial fibrosis was observed in 7% of the males and 22% of the females of L. bocagei, and 38% of the males and 21% of the females of P. duriense. When present, the histopathological changes were observed in the interstitial tissue of both sexes, surrounding the oocytes in females (Figure 2a), and in the germinative tissue of the testis in males (Figure 2b).

Table 1.
Prevalence (%) of histopathological changes identified in the gonads of L. bocagei and P. duriense at sampling points.
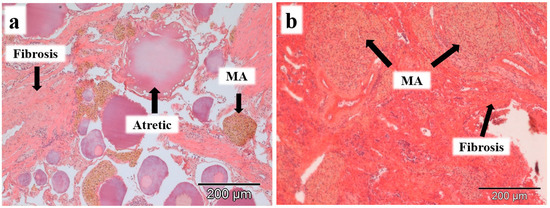
Figure 2.
Histopathological changes, such as macrophage aggregates (MA) and interstitial fibrosis were observed in females (a) and males (b) of L. bocagei and P. duriense. Scale bar 200 µm.
Considering the histopathological index (severity degree), the gonads showed a normal structure with slight histopathological changes (index ≤ 10), according to Zimmerli et al. [39] (Table 2). The histopathological changes and respective index (Gonad HI) only occurred after spawning. Moreover, both species showed higher Gonad HI downstream of the river (point I) than upstream (point III), with P. duriense presenting higher values than L. bocagei in all sampling points. Indeed, from upstream to downstream, the gonad histopathological index of P. duriense increased from 4 to 10 and that of L. bocagei from 0 to 4.8. During the histological examination of the gonadal tissue, no signs of intersex were found in either males or females.

Table 2.
Gonad histopathological index (severity degree) of L. bocagei and P. duriense sampled in the Summer and Winter.
3.2. Histopathological Indices of the Gill, Liver, and Gonad
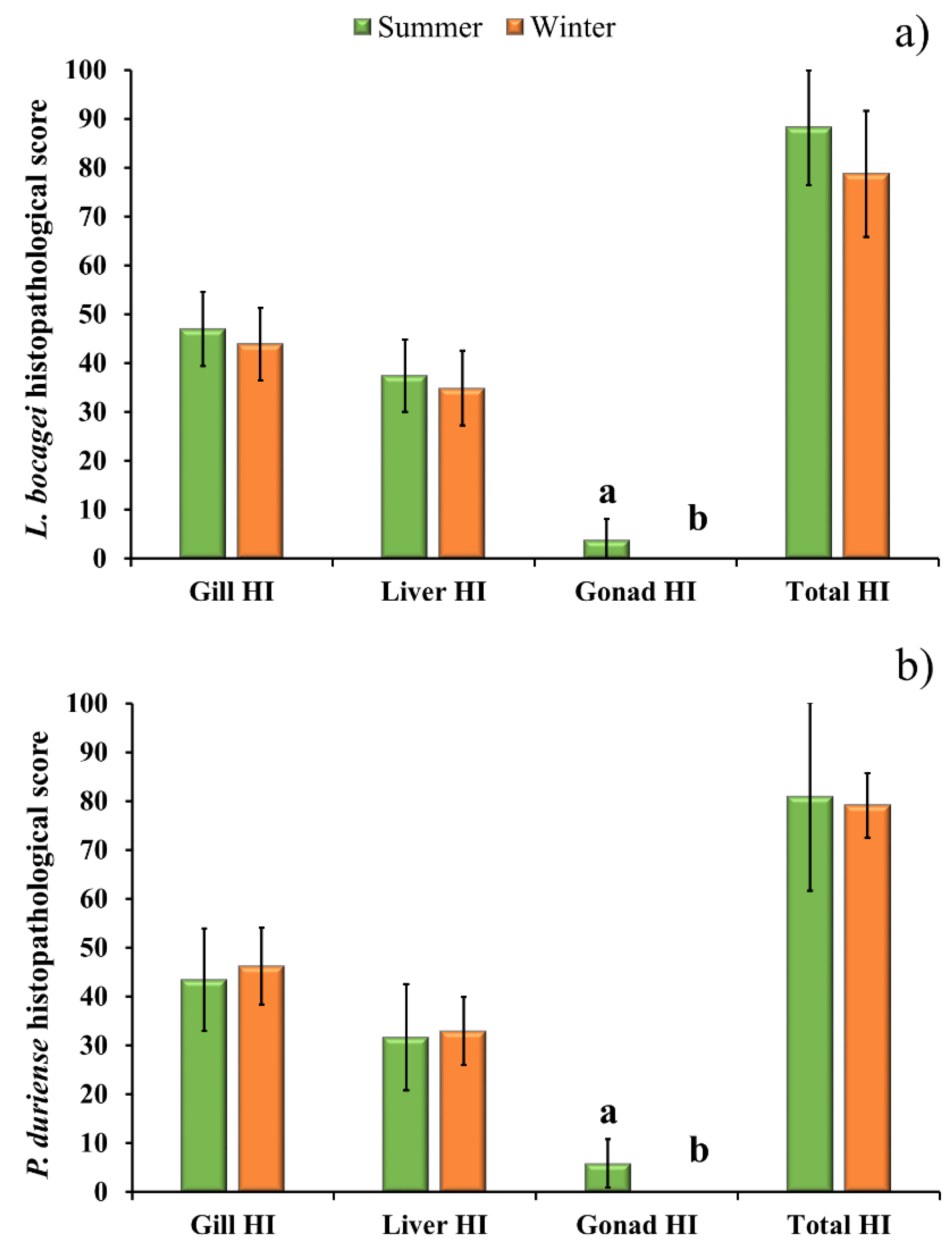
To evaluate the differences between seasons, the gill, liver, gonad, and total histopathological indices were statistically assessed for each species (Figure 3). Only the histopathological changes of the gonads showed statistical differences between seasons. Figure 3 also shows that among the three organs, the gill had the highest histopathological index, followed by the liver and the gonad. The Gill HI varied between 43.5 and 47, and the Liver HI varied between 31.6 and 37.5 for both species and seasons, while the Gonad HI was 3.8 and 5.8 for L. bocagei and P. duriense, respectively.
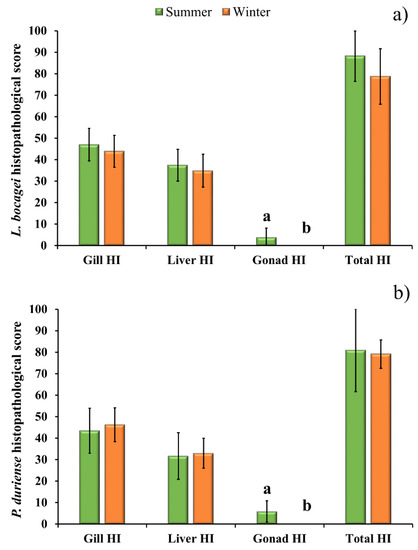
Figure 3.
