The Potential of rGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water
Abstract
1. Introduction
1.1. Challenges of TiO2 Semiconductor Photocatalysts
1.2. Why Coupling TiO2 with Graphene?
1.2.1. Synthesis of Graphene Oxide (GO)
1.2.2. Reduction of GO to rGO
1.2.3. Preparation of rGO-Based TiO2 Photocatalyst
2. Photodegradation of Organic Pollutants
Reusability of rGO@TiO2
3. Factors Affecting the Photodegradation of Organic Pollutants
3.1. The Effect of GO to TiO2 Weight Ratio
3.2. Effect of Catalyst Loading
3.3. Effect of Initial Pollutant Concentration
3.4. Effect of Initial pH
3.5. Effect of Water Matrix on the Photocatalytic Degradation of Pollutant
3.6. Effect of Intensity and Wavelength of Light Irradiation
3.7. Effect of Scavengers
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OMPs | organic micropollutants |
| MB | methylene blue |
| AOPs | advanced oxidation processes |
| TiO2 | titanium dioxide |
| UV | ultraviolet |
| e− | electron |
| h+ | hole |
| GO | graphene oxide |
| rGO | reduced graphene oxide |
| λ | lambda |
| HNO3 | nitric acid |
| KClO3 | potassium chlorate |
| H2SO4 | sulfuric acid |
| KMnO4 | potassium permanganate |
| NaNO3 | sodium nitrate |
| NO2 | nitrogen dioxide |
| N2O4 | dinitrogen tetroxide |
| ClO2 | chlorine dioxide |
| TTIP | titanium (IV) isopropoxide |
| WHO | World Health Organization |
| MO | methylene orange |
| Vis | visible |
| RhB | rhodamine B |
| Hg | mercury |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| ISI-MS | ion-spray ionization mass spectrometry |
| 2,4-DCP | 2,4-dichlorophenol |
| 2,4-DCR | dichlororesorcinol |
| TOC | total organic carbon |
| DCF | diclofenac |
| AO7 | acid orange 7 |
| •OH | hydroxyl radical |
| O2•− | superoxide radical |
| PZC | point of zero charge |
| Cl− | chloride ion |
| SO42− | sulphate ion |
| PO43− | phosphate ion |
| NO3− | nitrate ion |
| VB | valence band |
| CB | conduction band |
| EDTA | ethylenediaminetetraacetic acid |
| AgNO3 | silver nitrate |
| K2S2O8 | potassium persulfate |
| NaN3 | sodium azide |
| t-BuOH | tert-butyl alcohol |
References
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Karimi-Maleh, H. A review on magnetic sensors for monitoring of hazardous pollutants in water resources. Sci. Total Environ. 2022, 824, 153844. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Show, P.-L.; Orooji, Y.; Karimi-Maleh, H. Utilization of a double-cross-linked amino-functionalized three-dimensional graphene networks as a monolithic adsorbent for methyl orange removal: Equilibrium, kinetics, thermodynamics and artificial neural network modeling. Environ. Res. 2022, 207, 112156. [Google Scholar] [CrossRef] [PubMed]
