Efficacy of Biological Copper Oxide Nanoparticles on Controlling Damping-Off Disease and Growth Dynamics of Sugar Beet (Beta vulgaris L.) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Used and Cultural Conditions
2.2. Effect of Copper Oxide Nanoparticles on Plant Pathogens Microorganisms (In Vitro)
2.3. Effect of Copper Oxide Nanoparticles on Plant Growth in a Gnotobiotic Clay System of Sugar Beet
2.4. Sugar Beet Pot Experiments
2.4.1. Plants’ Vegetative Development
2.4.2. Physiological Characteristics
Photosynthetic Pigments
Total Soluble Sugars (TSS)
2.4.3. Antioxidant Enzymes
Peroxidase Activity
Polyphenol Oxidase Assay
Phenylalanine Ammonia Layase Assay
2.4.4. Disease Incidence Percentage
2.4.5. Chemical Contents of Sugar Beet Leaves
2.5. Statistical Analyses
3. Results
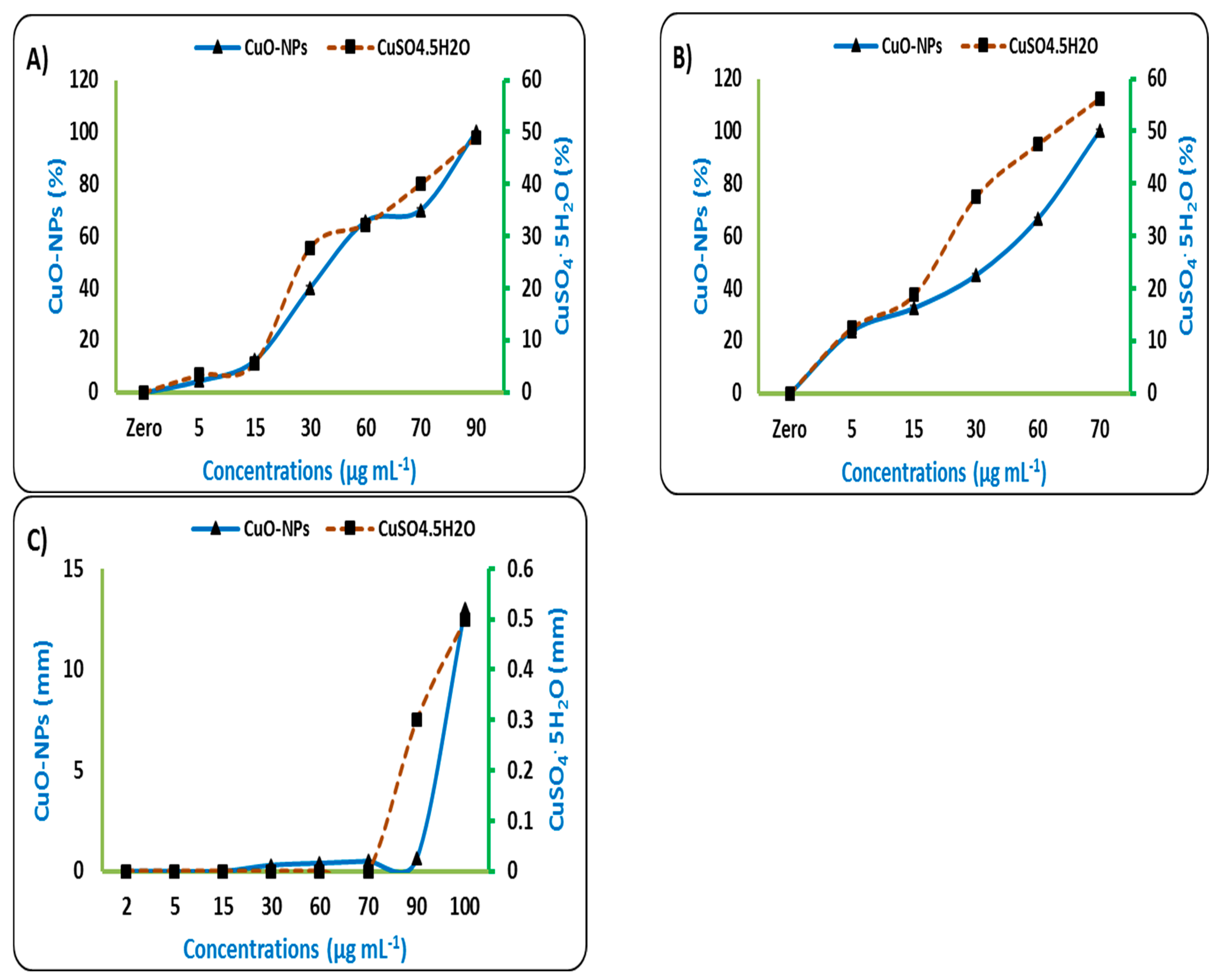
3.1. Effect of Copper Oxide Nanoparticles on Plant Pathogens Microorganisms (In Vitro)
3.2. Effect of Copper Oxide Nanoparticles on Plant Growth in a Gnotobiotic Clay System of Sugar Beet
3.3. Sugar Beet Pot Experiments
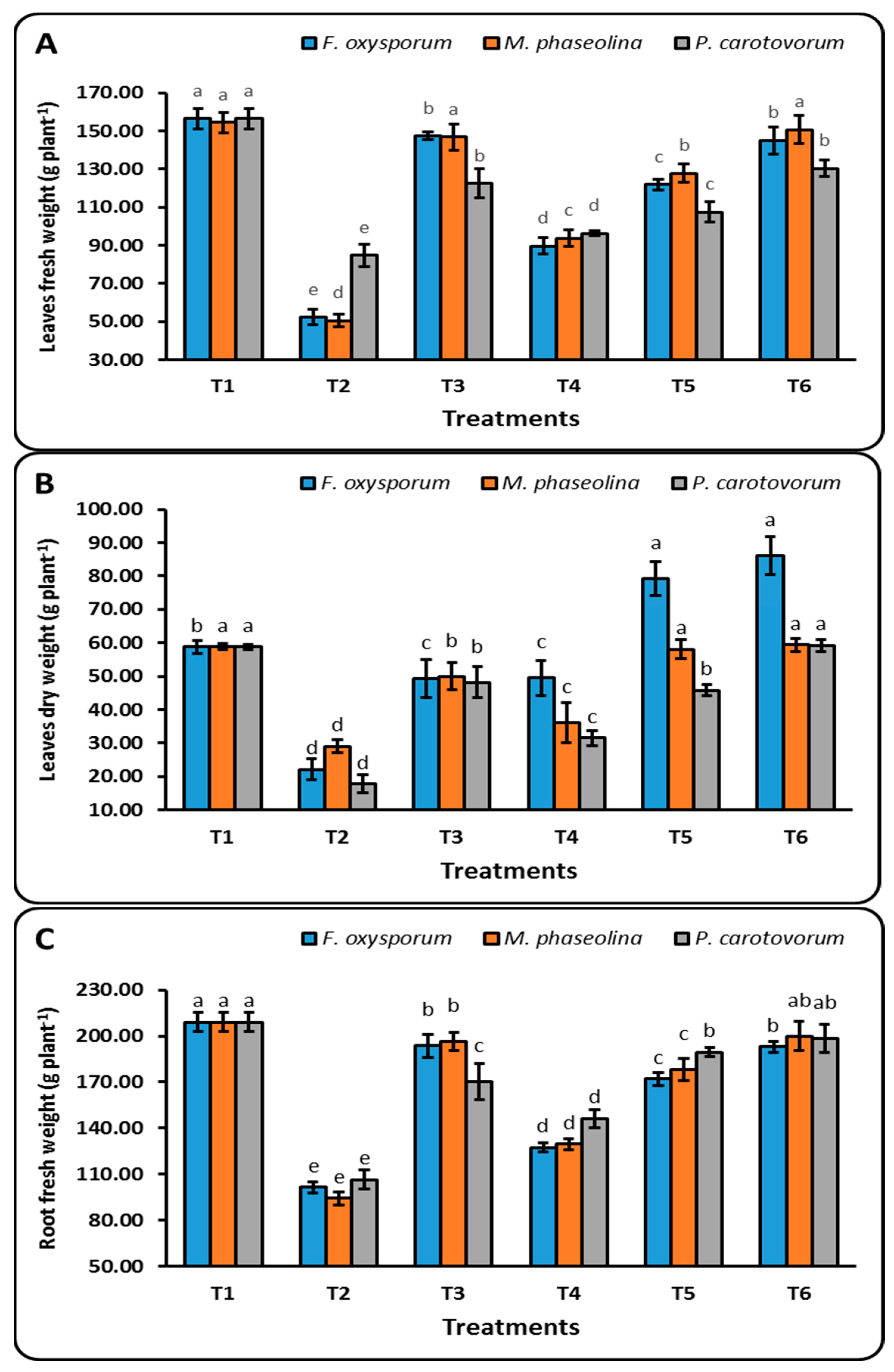
3.3.1. Plants’ Vegetative Development
3.3.2. Physiological Characteristics
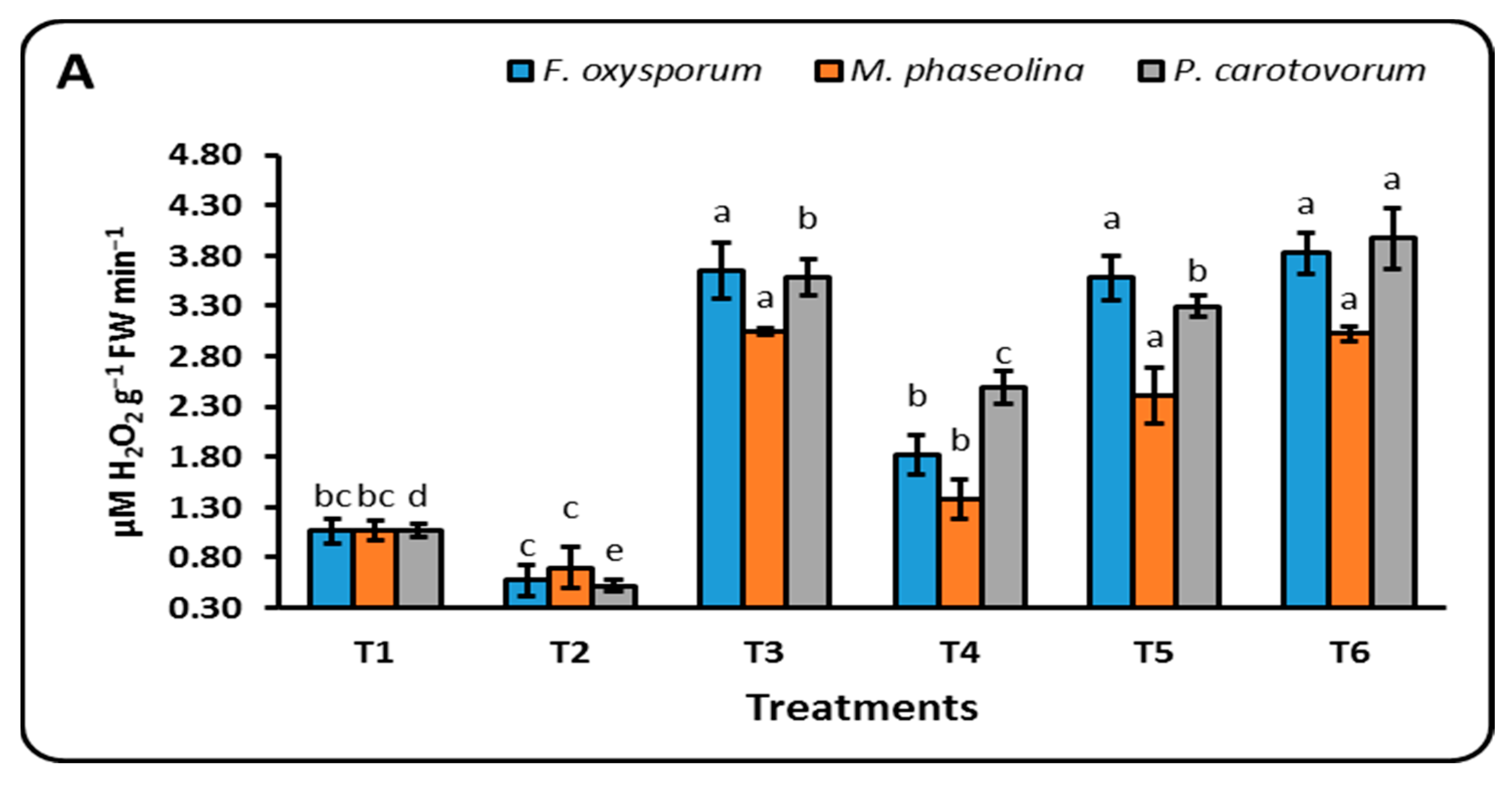
3.3.3. Antioxidant Enzymes
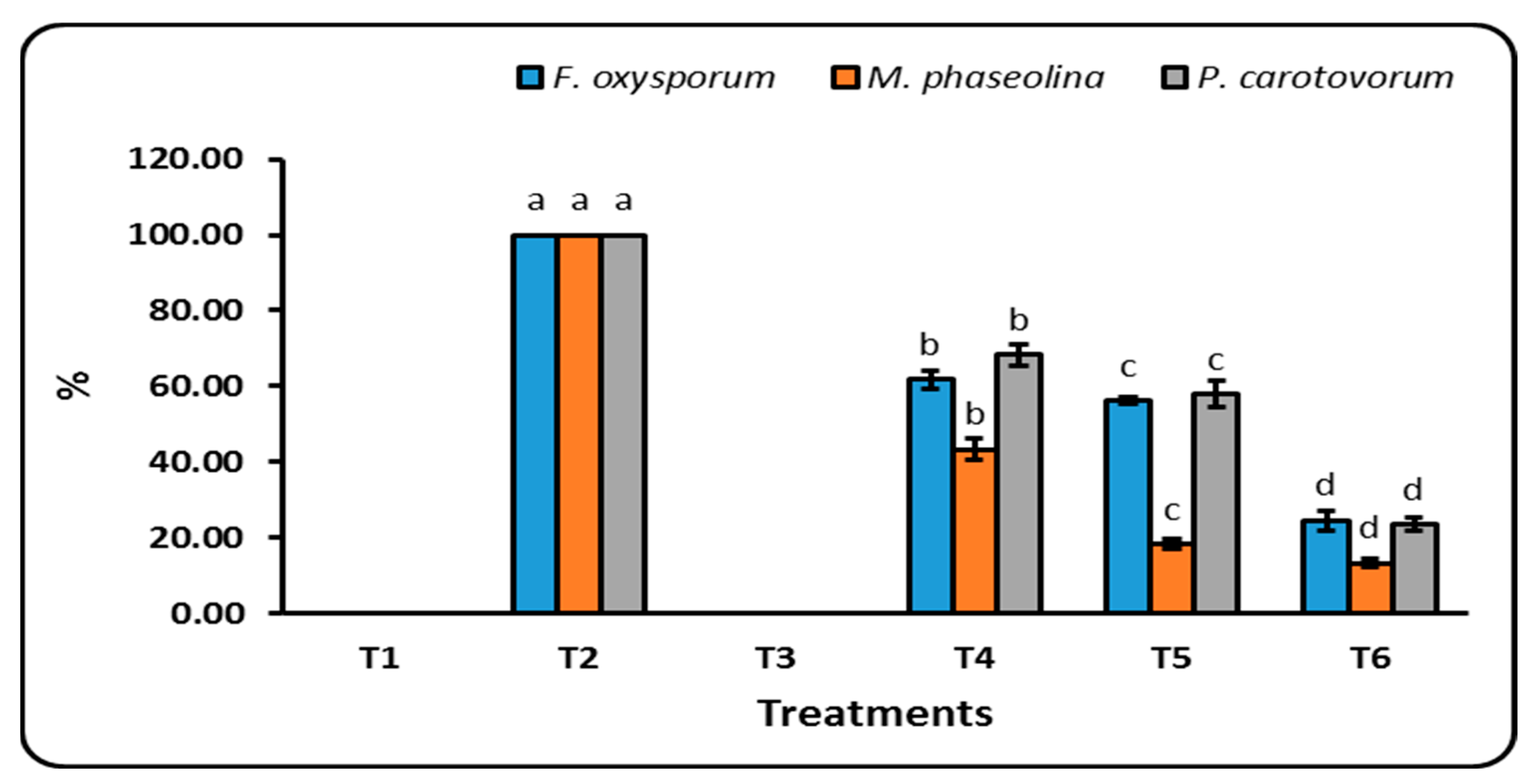
3.3.4. Disease Incidence Percent
3.3.5. Some Chemical Contents of Sugar Beet Leaves
4. Discussion
4.1. Effect of Cu-NPs on Plant Pathogens Microorganisms (In Vitro)
4.2. Effect of CuO-NPs on Growth Parameters of Sugar Beet under Soil Infected by Plant Pathogenic Microorganisms
4.3. Physiological Characteristics
4.4. Antioxidant Enzymes
4.5. Disease Incidence Percent