Gill Histopathological Index (Gill HI), liver Histopathological Index (Liver HI), gonad Histopathological Index (Gonad HI) and total Histopathological Index (Total HI) of L. bocagei (a), and P. duriense (b) presented as mean ± SE. Different letters are indicative of significant differences (p < 0.05) in the Gonad HI between seasons. Statistical analysis was conducted using a Mann-Whitney rank-sum test to detect differences between seasons for each species.
Table 3 shows the classification of the severity degree of the gill, liver, and gonad histopathological indices according to Zimmerli et al. [39]. The values represent the number and respective percentage of individuals of both species. The highest numbers of individuals with histopathological changes were observed in two classes: severe (class V) and pronounced (class IV). Additionally, 98% and 68% of the fish showed both pronounced and severe histopathological changes in the gills and liver, respectively, and 19% of the fish showed severe histopathological changes in both organs. Moreover, in a total of 28% of the fish with slight gonad histopathological changes, 21% of the fish had pronounced and severe histological alterations in the gill and liver (Table 3).

Table 3.
Classification of severity degree of the gill, liver, and gonad histopathological indices. The values represent the number (percentage) of individuals of both species. The gonad histopathological index showed a normal tissue structure with slight histopathological changes (Class I).
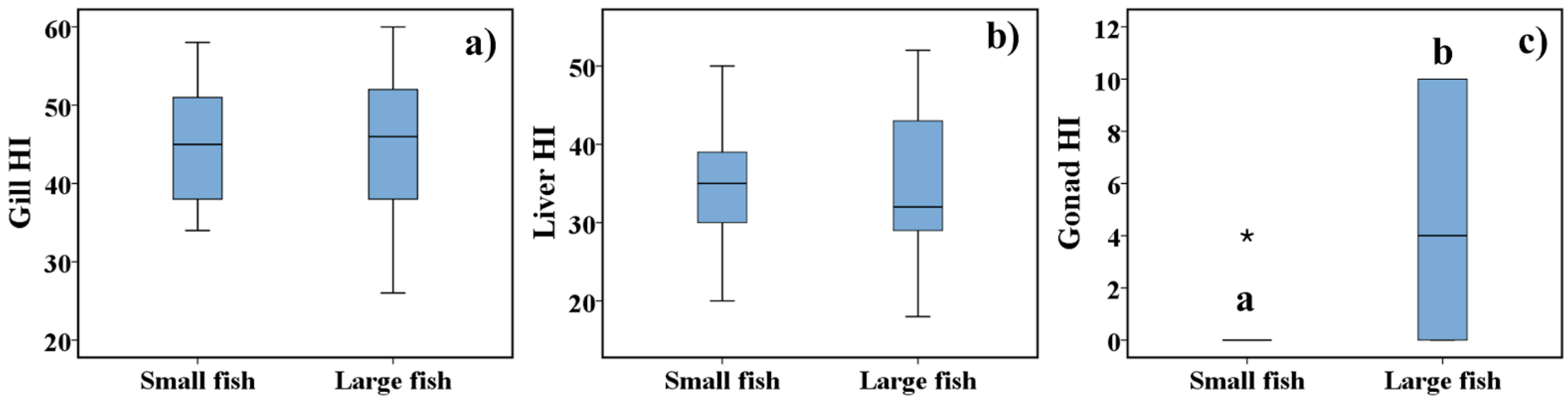
Figure 4 illustrates the statistical comparison of the Gill HI, Liver HI, and Gonad HI between small and large fish of both species. The results showed statistical differences in the Gonad HI due to the higher presence of macrophage aggregates and interstitial fibrosis in the ovary and testis of fish of large size. No statistical differences were observed in the Gill HI and Liver HI between the two classes of length.
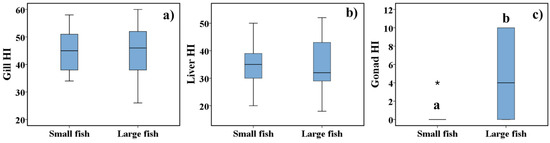
Figure 4.
Gill Histopathological Index (Gill HI) (a), Liver Histopathological Index (Liver HI) (b) and Gonad Histopathological Index (Gonad HI) (c) of small and large fish. The results are presented as box-plots. The boundaries of the box-plot indicate the 25th and 75th percentiles, the line within the box marks the median value, whiskers above and below the box indicate 10th and 90th percentiles, and an asterisk (*) indicates an extreme outlier. Different letters are indicative of significant differences (p < 0.05) in the Gonad HI between fish size classes. Statistical analysis was performed using a Mann-Whitney rank-sum test.