- Karaman, C.; Karaman, O.; Show, P.-L.; Karimi-Maleh, H.; Zare, N. Congo red dye removal from aqueous environment by cationic surfactant modified-biomass derived carbon: Equilibrium, kinetic, and thermodynamic modeling, and forecasting via artificial neural network approach. Chemosphere 2022, 290, 133346. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Tanhaei, B.; Ayati, A.; Tabrizi, S.H.; Alizadeh, M.; Bamoharram, F.F.; Karimi, F.; Salmanpour, S.; Rouhi, J.; Afshar, S.; et al. Heterogeneous UV-Switchable Au nanoparticles decorated tungstophosphoric acid/TiO2 for efficient photocatalytic degradation process. Chemosphere 2021, 281, 130795. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ranjbari, S.; Tanhaei, B.; Ayati, A.; Orooji, Y.; Alizadeh, M.; Karimi, F.; Salmanpour, S.; Rouhi, J.; Sillanpää, M.; et al. Novel 1-butyl-3-methylimidazomohammadim bromide impregnated chitosan hydrogel beads nanostructure as an efficient nanobio-adsorbent for cationic dye removal: Kinetic study. Environ. Res. 2021, 195, 110809. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC-Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Adeola, A.O.; Abiodun, B.A.; Adenuga, D.O.; Nomngongo, P.N. Adsorptive and photocatalytic remediation of hazardous organic chemical pollutants in aqueous medium: A review. J. Contam. Hydrol. 2022, 248, 104019. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71, 105367. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Noor, N.H.; Binti, M.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- Liu, Y.; Su, G.; Zhang, B.; Jiang, G.; Yan, B. Nanoparticle-based strategies for detection and remediation of environmental pollutants. Analyst 2011, 136, 872–877. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Shalan, A.E. An overview of nanomaterials for industrial wastewater treatment. Korean J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Habibah, N.; Serr, A.; Nasir, M.; Ibrahim, M. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Du, X.; Luo, J.; Qin, Q.; Zhang, J.; Fu, D. Modified TiO2-rGO Binary Photo-Degradation Nanomaterials: Modification, Mechanism, and Perspective. Catal. Surv. Asia 2022, 26, 16–34. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Wanag, A.; Kusiak-Nejman, E.; Czyżewski, A.; Moszyński, D.; Morawski, A.W. Influence of rGO and preparation method on the physicochemical and photocatalytic properties of TiO2/reduced graphene oxide photocatalysts. Catalysts 2021, 11, 1333. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Peter, A.; Al-Najar, B.; Vijaya, J.; Bououdina, M. Photocatalysis: Activity of Nanomaterials. In Nanotechnology in Environmental Science; Wiley: Hoboken, NJ, USA, 2018; pp. 209–292. [Google Scholar]