4.6. N, P, K and Cu of Sugar Beet Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green synthesis of copper nanoparticles using Celastrus aniculatus Willd. Leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.D.; El-Sadany, M.A.H. Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Abd El Aty, A.A. In-situ green myco-synthesis of silver nanoparticles onto cotton fabrics for broad spectrum antimicrobial activity. Int. J. Biol. Macromol. 2018, 118, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An Overview. Biol. Trace Elem. Res. 2020, 199, 344–370. [Google Scholar] [CrossRef]
- Cuevas, R.; Durán, N.; Diez, M.C.; Tortella, G.R.; Rubilar, O. extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from Chilean forests. J. Nanomater. 2015, 789089. [Google Scholar]
- Majumdar, T.D.; Singh, M.; Thapa, M.; Dutta, M.; Mukherjee, A.; Ghosh, C.K. Size-dependent antibacterial activity of copper nanoparticles against Xanthomonas oryzae pv. Oryzae—A synthetic and mechanistic approach. Colloid Interface Sci. Commun. 2019, 32, 100190. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant. Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Watson, L.; Dallwitz, M. The Families of Flowering Plants: Descriptions, Illustrations, Identification, and Information Retrieval. 1992. Available online: http://biodiversityunoedu/delta (accessed on 1 July 2021).
- Hassnein, A.M.; Azab, M.A.; El-Hawary, M.A.; Darwish, N.N. Effect of nano fertilization on sugar beet. Al-Azhar J. Agric. Res. 2019, 44, 194–201. [Google Scholar] [CrossRef]
- Abbas, M.S.; El-Hassanin, A.S.; Dewdar, M.D.H.; Abd Elaleem, H.A. Impact of nanomicronutrients as foliar fertilization on yield and quality of sugar beet roots. Pak. J. Biol. Sci. 2020, 23, 1416–1423. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. A review. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kanhed, P.; Sonal, B.; Swapnil, G.; Aniket, G.; Amedea, B.; Seabra, O.R.; Nelson, D.; Mahendra, R. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef]