3.3. Integrated Biomarker Response
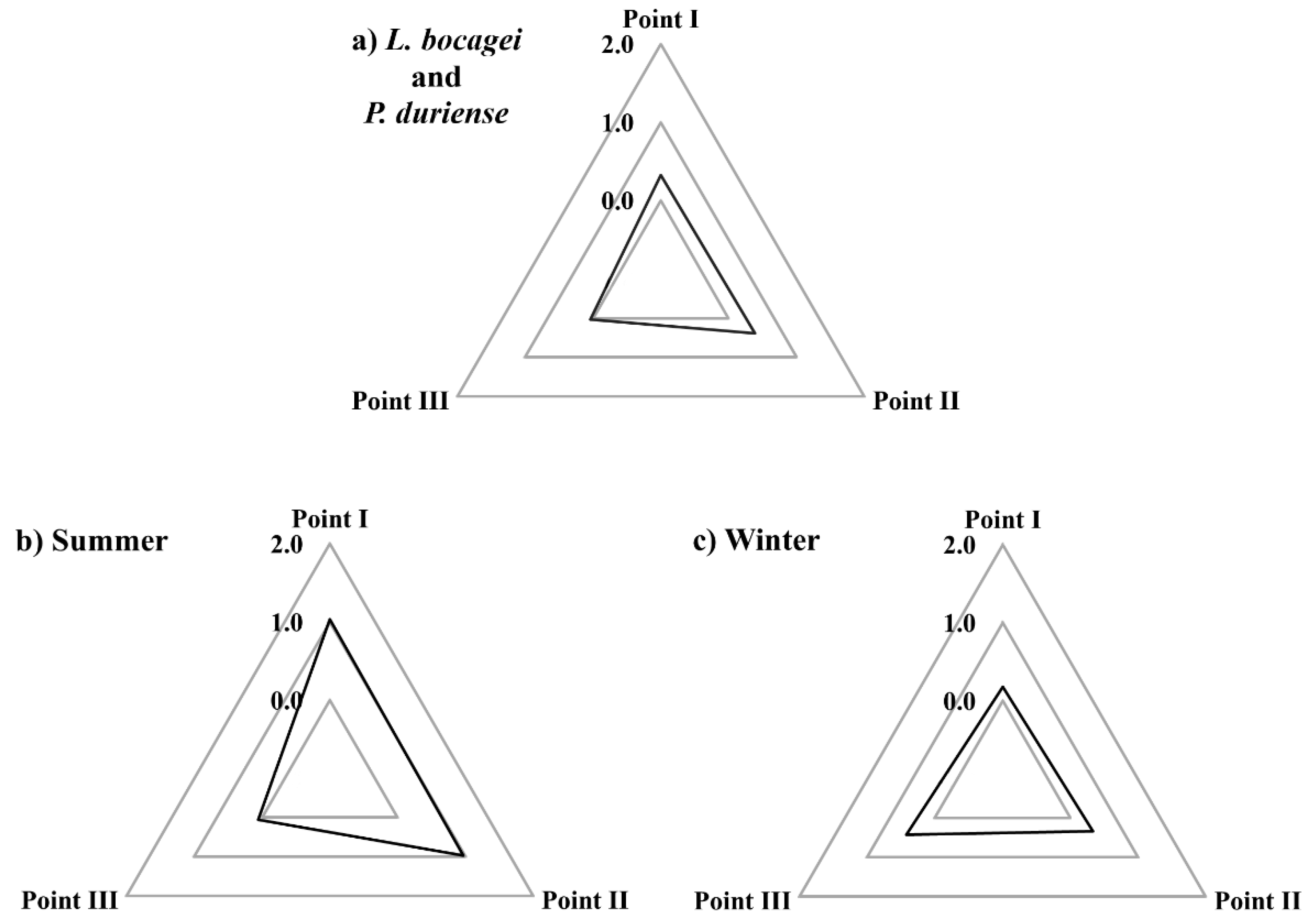
The IBR was determined using the histopathological changes assessed in the gill, liver, and gonad of L. bocagei and P. duriense as biomarkers. As a result of the IBR, the star plot area was compared across sampling sites for both species (Figure 5a) in the Summer (Figure 5b), and the Winter (Figure 5c). In general, the IBR computed for both species (in both seasons) was slightly higher in points I (0.33) and II (0.39) than in point III (0.04) (Figure 5a). Furthermore, the IBR of both species caught in the Summer was higher in points I (1) and II (0.97) (Figure 5b), while in the Winter, it was slightly higher in point III (0.42) (Figure 5c).
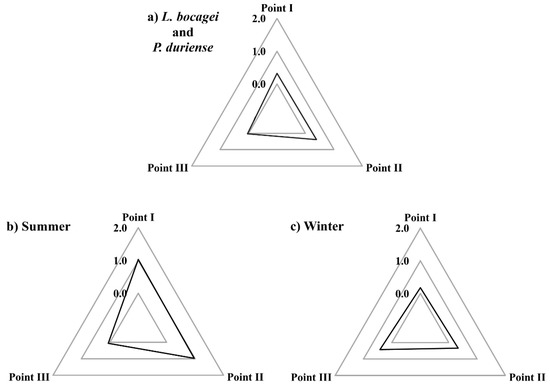
Figure 5.
Star plots of the integrated biomarker response (IBR) of both species (a), of the summer (b), and of the winter (c) for each sampling point (I, II and III).
3.4. Classification of Water Quality
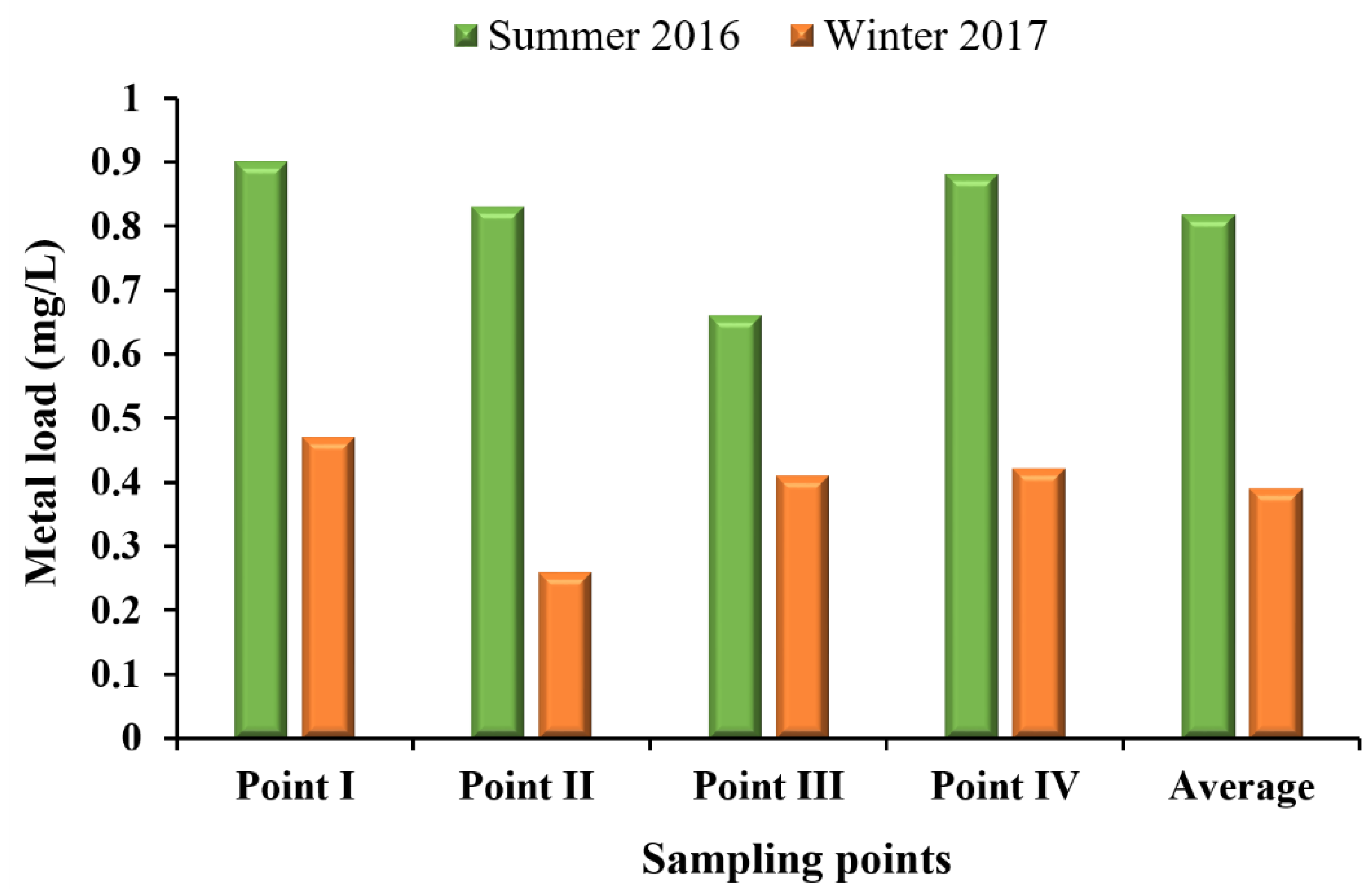
According to the acceptable limits established for good water quality for multiple uses, the water in the Vilariça River was classified as polluted in the Summer of 2016 and extremely polluted in the Winter of 2017 (Table S5). In the Summer, the water from sampling points I and II were classified as polluted and from points III and IV were classified as weakly polluted due to the low percentage of dissolved oxygen, high temperature, and high concentrations of Mn and As (Table S5). In the winter, the water was classified as extremely polluted in all sampling points due to the high TSS. Also, the percentage of dissolved oxygen, NO2−, and Cd concentrations exceeded the acceptable limits (Table S5). Moreover, the water classification showed that in all sampling sites and both seasons, there was near neutral pH (Table S5) and a low level of metal concentration (Figure 6). In addition, the values of metal load showed increasing concentrations from point III to point I (from upstream to downstream of the river) in both seasons, albeit with greater concentrations in the Summer (Figure 6).