- Xie, X.; Kretschmer, K.; Wang, G. Advances in graphene-based semiconductor photocatalysts for solar energy conversion: Fundamentals and materials engineering. Nanoscale 2015, 7, 13278–13292. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Allen, J.M.; Vincent, T.C.; Richard, K.B. Honeycomb carbon: A Review of Graphene What is graphene? Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene Derivatives in Photocatalysis. In Graphene-Based Energy Devices; Wiley: Hoboken, NJ, USA, 2015; pp. 249–276. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul, A.Y. Graphene oxide and derivatives: The place in graphene family. Front. Phys. 2019, 6. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Eigler, S. Mechanistic insights into the reduction of graphene oxide addressing its surfaces. Phys. Chem. Chem. Phys. 2014, 16, 19832–19835. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide–A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.T.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Method for the preparation of the graphite acid. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Al Kausor, M.; Chakrabortty, D. Graphene oxide based semiconductor photocatalysts for degradation of organic dye in waste water: A review on fabrication, performance enhancement and challenges. Inorg. Chem. Commun. 2021, 129, 108630. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.; Wilson, L. Preparation and Characterization of Graphene Oxide Cross-Linked Composites. In Proceedings of the CSME Conference Proceedings, Toronto, ON, Canada, 27–30 May 2018. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed]
- Paulchamy, B.; Arthi, G.; Lignesh, B.D. A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1–4. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Anandan, S.; Manivel, A.; Ashokkumar, M. One-step sonochemical synthesis of reduced graphene oxide/Pt/Sn hybrid materials and their electrochemical properties. Fuel Cells 2012, 12, 956–962. [Google Scholar] [CrossRef]
- Viinikanoja, A.; Wang, Z.; Kauppila, J.; Kvarnström, C. Electrochemical reduction of graphene oxide and its in situ spectroelectrochemical characterization. Phys. Chem. Chem. Phys. 2012, 14, 14003–14009. [Google Scholar] [CrossRef]
- Quezada Renteria, J.A.; Ruiz-Garcia, C.; Sauvage, T.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R.; Ania, C.O. Photochemical and electrochemical reduction of graphene oxide thin films: Tuning the nature of surface defects. Phys. Chem. Chem. Phys. 2020, 22, 20732–20743. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Stepić, K.; Ljupković, R.; Ickovski, J.; Zarubica, A. A short review of titania-graphene oxide-based composites as a photocatalysts. Adv. Technol. 2021, 10, 51–60. [Google Scholar] [CrossRef]
- Das, A.; Adak, M.K.; Mahata, N.; Biswas, B. Wastewater treatment with the advent of TiO2 endowed photocatalysts and their reaction kinetics with scavenger effect. J. Mol. Liq. 2021, 338, 116479. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, C.; Wang, H.; Chen, M.; Zeng, G.; Liu, Z.; Liu, Y.; Wang, W.; Wu, T.; Shao, B.; et al. Recent advance of graphene/semiconductor composite nanocatalysts: Synthesis, mechanism, applications and perspectives. Chem. Eng. J. 2021, 414, 128795. [Google Scholar] [CrossRef]