- Nabila, M.I.; Krishnan, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Ganesan, P.; Reegan, A.D.; David, R.H.A.; Gandhi, M.R.; Paulraj, M.G.; Al-Dhabi, N.A.; Ignacimuthu, S. Antimicrobial activity of some actinomycetes from Western Ghats of Tamil Nadu. India Alex. J. Med. 2017, 53, 101–110. [Google Scholar] [CrossRef]
- Whipps, J.M. Effect of media on growth and interactions between a range of soil-borne glasshouse pathogens and antagonistic fungi. New Phytol. 1987, 107, 127–142. [Google Scholar] [CrossRef]
- El-Shabrawy, E.; Heba, S. Controlling maize late-wilt and enhancing plant salinity tolerance by some rhizobacterial strains. Egypt. J. Phytopathol. 2018, 46, 235–255. [Google Scholar] [CrossRef]
- Abdalla, M.E.; Seadh, S.E.; Hamza, S. Inhibitory effect and morphological changes by organic acids to bacterial strains causing sugar beet soft root rot in Vitro. J. Plant Prot. Pathol. 2019, 10, 187–193. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Hendrix, D.L. Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop. Sci. 1993, 33, 1306–1311. [Google Scholar] [CrossRef]
- Srivastava, S.K. Peroxidase and polyphenol-oxidase in Brassica juncea plants infected with Macrophomina phaseolina (Tassi): Goid of and their implication in disease resistance. Phytopathology 1987, 120, 249–254. [Google Scholar] [CrossRef]
- Matta, A.I.; Dimond, A.F. Symptoms of Fusarium wilt in relation to quantity of fungus and enzyme activity in tomato stems. Phytopathology 1963, 53, 574–578. [Google Scholar]
- Zucker, M. Sequential induction of phenylalanine ammonia layse and lyase in inactivating system in potato tuber disks. Plant Physiol. 1968, 43, 365–374. [Google Scholar] [CrossRef]