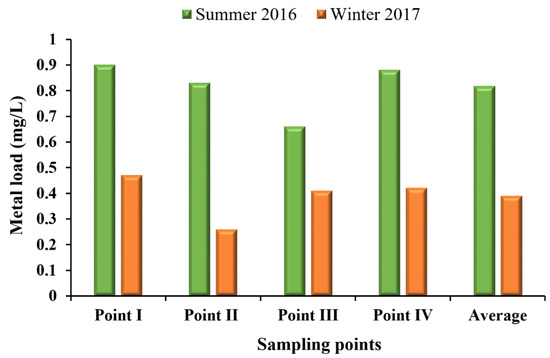
Figure 6.
Metal (As+Al+Cr+Cd+Pb+Cu+Mn+Zn+Ni+Fe+Co mg/L) concentrations measured in the Summer of 2016 and Winter of 2017 at sampling point (I, II, III and IV).
4. Discussion
The gonad histopathological changes included interstitial fibrosis and macrophage aggregates with exclusive prevalence in the post-spawning phase (Summer). These alterations were common in both species and both genders, although they were more prevalent in males of larger size. The observation of MA in ovaries and testes during the regression of the gonad has been associated with the degradation and resorption of unspawned atretic oocytes and sperm, respectively [19,42]. However, the area occupied by MA exclusively in mature testes suggested that these might be associated with factors other than the regression of the gonads. It has been demonstrated that MA may become larger and/or more numerous following exposure to xenobiotics, such as endocrine-disrupting chemicals [43] and toxicants or infectious agents [44,45]. Zorita et al. [45] observed a high frequency of MA in males of Mullus barbatus from polluted sites in the NW Mediterranean, mainly at the end of the spawning season. Identical results were also obtained by Micale et al. [19] also Mullus barbatus in the post-spawning phase in the NW Mediterranean, who reported that gonadal MA was significantly higher in larger males than smaller ones. The significant difference in MA between larger (old) males and smaller ones could be justified by cell necrosis or apoptosis associated with ageing [46]. These authors suggest that ovarian MA may be associated with ovary regression after spawning and testicular MA with environmental factors. Interstitial fibrosis was observed within the testicular or ovarian interstitium (stroma) with a higher prevalence in P. duriense. The IF is characterized by the thickening of the interstitium, indicative of collagenous fibers, fibrocytes or fibroblastic proliferation [42,44]. In most fish, the IF was observed surrounding MA, and this has also been reported by Blazer [44]. Many researchers have suggested that IF is a chronic tissue response to injuries from things such as agricultural pesticides [22], urban effluents [13], and endocrine-disrupting chemicals [43,47].
The integration of the histopathological changes of the gill, liver, and gonad allowed us to assess the general health condition of fish with more reliability than the evaluation based only on one of those organs. According to the results, the highest histopathological score was found in the gill, followed by the liver and gonad. Similar results were obtained by Rašković et al. [48] in a work developed with Barbus barbus from the Danube River, where the metal concentrations exceeded the maximum acceptable, as well as by Javed [4] at the Satha canal water, India, with Channa punctatus exposed to industrial effluents with metals concentrations exceeding the permissible limits. Given that the gill is the organ responsible for ion exchanges with the environment, the histopathological changes could be interpreted as a defense mechanism to increase the distance that heavy metals dissolved in water must diffuse across to reach the bloodstream. Furthermore, it has also been reported that histopathological changes are more severe in the liver than in the gills of fish exposed to a wide range of heavy metals and organic compounds. This is the case in the work developed by Poleksic et al. [49] with Acipenser ruthenus from the Danube River basin and by Abdel-Moneim et al. [17] with Oreochromis niloticus from at Al-Hassa springs and irrigation channels in Saudi Arabia. This is possible because the liver is a fundamental organ in the detoxification of different environmental contaminants [15], and the exposure of fish to metals stimulates the production of hepatic proteins, mainly the metallothionein that is involved in the binding and regulation of essential metals such as copper and zinc, and in the detoxification of toxic metals such as arsenic [50]. The lower histopathological changes were observed in the gonad. Similar results were obtained with Squalius vardarensis in which lower levels of metals and metalloids were detected in gonads compared to the liver [51]. This can indicate that gonads were probably less exposed to metals. However, it is noteworthy that in 28% of the fish with gonad histopathological changes, 21% had pronounced and severe histopathological changes in the gill and liver. These results reveal that the presence of histopathological changes in the gonad may be indicative of the presence of injuries in the gill and liver.
The IBR was calculated based on histopathological changes found in the gills, liver, and gonads, effectively reproducing well variations in metal concentrations. Indeed, the IBR values were higher in the Summer than in Winter, and higher in the middle stream and downstream than upstream of the river, as well as in the metal concentrations.