- Preetha, S.; Pillai, R.; Ramamoorthy, S.; Mayeen, A.; Archana, K.M.; Kalarikkal, N.; Narasimhamurthy, B.; Lekshmi, I.C. TiO2–rGO Nanocomposites with high rGO content and Luminescence Quenching through Green Redox Synthesis. Surf. Interfaces 2022, 30, 101812. [Google Scholar] [CrossRef]
- Dutta, V.; Singh, P.; Shandilya, P.; Sharma, S.; Raizada, P.; Saini, A.K.; Gupta, V.K.; Hosseini-Bandegharaei, A.; Agarwal, S.; Rahmani-Sani, A. Review on advances in photocatalytic water disinfection utilizing graphene and graphene derivatives-based nanocomposites. J. Environ. Chem. Eng. 2019, 7, 103132. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]
- Sohail, M.; Xue, H.; Jiao, Q.; Li, H.; Khan, K.; Wang, S.; Zhao, Y. Synthesis of well-dispersed TiO2@reduced graphene oxide (rGO) nanocomposites and their photocatalytic properties. Mater. Res. Bull. 2017, 90, 125–130. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Kale, D.P.; Kar, S.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K.; Sonawane, S.H. Ultrasound assisted preparation of rGO/TiO2 nanocomposite for effective photocatalytic degradation of methylene blue under sunlight. Nano-Struct. Nano-Objects 2020, 21, 100407. [Google Scholar] [CrossRef]
- Garrafa-Gálvez, H.E.; Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Hurtado-Macías, A.; Garzon-Fontecha, A.M.; Luque, P.A.; Castro-Beltrán, A. Graphene role in improved solar photocatalytic performance of TiO2-RGO nanocomposite. Chem. Phys. 2019, 521, 35–43. [Google Scholar] [CrossRef]
- Deepthi, J.; Rajalakshmi, A.S.; Lopez, R.M.; Achari, V.S. TiO2-reduced graphene oxide nanocomposites for the trace removal of diclofenac. SN Appl. Sci. 2020, 2, 480. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2−x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhong, Y.H.; Chen, X.; Huang, X.J.; Wu, Y.C. Mesoporous anatase TiO2/reduced graphene oxide nanocomposites: A simple template-free synthesis and their high photocatalytic performance. Mater. Res. Bull. 2014, 51, 244–250. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide-TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, Á.; Manassero, A.; Satuf, M.L.; Alfano, O.M.; Casas, J.A.; Bahamonde, A. Influence of TiO2-rGO optical properties on the photocatalytic activity and efficiency to photodegrade an emerging pollutant. Appl. Catal. B Environ. 2019, 246, 1–11. [Google Scholar] [CrossRef]
- Leal, J.F.; Cruz, S.M.A.; Almeida, B.T.A.; Esteves, V.I.; Marques, P.A.A.P.; Santos, E.B.H. TiO2-rGO nanocomposite as an efficient catalyst to photodegrade formalin in aquaculture’s waters, under solar light. Environ. Sci. Water Res. Technol. 2020, 6, 1018–1027. [Google Scholar] [CrossRef]
- Sun, X.; Ji, S.; Wang, M.; Dou, J.; Yang, Z.; Qiu, H.; Kou, S.; Ji, Y.; Wang, H. Fabrication of porous TiO2-rGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes. J. Alloys Compd. 2020, 819, 153033. [Google Scholar] [CrossRef]