- Crowe, F.J.; Debons, J.; Darnell, T.; McGrath, D.; Koepsell, P.; Laborde, J.; Redondo, J. Control of Allium white rot with DADS and related products. In Proceedings of the Fifth International Workshop on Allium White Rot, Cordoba, Spain, April 1994; Entwistle, A.R., Melero-Vara, J.M., Eds.; HRI: Norwich, UK; pp. 15–19. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis, Part II, Agronomy Monographs ASA and SSSA, 2nd ed.; Madison Book Company: Madison, WI, USA, 1982. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- USDA. Soil Survey Laboratory Methods Manual Soil Survey Investigation Report, No. 42, Version 4; USDA-NRCS: Lincoln, NE, USA, 2004. [Google Scholar]
- Gomez, K.A.; Gomez, A. Statistical Procedure for Agricultural Research—Handbook; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Usman, M.S.; El-Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar]
- Patel, B.H.; Channiwala, M.Z.; Chaudhari, S.B.; Mandot, A.A. Biosynthesis of copper nanoparticles; its characterization and efficacy against human pathogenic bacterium. J. Environ. Chem. Eng. 2016, 4, 2163–2169. [Google Scholar] [CrossRef]
- Muralisankar, T.; Bhavan, P.S.; Gandimathy, S.; Radhakrishnan, S.; Seenivasan, C.; Aarumugam, P. In-vitro antibacterial activity of Zinc and nanoparticles against Gram-negative bacteria Aeromonas hydrophila. Malaya J. Biosci. 2015, 1, 196–200. [Google Scholar]
- El-Batal, A.I.; Gharieb, S.E.; Farag, M.M.; Rasha, M.F. Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J. Clust. Sci. 2020, 31, 79–90. [Google Scholar] [CrossRef]
- Lequeux, H.; Hermans, C.; Lutts, S.; Verbruggen, N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant. Physiol. Biochem. 2010, 48, 673–682. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, Á.; Ördög, A.; Szepesi, Á.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar]