In the Summer, the water quality was classified as polluted due to the low percentage of dissolved oxygen, high temperature, and high concentrations of Mn and As. Dissolved oxygen represents the oxygen that is available to aquatic life, and its solubility in water increases with decreasing temperature [52]. Thus, as expected, the lowest dissolved oxygen and the highest temperature values were measured in the Summer. The values of dissolved oxygen ranged from 50.2 to 89.4% and 88.5 to 128.7% in the summer and winter, respectively, and the values of temperature ranged from 21.2 to 26.2 °C and 10.1 to 14.5 °C in the Summer and Winter, respectively (Table S5). Low precipitation, reduced streamflow (due in part to water diversion for irrigation), and high air temperature could explain the rise in water temperature and, consequently, the decrease in dissolved oxygen. In addition, the source of metals in the river water may be due to agricultural fertilizers that have heavy metals in their composition and that reach water bodies through soil leaching during irrigation [53,54,55]. Moreover, the temperature is one of the factors influencing the toxicity of metals [2,7,56]. Thus, when the water temperature increases, the toxicity of the metals for several aquatic animal species, including fish, also increases. This is also the case of the metals As, Mn, and Cd that were in higher concentrations in the Vilariça River. The work developed by Han et al. [7] with Platichthys stellatus, following exposure to varying As concentrations present at different water temperatures, showed that the toxic effects of As exposure were higher at the higher temperature. The same result was obtained by Aliko et al. [56] with the Mn contamination on Carassius auratus, as well as by Abdel-Tawwab and Wafeek [2] with the Cd-exposed on Oreochromis niloticus. These works observed the consequences on the growth of the fish [2,7], altered the immune system, and compromised the blood oxygen [56] and carrying capacity and overall health status in fish [2,56]. Several studies mention that the uptake of Mn by fish significantly increases with temperature [56] and that the liver, kidney, and gills were the main organs where Cd accumulated [2,57,58]. The authors argue that fish accumulate more metals at higher temperatures due to a faster metabolic rate, which includes a higher rate of metal uptake and binding [57,59]. Thus, the higher water temperatures registered in the Summer could have had responsibility for the increased metal toxicities for both species, in particular As and Mn, because they exceed the status of surface water bodies for multiple uses, whereas Cd could not have had its toxicity increased because it presented higher concentrations in the Winter, i.e., when the water temperature is lower.
In the Winter, the IBR values were lower, with a small variation between sampling points, as well as lower metal concentrations. However, the water was classified as extremely polluted in all sampling points due to the high TSS, as well as the lower percentage of dissolved oxygen and high concentrations of NO2− and Cd. The lower percentage of dissolved oxygen was measured at one sampling point located upstream of the river (sampling point IV), where the streamflow is lower and there are some pools of still water. The high concentration of TSS, NO2−, and Cd was probably due to soil erosion by water [53,60]. The precipitation in the mountain area with thin soil layers, low vegetation cover, and steep topography promotes soil erosion. In addition, the intensive management of agricultural areas such as herbicide application, regular tillage, and the reduced riparian zone has also contributed to soil loss, as well as the siltation in the water bodies during peak flows [34,40].
Once the causes that compromise water quality and fish health have been identified, it is important to propose mitigation strategies, such as (a) the use of irrigation methods and techniques suitable for crops to reduce water loss through runoff, thus ensuring production systems and ecosystem services; (b) practices and techniques based on the use of machines that eliminate vegetation cover and mobilize the soil, leaving it vulnerable to erosion from precipitation and wind, and should thus be avoided or reduced to a minimum. Alternatively, vegetation cover (grasses) should be promoted in the spaces between the rows of orchards and vineyards because it allows greater protection of the soil against erosion, provides nutrients to the soil, favours greater water infiltration, and reduces surface runoff. Vegetation cover also contributes to increasing organic matter, fertility, and soil structure, thus helping to reduce the need to use chemical fertilizers and perform soil recovery tasks; (c) reforestation is one strategy to prevent soil erosion and improve the water balance of hydrographic basins. Thus, the reforestation of abandoned farmland, degraded areas, and burned areas should be promoted, as well as the use of bushes as facilitators in reforestation; and (d) proceed to replant riparian vegetation where it is absent with native species to promote the nutrient retention and suspended sediment produced by agricultural soil erosion and leaching.
5. Conclusions
The analysis of the relationship between the water quality and the histopathological changes observed in the different organs of two cyprinid species was the focus of the present work. The analysis of the gonad histopathology showed that changes such as interstitial fibrosis and macrophage aggregates, more prevalent in males, may be associated with the size and age of the fish. However, the histopathological analysis of the gills and liver revealed that L. bocagei and P. duriense live under adverse environmental conditions, with more than 68% of the fish exhibiting pronounced and severe histopathological changes in both organs. Furthermore, using histopathological changes as biomarkers in the calculation of the IBR allowed us to confirm that the changes observed in fish organs had a similar spatial and temporal distribution as metal concentrations. Thus, higher IBR areas were observed in the Summer and in the middle stream and downstream, as well as metal concentrations. These results allow us to argue that the histopathological changes observed in fish could be justified by the higher metal concentrations in the river.
The current study provides evidence that biomarkers are essential for identifying early deleterious impacts on populations before a system may falter and reach a point where recovery is challenging, expensive, or unattainable. As one of the biggest and most dangerous ecological hazards, chronic pollution alters ecosystems abruptly, with unpredicted ecological, economic, and social repercussions. These effects are anticipated to be acute in Mediterranean rivers due to their high level of endemicity and lengthy history of anthropogenic influences that may impair their capacity to cope with upcoming stressors. Therefore, measures like proper agricultural practices and reforestation should be implemented to lower the ecological risk in systems where several species are affected by health issues.
For future studies, the following is recommended: (a) increase the sample size per sampling point to allow a correlation analysis between the IBR and the concentration of metals in the river water, as well as a more robust statistical analysis between the sampling points and the seasons; (b) in addition to metals, pesticides must also be monitored, as they are equally responsible for the presence and prevalence of histopathological changes in the different organs of fish [14,61]; and (c) the research could be improved through the use of biochemical indicators which are also used to identify possible environmental contamination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su141912596/s1, Table S1: The analytical method, detection limit, and equipment used to determine the physicochemical parameters at the sampling points in the Vilariça River; Table S2: The five classifications of surface water bodies for multiple uses; Table S3: Gill and liver histopathological changes observed in L. bocagei and P. duriense were grouped into four reaction patterns; Table S4: Biometric parameters (average length and weight) of L. bocagei and P. duriense (females and males) captured in the Vilariça River; Table S5: Water physicochemical parameters collected and measured in the sampling points (I, II, III and IV) of the Vilariça River. References [10,15,22,62,63,64,65,66,67] are cited in the supplementary materials.