- H.H. Ali, M.; E Goher, M.; D.G. Al-Afify, A. Kinetics and Adsorption Isotherm Studies of Methylene Blue Photodegradation Under UV Irradiation Using Reduced Graphene Oxide-TiO2 Nanocomposite in Different Wastewaters Effluents. Egypt. J. Aquat. Biol. Fish. 2019, 23, 253–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Zhou, F.; Yang, X.; Zhang, D.; Chen, Y. Synergistic effect of rGO/TiO2 nanosheets with exposed (001) facets for boosting visible light photocatalytic activity. Appl. Surf. Sci. 2020, 510, 145451. [Google Scholar] [CrossRef]
- Duan, Y.; Gou, M.L.; Guo, Y.; Cai, J.; Song, W.; Liu, Z.; Zhou, E. In situ hydrothermal synthesis of TiO2–rGO nanocomposites for 4-nitrophenol degradation under sunlight irradiation. J. Mater. Res. 2021, 36, 906–915. [Google Scholar] [CrossRef]
- Maruthamani, D.; Divakar, D.; Kumaravel, M. Enhanced photocatalytic activity of TiO2 by reduced graphene oxide in mineralization of Rhodamine B dye. J. Ind. Eng. Chem. 2015, 30, 33–43. [Google Scholar] [CrossRef]
- Abd Elnaby, M.; Ahmed, M.A.; Abdel-Samad, H.S.; Hesham Medien, M. Exceptional removal of methylene blue dye over novel TiO2/rGO nanocomposites by tandem adsorption-photocatalytic processes. Mater. Sci. Energy Technol. 2022, 5, 217–231. [Google Scholar] [CrossRef]
- Fan, H.; Yi, G.; Zhang, Z.; Zhang, X.; Li, P.; Zhang, C.; Chen, L.; Zhang, Y.; Sun, Q. Binary TiO2/rGO photocatalyst for enhanced degradation of phenol and its application in underground coal gasification wastewater treatment. Opt. Mater. 2021, 120, 111482. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Ma, F.; Xu, Y. Synergistic effects and kinetics of rGO-modified TiO2 nanocomposite on adsorption and photocatalytic degradation of humic acid. J. Environ. Manag. 2019, 235, 293–302. [Google Scholar] [CrossRef]
- Sun, N.; Ma, J.; Wang, C.; Xue, J.; Qiang, L.; Tang, J. A facile and efficient method to directly synthesize TiO2/rGO with enhanced photocatalytic performance. Superlattices Microstruct. 2018, 121, 1–8. [Google Scholar] [CrossRef]
- Ruidíaz-Martínez, M.; Álvarez, M.A.; López-Ramón, M.V.; Cruz-Quesada, G.; Rivera-Utrilla, J.; Sánchez-Polo, M. Hydrothermal Synthesis of rGO-TiO2 Composites as High-Performance UV Photocatalysts for Ethylparaben Degradation. Catalysts 2020, 10, 520. [Google Scholar] [CrossRef]
- Yu, F.; Bai, X.; Yang, C.; Xu, L.; Ma, J. Reduced Graphene Oxide–P25 Nanocomposites as Efficient Photocatalysts for Degradation of Bisphenol A in Water. Catalysts 2019, 9, 607. [Google Scholar] [CrossRef]
- Balsamo, S.A.; Fiorenza, R.; Condorelli, M.; Pecoraro, R.; Brundo, M.V.; Presti, F.L.; Scir, S. One-Pot Synthesis of TiO2-rGO Photocatalysts for the Degradation of Groundwater Pollutants. Materials 2021, 14, 5938. [Google Scholar] [CrossRef]