- Mourato, M.P.; Moreira, I.N.; Leitão, I.; Pinto, F.R.; Sales, J.R.; Martins, L.L. Effect of heavy metals in plants of the genus Brassica. Int. J. Mol. Sci. 2015, 16, 17975–17998. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, F.; Razzaq, A.; Iqbal, M.N.; Jhanzab, H.M. Effect of silver, copper and iron nanoparticles on wheat germination. Int. J. Biosci. 2015, 6, 112–117. [Google Scholar]
- Zuverza-Mena, N.; Medina-Velo, I.A.; Barrios, A.C.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ. Sci. Process. Impacts 2015, 17, 1783–1793. [Google Scholar] [CrossRef]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar]
- Baskar, V.; Nayeem, S.; Kuppuraj, S.P.; Muthu, T.; Ramalingam, S. Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in vitro grown eggplant (Solanum melongena). 3 Biotech. 2018, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Nilly, A.H.A.; Salem, S.S.; Mohamed, F.A.; Amr, F. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Amin, M.A.; Badawy, A. Metabolic changes in common bean plants in response to zinc nanoparticles and zinc sulfate. Int. J. Innov. Sci. Eng. Technol. 2017, 4, 321–335. [Google Scholar]
- Ewais, E.; Ismail, M.; Badawy, A. Vegetative growth, photosynthetic pigments and yield of Phaseolus vulgaris (L.) plants in response to the application of biologically-synthesized zinc oxide nanoparticles and zinc sulfate. Al Azhar Bull. Sci. 2017, 9, 33–46. [Google Scholar]
- Shobha, G.; Moses, V.; Ananda, S. Biological synthesis of copper nanoparticles and its impact. Int. J. Pharm. Sci. Invent. 2014, 3, 6–28. [Google Scholar]
- Kasana, R.C.; Panwar, N.R.; Kaul, R.K.; Kumar, P. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Lett. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef]
- Ochoa, L.; Zuverza-Mena, N.; Medina-Velo, I.A.; Flores-Margez, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Copper oxide nanoparticles and bulk copper oxide, combined with indole-3-acetic acid, alter aluminum, boron, and iron in Pisum sativum seeds. Sci. Total Environ. 2018, 634, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Kasana, A.; AliNiazee, M.T. A thermal unit summation model for the phenology of Rhagoietis completa (Diptera: Tephritidae). J. Entomol. Soc. Br. Columbia 1997, 94, 13–18. [Google Scholar]