Author Contributions
Conceptualization, R.M.B.S. and S.M.V.M.; methodology, R.M.B.S. and S.M.V.M.; software, R.M.B.S.; validation, R.M.B.S., S.M.V.M. and R.M.V.C.; formal analysis, R.M.B.S.; investigation, R.M.B.S.; resources, R.M.B.S., S.D.G.P.V. and S.M.V.M.; data curation, R.M.B.S. and S.M.V.M.; writing—original draft preparation, R.M.B.S.; writing—review and editing, R.M.B.S. and R.M.V.C.; visualization, S.D.G.P.V., S.M.V.M., R.M.V.C., F.A.L.P. and L.F.S.F.; supervision, S.M.V.M., R.M.V.C., F.A.L.P., S.D.G.P.V. and L.F.S.F.; project administration, R.M.V.C., F.A.L.P. and L.F.S.F.; funding acquisition, R.M.V.C., F.A.L.P. and L.F.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support for the identification of gonad histopathology changes, analysis of results and article writing from Portuguese Foundation for Science and Technology (FCT), Ministry of Science, Technology, and Higher Education (MCTES), European Social Fund (FSE) through NORTE 2020 (North Regional Operational Program 2014/2020) and European Union (EU) to Regina Santos (Grant: SFRH/BD/141503/2018). And also received financial support for the water and fish data collection from the INTERACT project—“Integrated Research in Environment, Agro-Chain and Technology”, no. NORTE-01-0145-FEDER-000017, in its line of research entitled BEST, co-financed by the European Regional Development Fund (ERDF) through NORTE 2020 (North Regional Operational Program 2014/2020). For the author integrated in the CITAB Research Centre, this work was supported by the National Funds of FCT, under the project UIDB/04033/2020. For the author integrated in the CQVR, the research was additionally supported by National Funds of FCT, under the projects UIDB/QUI/00616/2020 and UIDP/00616/2020.
Institutional Review Board Statement
Fish sampling was conducted in accordance with the Ethical Guidelines of the European Union Council (European Directive 2010/63/EU, 22/09/2010) and the Portuguese Agricultural Ministry (Portaria no 1005/92, 23/10/1992) for the protection of animals used for experimental and other scientific purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Abdel-tawwab, M.; Wafeek, M. Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein–cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L.). J. Therm. Biol. 2014, 45, 157–162. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Dai, M.; Diao, X.; Dai, S.; Fang, T.; Dong, X. Input of Cd from agriculture phosphate fertilizer application in China during 2006–2016. Sci. Total Environ. 2020, 698, 134149. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, I.; Usmani, N.; Ahmad, M. Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemosphere 2016, 151, 210–219. [Google Scholar] [CrossRef]
- Saran, L.; Pissarra, T.; Silveira, G.; Constancio, M.; de Melo, W.J.; Alves, L. Land use impact on potentially toxic metals concentration on surface water and resistant microorganisms in watersheds. Ecotoxicol. Environ. Saf. 2018, 166, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Assessment of toxic metals in water and sediment of Pasur River in Bangladesh. Water Sci. Technol. 2018, 77, 1418–1430. [Google Scholar] [CrossRef]
- Han, J.; Park, H.; Kim, J.; Jeong, D.; Kang, J. Toxic effects of arsenic on growth, hematological parameters, and plasma components of starry flounder, Platichthys stellatus, at two water temperature conditions. Fish. Aquat. Sci. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Li, J.; Gao, X.; Zhang, J.; Liu, Z. Ecological and health risk assessments and water quality criteria of heavy metals in the Haihe River. Environ. Pollut. 2021, 290, 117971. [Google Scholar] [CrossRef]
- Colin, N.; Porte, C.; Fernandes, D.; Barata, C.; Padrós, F.; Carrassón, M.; Monroy, M.; Cano-Rocabayera, O.; de Sostoa, A.; Piña, B.; et al. Ecological relevance of biomarkers in monitoring studies of macro-invertebrates and fish in Mediterranean rivers. Sci. Total Environ. 2016, 540, 307–323. [Google Scholar] [CrossRef]
- Prasanna, M.V.; Praveena, S.M.; Chidambaram, S.; Nagarajan, R.; Elayaraja, A. Evaluation of water quality pollution indices for heavy metal contamination monitoring: A case study from Curtin Lake, Miri City, East Malaysia. Environ. Earth Sci. 2012, 67, 1987–2001. [Google Scholar] [CrossRef]
- Arcy, B.D.; Frost, A. The role of best management practices in alleviating water quality problems associated with diffuse pollution. Sci. Total Environ. 2001, 265, 359–367. [Google Scholar] [CrossRef]
- Barisie, J.; Dragun, Z.; Ramani, S.; Marijie, V.F.; Krasniei, N.; Coz-Rakovac, R.; Kostov, V.; Rebok, K.; Jordanova, M. Evaluation of histopathological alterations in the gills of Vardar chub (Squalius vardarensis Karaman) as an indicator of river pollution. Ecotoxicol. Environ. Saf. 2015, 118, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kaptaner, B.; Kankaya, E.; Dogan, A.; Durmuş, A. Alterations in histology and antioxidant defense system in the testes of the lake Van fish (Alburnus tarichi Güldenstädt, 1814). Environ. Monit. Assess. 2016, 188, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environ. Toxicol. Pharmacol. 2017, 53, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Raibeemol, K.P.; Chitra, K.C. Fish and Shellfish Immunology Induction of immunological, hormonal and histological alterations after sublethal exposure of chlorpyrifos in the freshwater fish, Pseudetroplus maculatus (Bloch, 1795). Fish Shellfish Immunol. 2020, 102, 1–12. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; Al-kahtani, M.A.; Elmenshawy, O.M. Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 2012, 88, 1028–1035. [Google Scholar] [CrossRef]