- Sohail, M.; Saleem, M.; Ullah, S.; Saeed, N.; Afridi, A.; Khan, M.; Arif, M. Modified and improved Hummer’s synthesis of graphene oxide for capacitors applications. Mod. Electron. Mater. 2017, 3, 110–116. [Google Scholar] [CrossRef]
- Liu, Y. Hydrothermal synthesis of TiO2-RGO composites and their improved photocatalytic activity in visible light. RSC Adv. 2014, 4, 36040–36045. [Google Scholar] [CrossRef]
- Mohammadi, M.; Rezaee Roknabadi, M.; Behdani, M.; Kompany, A. Enhancement of visible and UV light photocatalytic activity of rGO-TiO2 nanocomposites: The effect of TiO2/Graphene oxide weight ratio. Ceram. Int. 2019, 45, 12625–12634. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica- Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2019, 357, 630–637. [Google Scholar] [CrossRef]
- Wu, S.; Jia, Q.; Dai, W. Synthesis of rGO/TiO2 hybrid as a high performance photocatalyst. Ceram. Int. 2017, 43, 1530–1535. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar photocatalytic degradation of pesticides over TiO2-rGO nanocomposites at pilot plant scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef]
- Ton, N.N.T.; Dao, A.T.N.; Kato, K.; Ikenaga, T.; Trinh, D.X.; Taniike, T. One-pot synthesis of TiO2/graphene nanocomposites for excellent visible light photocatalysis based on chemical exfoliation method. Carbon 2018, 133, 109–117. [Google Scholar] [CrossRef]
- Liang, D.; Cui, C.; Hub, H.; Wang, Y.; Xu, S.; Ying, B.; Li, P.; Lu, B.; Shen, H. One-step hydrothermal synthesis of anatase TiO2/reduced graphene oxide nanocomposites with enhanced photocatalytic activity. J. Alloys Compd. 2014, 582, 236–240. [Google Scholar] [CrossRef]
- Li, T.; Wang, T.; Qu, G.; Liang, D.; Hu, S. Synthesis and photocatalytic performance of reduced graphene oxide–TiO2 nanocomposites for orange II degradation under UV light irradiation. Environ. Sci. Pollut. Res. 2017, 24, 12416–12425. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Chen, C.; Wang, X. Enhanced photocatalytic degradation of methylene blue under visible irradiation on graphene@TiO2 dyade structure. Appl. Catal. B Environ. 2012, 111–112, 303–308. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Wang, X.; Zhang, J.; Long, T. A critical review on challenges and trend of ultrapure water production process. Sci. Total Environ. 2021, 785, 147254. [Google Scholar] [CrossRef] [PubMed]
- Díez, A.M.; Pazos, M.; Sanromán, M.A. Synthesis of magnetic-photo-Fenton catalyst for degradation of emerging pollutant. Catal. Today 2019, 328, 267–273. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Chang, K.C.; Lin, K.L.; Ma, C.M. Study on isopropanol degradation by UV/TiO2 nanotube. AIP Conf. Proc. 2018, 1946, 020006. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; Gonçalves, G.; Radošević, T.; Vengust, D.; Podlogar, M. Immobilised rGO/TiO2 Nanocomposite for Multi-Cycle Removal of Methylene Blue Dye from an Aqueous Medium. Appl. Sci. 2022, 12, 385. [Google Scholar] [CrossRef]