- Wang, S.-H.; Yang, Z.-M.; Yang, H.; Lu, B.; Li, S.-Q.; Lu, Y.-P. Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Bot. Bull. Acad. Sin. 2004, 45, 203–212. [Google Scholar]
- Ananda, S.; Shobha, G.; Shashidhara, K.; Mahadimane, V. Nano-cuprous oxide enhances seed germination and seedling growth in Lycopersicum esculentum plants. J. Drug Deliv. Ther. 2019, 9, 296–302. [Google Scholar]
- Nair, P.M.G.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2014, 21, 12709–12722. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef]
- Ismail Ahmed, M. Efficacy of Copper Oxide and Magnesium Oxide Nanoparticles on Controlling Black Scurf Disease on Potato. Egypt. J. Phytopathol. 2021, 49, 116–130. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Yawar, A.W.; Masood ul Hasan, M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Oussou-Azo, A.F.; Nakama, T.; Nakamura, M.; Futagami, T.; Vestergaard, M.C.M. Antifungal potential of nanostructured crystalline copper and its oxide forms. Nanomaterials 2020, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- El-Shewy, E.S.A.; Mohemed, F.G.; Abd-latif, F.M.; Hafez, E.M.; Mansour, S.A. The efficacy of copper oxide, tri-calcium phosphate and silicon dioxide nanoparticles in controlling black scurf disease of potato. Ann. Agric. Sci. Moshtohor 2019, 57, 129–138. [Google Scholar] [CrossRef]
- Giannousi, K.; Avramidis, I.; Dendrinou-Samara, C. Synthesis, characterization and evaluation of copper-based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv. 2013, 3, 21743–21752. [Google Scholar] [CrossRef]