- Luzio, A.; Monteiro, S.M.; Garcia-santos, S.; Rocha, E.; Fontaínhas-fernandes, A.A.; Coimbra, A.M. Zebrafish sex differentiation and gonad development after exposure to 17-ethinylestradiol, fadrozole and their binary mixture: A stereological study. Aquat. Toxicol. 2015, 166, 83–95. [Google Scholar] [CrossRef]
- Micale, V.; Perdichizzi, A.; Muglia, U.; Rinelli, P.; Cosenza, A.; Mita, D.G. Gonadal macrophage aggregates in fish: A preliminary quantitative study in red mullet. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 357–361. [Google Scholar] [CrossRef]
- Saraiva, A.; Costa, J.; Serrão, J.; Eiras, J.C.; Cruz, C. Study of the gill health status of farmed sea bass (Dicentrarchus labrax L., 1758) using different tools. Aquaculture 2015, 441, 16–20. [Google Scholar] [CrossRef]
- van Dyk, J.C.; Cochrane, M.J.; Wagenaar, G.M. Liver histopathology of the sharptooth catfish Clarias gariepinus as a biomarker of aquatic pollution. Chemosphere 2012, 87, 301–311. [Google Scholar] [CrossRef]
- Agbohessi, P.T.; Toko, I.I.; Atchou, V.; Tonato, R.; Mandiki, S.N.M.; Kestemont, P. Pesticides used in cotton production affect reproductive development, endocrine regulation, liver status and offspring fitness in African catfish Clarias gariepinus (Burchell, 1822). Comp. Biochem. Physiol. Part C 2015, 167, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Kostić, J.; Kolarević, S.; Kračun-Kolarević, M.; Aborgiba, M.; Gačić, Z.; Paunović, M.; Višnjić-Jeftić, Ž.; Rašković, B.; Poleksić, V.; Lenhardt, M.; et al. The impact of multiple stressors on the biomarkers response in gills and liver of freshwater breams during different seasons. Sci. Total Environ. 2017, 602, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. Int. J. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Devin, S.; Burgeot, T.; Giambérini, L.; Minguez, L.; Pain-Devin, S. The integrated biomarker response revisited: Optimization to avoid misuse. Environ. Sci. Pollut. Res. 2014, 24, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Iturburu, F.G.; Bertrand, L.; Mendieta, J.R.; Amé, M.V.; Menone, M.L. An integrated biomarker response study explains more than the sum of the parts: Oxidative stress in the fi sh Australoheros facetus exposed to imidacloprid. Ecol. Indic. 2018, 93, 351–357. [Google Scholar] [CrossRef]
- Madeira, C.; Madeira, D.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Thermal acclimation in clownfish: An integrated biomarker response and multi-tissue experimental approach. Ecol. Indic. 2016, 71, 280–292. [Google Scholar] [CrossRef]
- Samanta, P.; Im, H.; Na, J.; Jung, J. Ecological risk assessment of a contaminated stream using multi-level integrated biomarker response in Carassius auratus *. Environ. Pollut. 2018, 233, 429–438. [Google Scholar] [CrossRef]
- Santos, R.M.B.; Fernandes, L.S.; Cortes, R.M.V.; Varandas, S.G.P.; Jesus, J.J.B.; Pacheco, F.A.L. Integrative assessment of river damming impacts on aquatic fauna in a Portuguese reservoir. Sci. Total Environ. 2017, 601–602, 1108–1118. [Google Scholar] [CrossRef]
- Boavida, I.; Jesus, J.B.; Pereira, V.; Santos, C.; Lopes, M.; Cortes, R.M. Fulfilling spawning flow requirements for potamodromous cyprinids in a restored river segment. Sci. Total Environ. 2018, 635, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Fernandes, L.; Pacheco, F.; Monteiro, M.; Jesus, J. River restoration for the replacement of lost spawning grounds due to dam construction. In Proceedings of the Sustainable Water Resources Management XI, online, 18–20 May 2021; WIT Press: Southampton, UK, 2021; Volume 250, pp. 35–44. [Google Scholar]
- DGT Directorate-General of Territory. Available online: http://www.dgterritorio.pt/ (accessed on 12 July 2021).
- Santos, R.M.B.; Sanches Fernandes, L.F.; Cortes, V.M.R.; Pacheco, F.A.L. Development of a Hydrologic and Water Allocation Model to Assess Water Availability in the Sabor River Basin ( Portugal ). Int. J. Environ. Res. Public Health 2019, 16, 2419. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Monteiro, S.M.; Cortes, R.M.V.; Pacheco, F.A.L.; Fernandes, L.F.S. Seasonal effect of land use management on gill histopathology of Barbel and Douro Nase in a Portuguese watershed. Sci. Total Environ. 2021, 764, 142869. [Google Scholar] [CrossRef]
- European Commision Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; L276/33; Official Journal European Union: Brussels, Belgium, 2010.
- Portaria no 1005/92; Ministérios da Agricultura, da Educação, da Saúde e do Comércio e Turismo. Diário da Répública I: Lisboa, Portugal, 1992.
- Pereira, S.; Pinto, A.L.; Cortes, R.; Fontaínhas-Fernandes, A.; Coimbra, A.M.; Monteiro, S.M. Gill histopathological and oxidative stress evaluation in native fish captured in Portuguese northwestern rivers. Ecotoxicol. Environ. Saf. 2013, 90, 157–166. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Rocha, E.; Fontaínhas-Fernandes, A.; Sousa, M. Quantitative histopathology of Oreochromis niloticus gills after copper exposure. J. Fish Biol. 2008, 73, 1376–1392. [Google Scholar] [CrossRef]
- Zimmerli, S.; Bernet, D.; Burkhardt-Holm, P.; Schmidt-Posthaus, H.; Vonlanthen, P.; Wahli, T.; Segner, H. Assessment of fish health status in four Swiss rivers showing a decline of brown trout catches. Aquat. Sci. 2007, 69, 11–25. [Google Scholar] [CrossRef]
- Santos, R.M.B.; Monteiro, S.M.V.; Cortes, R.M.V.; Pacheco, F.A.L.; Fernandes, L.F.S. Seasonal Differences in Water Pollution and Liver Histopathology of Iberian Barbel (Luciobarbus bocagei) and Douro Nase (Pseudochondrostoma duriense) in an Agricultural Watershed. Water 2022, 14, 444. [Google Scholar] [CrossRef]
- SPSS Statistical Product and Service Solutions, Statistical software, Version 26; IBM SPSS: Chicago, IL, USA, 2019.