- Muthirulan, P.; Devi, C.N.; Sundaram, M.M. TiO2 wrapped graphene as a high performance photocatalyst for acid orange 7 dye degradation under solar/UV light irradiations. Ceram. Int. 2014, 40, 5945–5957. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting Photocatalytic Activity Using Reduced Graphene Oxide (rGO)/Semiconductor Nanocomposites: Issues and Future Scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef]



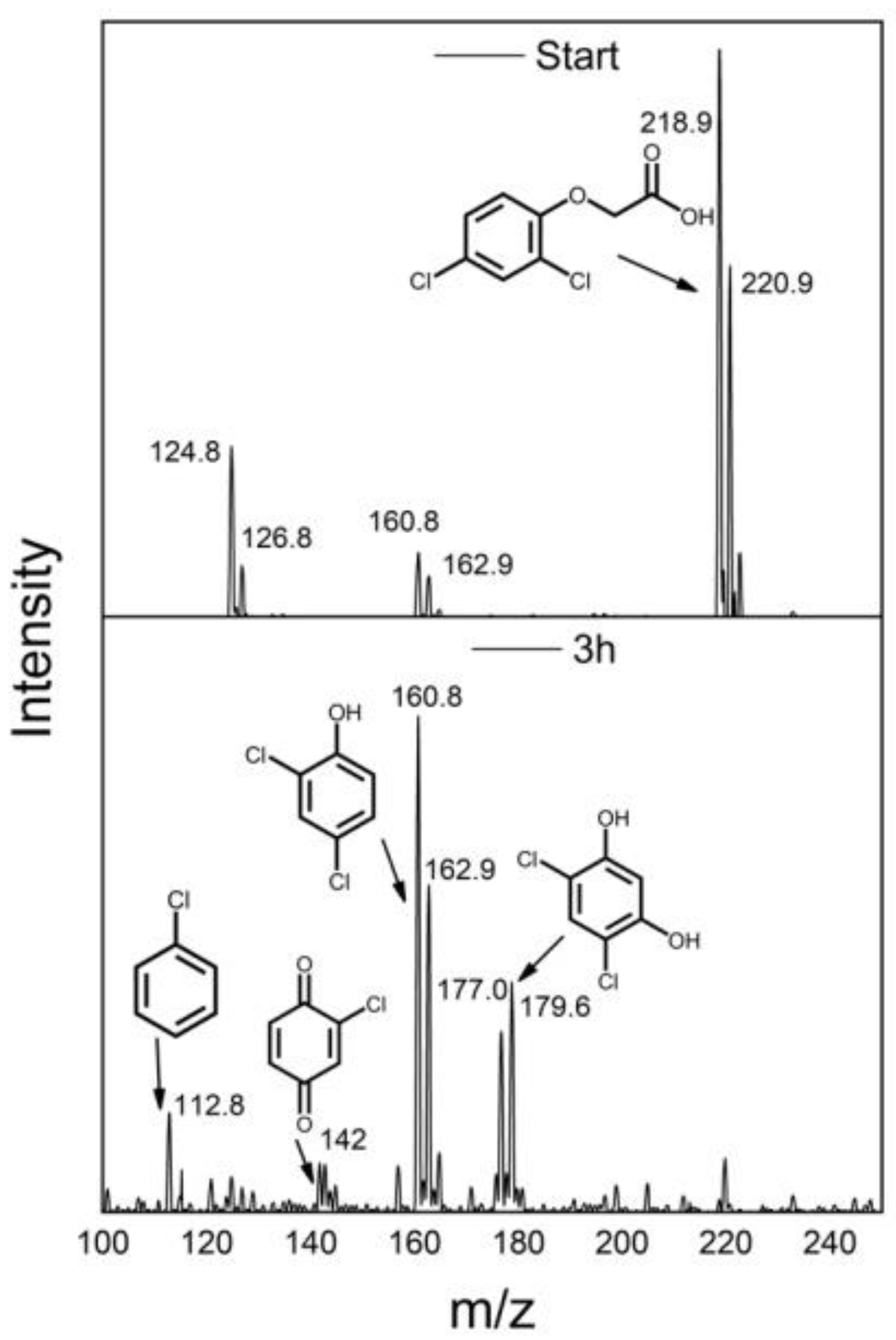
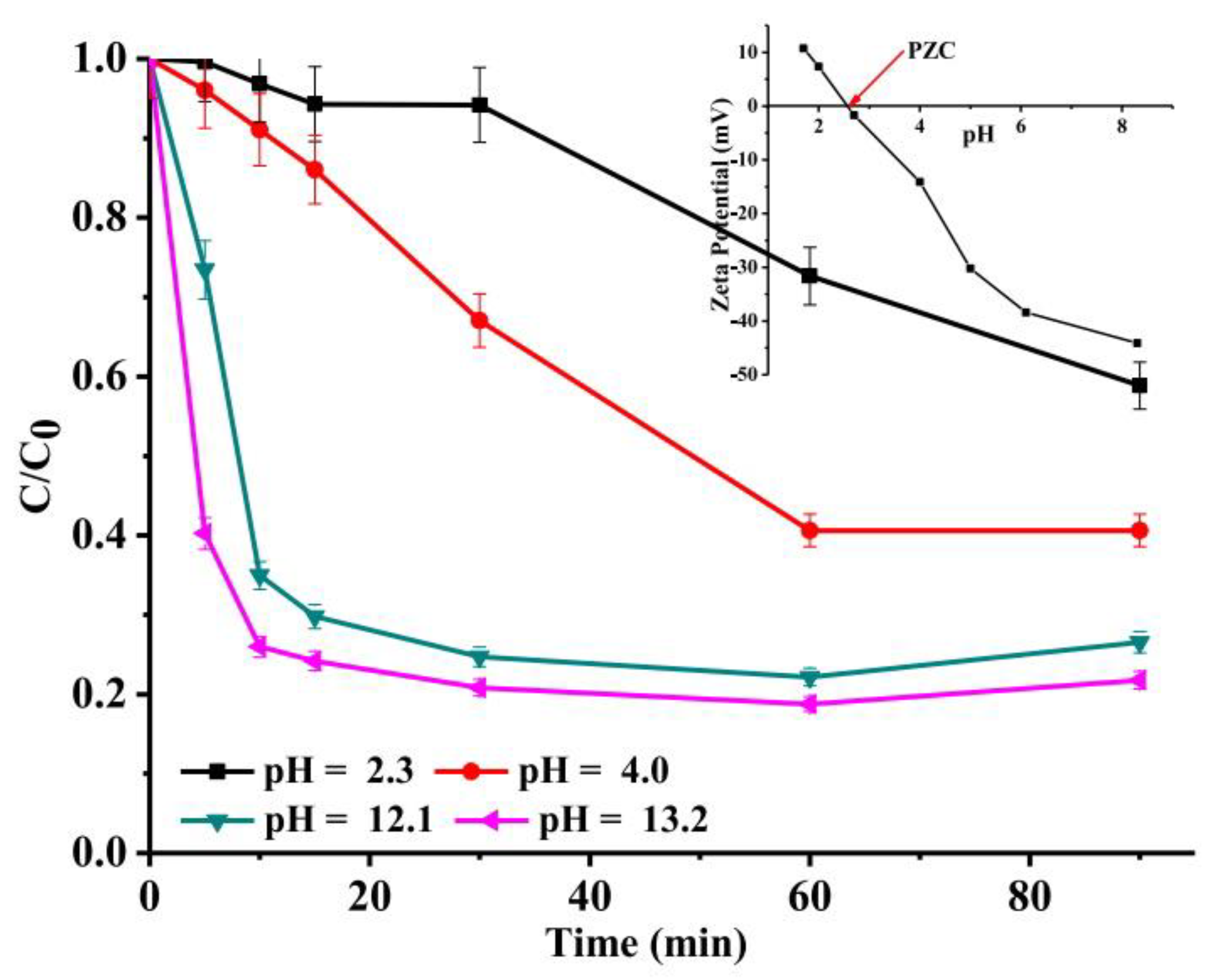
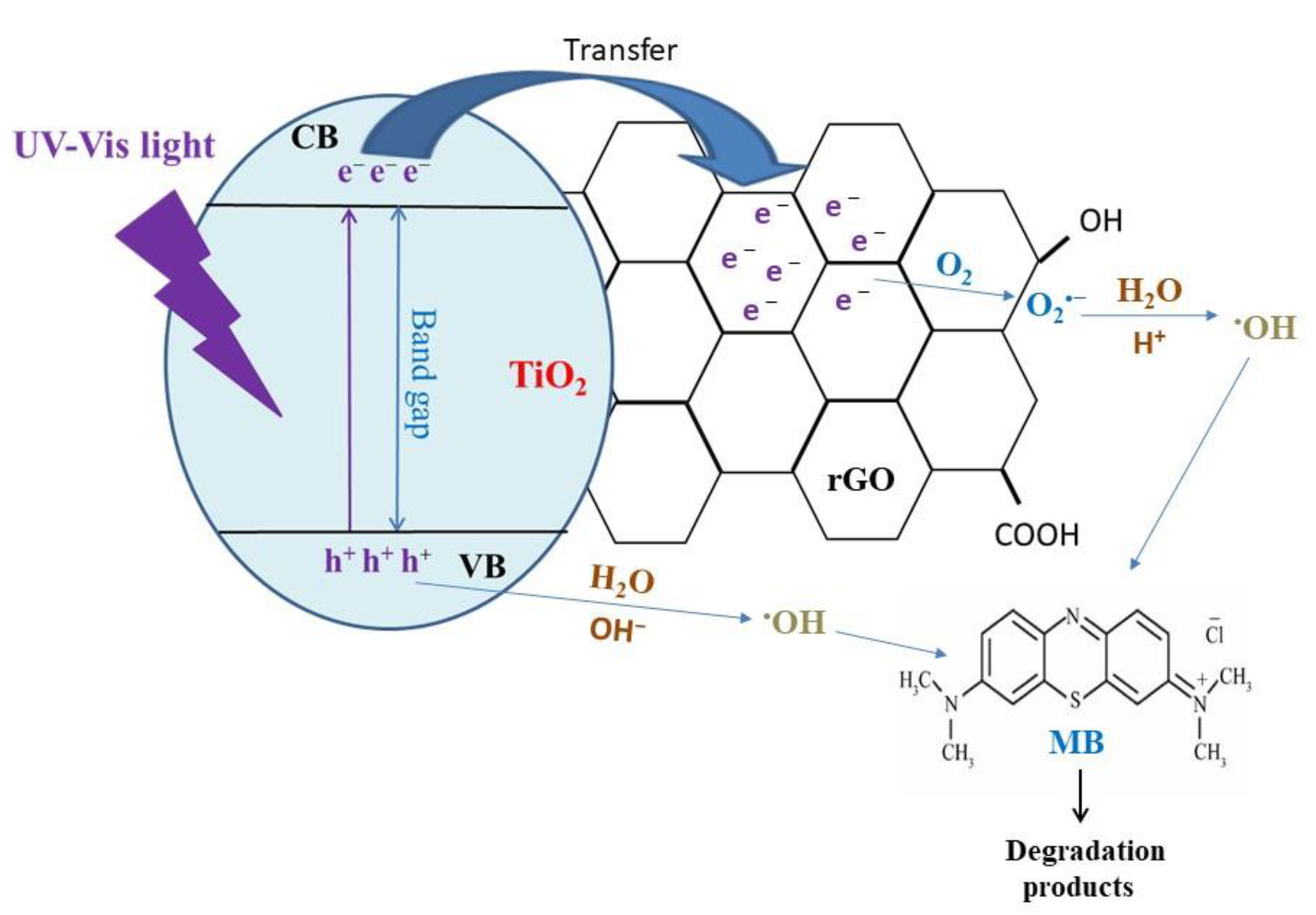
| Synthesis Method | Catalyst Dosage | Light Source | Pollutant and Initial Concentration | Irradiation Time | Kinetic /10−3 | Removal Efficiency | Reference |
|---|---|---|---|---|---|---|---|
| Solvothermal | 0.8 g/L | UV light | Methylene blue, 10 mg/L | 90 min | - | 100.0% | [25] |
| Sol-gel method | 0.5 g/L | Natural sunlight | Alizarin Yellow GG, 10 mg/L | 150 min | - | 100.0% | [61] |
| Hydrothermal | 0.5 g/L | Simulated solar light | Rhodamine B, 10 mg/L | 120 min | 53.1 min−1 | 99.8% | [63] |
| Hydrothermal | 0.5 g/L | Simulated solar light | Methylene blue, 10 mg/L | 120 min | 32.84 min−1 | 98.1% | [63] |
| Sonochemical | 2.0 g/L | Natural sunlight | Methylene blue, 20 mg/L | 30 min | 18.8 min−1 | 91.3% | [65] |
| Solvothermal | 0.1 g/L | Natural sunlight | Methylene blue, 15 mg/L | 60 min | 21.2 min−1 | 85.0% | [66] |
| Solvothermal | 0.1 g/L | UV lamp | Methylene blue, 15 mg/L | 60 min | 89.1 min−1 | 100.0% | [66] |
| Hydrothermal | 0.075 g/L | Natural sunlight | Diclofenac, 25 mg/L | 60 min | 50.4 min−1 | ~100.0% | [67] |
| Hydrothermal | 0.1 g/L | UV light | Methylene blue, 12 mg/L | 180 min | 13.1 min−1 | ~90.0% | [68] |
| Hydrothermal | 0.0004 g/L | UV light | Methyl orange, 30 mg/L | 30 min | 122.0 min−1 | 95.0% | [69] |
| Hydrothermal | 0.5 g/L | Visible light | Methyl orange, 10 mg/L | 240 min | 126.0 min−1 | 87.4% | [70] |
| Hydrothermal | 0.1 g/L | UV-A lamp | Clofibric acid, 20 mg/L | 360 min | 11.2 min−1 | 100.0% | [71] |
| Hydrothermal | 0.18 g/L | Simulated sunlight | Formalin, 40 mg/L | 90 min | 17.9 min−1 | 93.8% | [72] |
| Freeze-drying | 0.4 g/L | Simulated solar lamp | Rhodamine B, 10 mg/L | 240 min | - | 84.6% | [73] |
| Ultrasonication | 0.75 g/L | UV light source | Methylene blue, 30 mg/L | 120 min | - | 99.3% | [74] |
| Solvothermal | 0.1 g/L | Visible light | Methyl orange, 10 mg/L | 180 min | - | 94.1% | [75] |
| Hydrothermal | 0.25 g/L | Simulated sunlight | 4-nitrophenol, 45 mg/L | 360 min | 11.8 min−1 | 95.0% | [76] |
| Solvothermal | 1.0 g/L | UV light | Rhodamine B, 0.125 mM | 300 min | 6.3 min−1 | 94.8% | [77] |
| Hydrothermal | 0.5 g/L | UV-A lamp | Methylene blue, 6.4 mg/L | 60 min | 23.0 min−1 | 86.0% | [78] |
| Hydrothermal | 0.5 g/L | Natural sunlight | Methylene blue, 6.4 mg/L | 60 min | 20.0 min−1 | 81.0% | [78] |
| Sol-anaerobic calcination | 0.4 g/L | UV light | Phenol, 10 mg/L | 180 min | - | 97.9% | [79] |
| Hydrothermal | 1.2 g/L | Visible light | Humic acid, 30 mg/L | 180 min | - | 88.0% | [80] |
| Hydrothermal | 0.5 g/L | Visible light | Methylene blue, 0.01 mM | 130 min | 37.4 min−1 | ~99.0% | [81] |
| Hydrothermal | 0.7 g/L | UV light | Ethylparaben, 0.3 mM | 40 min | 96.0 min−1 | 98.6% | [82] |
| Hydrothermal | 0.5 g/L | Natural sunlight | Bisphenol A, 0.01 mol/L | 30 min | - | 100.0% | [83] |
| Hydro-solvothermal | 1.0 g/L | Solar light | 2,4-Dichlorophenoxyacetic acid, 0.5 mM | 180 min | 9.6 min−1 | 97.0% | [84] |
| Parameter | Impact on Photocatalysis |
|---|---|
| Catalyst concentration | The photodegradation rate increases with the increase of the amount of catalyst. Above a certain amount of catalyst, the photodegradation rate decreases as the catalyst amount increases. |
| Initial pollutant concentration | The photodegradation rate increases with the increase of the initial concentration of pollutant. Above a certain initial concentration of pollutant, the photodegradation rate decreases as the initial concentration of pollutant increases. |
| pH | The photodegradation rate depends significantly on the pH value. |
| Light source | Light source supplies irradiation of different wavelengths (UV-C, UV-B, UV-A, visible light, simulated solar light, natural sunlight). |
| Intensity of irradiation | Light intensity depends on the light source and enhances the photocatalytic reaction. |
| Water matrix | Presence of different pollutants/organic matter that can act as inhibitors or competitors of the photodegradation rate. |
| Weight ratio of graphene oxide to TiO2 | The photodegradation rate increases with the increasing amount of GO to TiO2. Above a certain threshold the photodegradation rate decreases as the amount of GO increases. |
| Scavengers | Improve the separation rate of photogenerated holes and electrons and enhance the photocatalytic reaction. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocijan, M.; Ćurković, L.; Gonçalves, G.; Podlogar, M. The Potential of rGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water. Sustainability 2022, 14, 12703. https://doi.org/10.3390/su141912703
Kocijan M, Ćurković L, Gonçalves G, Podlogar M. The Potential of rGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water. Sustainability. 2022; 14(19):12703. https://doi.org/10.3390/su141912703
Chicago/Turabian StyleKocijan, Martina, Lidija Ćurković, Gil Gonçalves, and Matejka Podlogar. 2022. "The Potential of rGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water" Sustainability 14, no. 19: 12703. https://doi.org/10.3390/su141912703
APA StyleKocijan, M., Ćurković, L., Gonçalves, G., & Podlogar, M. (2022). The Potential of rGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water. Sustainability, 14(19), 12703. https://doi.org/10.3390/su141912703