- Johnson, R.; Wolf, J.; Braunbeck, T. OECD Guidance Document for the Diagnosis of Endocrine-Related Histopathology of Fish Gonads; Organisation de Coopération et de Développement Économiques: Paris, France, 2010. [Google Scholar]
- Martin-skilton, R.; Lavado, R.; Thibaut, R.; Minier, C.; Porte, C. Evidence of endocrine alteration in the red mullet, Mullus barbatus from the NW Mediterranean. Environ. Pollut. 2006, 141, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S. Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol. Biochem. 2002, 26, 85–101. [Google Scholar] [CrossRef]
- Zorita, I.; Ortiz-zarragoitia, M.; Apraiz, I.; Cancio, I.; Orbea, A.; Cajaraville, M.P.; Soto, M.; Marigómez, I.; Cajaraville, M.P. Assessment of biological effects of environmental pollution along the NW Mediterranean Sea using red mullets as sentinel organisms. Environ. Pollut. 2008, 153, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Schwindt, A.R.; Truelove, N.; Schreck, C.B.; Fournie, J.W.; Landers, D.H.; Kent, M.L. Quantitative evaluation of macrophage aggregates in brook trout Salvelinus fontinalis and rainbow trout Oncorhynchus mykiss. Dis. Aquat. Organ. 2006, 68, 101–113. [Google Scholar] [CrossRef]
- Silva, P.; Rocha, M.J.; Cruzeiro, C.; Malhão, F.; Reis, B.; Urbatzka, R.; Monteiro, R.A.F.; Rocha, E. Testing the effects of ethinylestradiol and of an environmentally relevant mixture of xenoestrogens as found in the Douro River (Portugal) on the maturation of fish gonads—A stereological study using the zebrafish (Danio rerio) as model. Aquat. Toxicol. 2012, 124–125, 1–10. [Google Scholar] [CrossRef]
- Rašković, B.; Poleksić, V.; Višnjić-Jeftić, Ž.; Skorić, S.; Gačić, Z.; Djikanović, V.; Jaric, I.; Lenhardt, M. Use of histopathology and elemental accumulation in different organs of two benthophagous fish species as indicators of river pollution. Environ. Toxicol. 2015, 30, 1153–1161. [Google Scholar] [CrossRef]
- Poleksic, V.; Lenhardt, M.; Jaric, I.; Djordjevic, D.; Gacic, Z.; Cvijanovic, G.; Raskovic, B. Liver, gills, and skin histopathology and heavy metal content of the Danube sterlet (Acipenser ruthenus Linnaeus, 1758). Environ. Toxicol. Chem. An Int. J. 2010, 29, 515–521. [Google Scholar] [CrossRef]
- Weber, A.; Sales, C.; Faria, F.; Melo, R.; Bazzoli, N.; Rizzo, E. Effects of metal contamination on liver in two fish species from a highly impacted neotropical river: A case study of the Fundão dam, Brazil. Ecotoxicol. Environ. Saf. 2020, 190, 110165. [Google Scholar] [CrossRef]
- Jordanova, M.; Rebok, K.; Dragun, Z.; Ramani, S.; Ivanova, L.; Kostov, V.; Valić, D.; Krasnići, N.; Marijić, V.; Kapetanovi, D. Histopathology investigation on the Vardar chub (Squalius vardarensis) populations captured from the rivers impacted by mining activities. Ecotoxicol. Environ. Saf. 2016, 129, 35–42. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Lin, L. Dissolved oxygen concentration predictions for running waters with different land use land cover using a quantile regression forest machine learning technique. J. Hydrol. 2021, 597, 126213. [Google Scholar] [CrossRef]
- McDowell, R.W. Assessing the leaching of cadmium in an irrigated and grazed pasture soil. Environ. Pollut. 2022, 292, 118430. [Google Scholar] [CrossRef]
- Niño-savala, A.G.; Zhuang, Z.; Ma, X.; Fangmeier, A.; Li, H.; Tang, A.; Liu, X. Cadmium pollution from phosphate fertilizers in arable soils and crops: An overview. Front. Agric. Sci. Eng. 2019, 6, 419–430. [Google Scholar] [CrossRef]
- Wan, L.; Lv, H.; Qasim, W.; Xia, L.; Yao, Z.; Hu, J.; Zhao, Y.; Ding, X.; Zheng, X.; Li, G.; et al. Heavy metal and nutrient concentrations in top- and sub-soils of greenhouses and arable fields in East China—Effects of cultivation years, management, and shelter. Environ. Pollut. 2022, 307, 119494. [Google Scholar] [CrossRef] [PubMed]
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant defense system, immune response and erythron pro fi le modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Köck, G.; Triendl, M.; Hofer, R. Seasonal patterns of metal accumulation in Arctic char (Salvelinus alpinus) from an oligotrophic Alpine lake related to temperature. Can. J. Fish. Aquat. Sci. 1996, 53, 780–786. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H. Uptake and Elimination of Cadmium by Japanese Eel, Anguilla japonica, at Various Temperatures. Bull. Environ. Contam. Toxicol. 1996, 56, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, B.; Witeska, M. The metal uptake and accumulation in fish living in polluted waters. In Soil and Water Pollution Monitoring, Protection and Remediation; Twardowska, I., Allen, H.E., Häggblom, M.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 107–114. ISBN 9781402047275. [Google Scholar]
- Zhang, J.; Wang, K.; Yi, Q.; Zhang, T.; Shi, W.; Zhou, X. Transport and partitioning of metals in river networks of a plain area with sedimentary resuspension and implications for downstream lakes. Environ. Pollut. 2022, 294, 118668. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.C.; Silveira, A.W.; Silva, A.L.; Souza, A.I.; Povh, J.A.; Jaques, J.A.; Santos, E.A.; Yonekawa, M.K.; Penteadoall, B.B.; Franco-Belussi, L. Osmoregulatory profiles and gill histological changes in Nile tilapia (Oreochromis niloticus) exposed to lambda-cyhalothrin. Aquat. Toxicol. 2020, 227, 105612. [Google Scholar] [CrossRef]
- Pereira, L. Qualidade das águas superficiais na bacia hidrográfica do rio Almonda; CCDR–LVT – Comissão de Coordenação e Desenvolvimento Regional de Lisboa e Vale do Tejo. Divisão de Monitorização Ambiental; Rede de Monitorização de Recursos Hídricos Superficiais: Lisboa, Portugal, 2004. [Google Scholar]
- Rodrigues, R.; Quadrado, F.; Lopes, A.R. A Qualidade da Água em Portugal; Instituto da Água: Lisboa, Portugal, 2001.
- SNIRH National Information System for Water Resources. Available online: https://snirh.apambiente.pt/ (accessed on 6 March 2021).
- Decreto-Lei n. º, 236/98. Regulamentação da Qualidade Com a Finalidade de Proteger o Meio Aquático e Melhorar a Qualidade Das Águas em Função dos Seus Principais Usos. 1998, Volume 176. Available online: https://dre.pt/dre/legislacao-consolidada/decreto-lei/1998-75031534 (accessed on 15 September 2021).
- WFD Directive of the European Parliament and of the Council 2000/60/EC. Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Parliam. 2000, L327, 1–82.
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 2002, 57, 295–304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).