- Ouda, S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef]
- Elmer, W.H.; White, J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Ortas, I.; Rafique, M.; Akpinar, C.; Kacar, Y.A. Growth media and mycorrhizal species effect on acclimatization and nutrient uptake of banana plantlets. Sci. Hortic. 2017, 217, 55–60. [Google Scholar] [CrossRef]
- Huo, C.; Ouyang, J.; Yang, H. CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous materials via direct synthetic route. Sci. Rep. 2014, 4, 3682. [Google Scholar] [CrossRef]
- Dey, J.K.; Das, S.; MawlongInt, L.G. Nanotechnology and its importance in micronutrient fertilization. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2306–2325. [Google Scholar]
- Shalaby, T.A.; Said, M.E.; Mohammed, E.E.; Alaa, E.O.; Hossam, S.E.; Hassan, E. Acclimatization of In Vitro Banana Seedlings Using Root-Applied Bio-Nanofertilizer of Copper and Selenium. Agronomy 2022, 12, 539. [Google Scholar] [CrossRef]


| Mechanical Analysis (%) | Texture | pH (1:2.5) | EC (dSm–1) | OM (g Kg−1) | Available Elements (mg Kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | N | P | K | Cu | ||||
| 21.65 | 25.14 | 53.21 | Clayey | 7.78 | 2.67 | 16.98 | 8.91 | 8.28 | 396.39 | 12.35 |
| Concentration (µg mL−1) | Length (cm) | Fresh Weight (g) | Dry Weight (g) | |||
|---|---|---|---|---|---|---|
| Root | Leaves | Root | Leaves | Root | Leaves | |
| F. oxysporum | ||||||
| C (uninfected) | 15.94 ± 0.76 a | 15.33 ± 0.33 a | 3.38 ± 0.23 bc | 7.25 ± 0.31 a | 0.52 ± 0.05 a | 0.63 ± 0.04 a |
| C (Infected) | 7.13 ± 0.12 i | 7.15 ± 0.17 f | 0.87 ± 0.07 h | 1.26 ± 0.22 g | 0.11 ± 0.03 f | 0.12 ± 0.02 g |
| 5 | 8.46 ± 0.42 h | 9.40 ± 0.44 e | 1.27 ± 0.07 g | 1.99 ± 0.12 f | 0.19 ± 0.04 e | 0.24 ± 0.02 f |
| 15 | 9.32 ± 0.45 g | 9.74 ± 0.23 e | 1.71 ± 0.23 f | 3.12 ± 0.11 e | 0.17 ± 0.02 e | 0.28 ± 0.02 ef |
| 30 | 10.24 ± 0.28 f | 10.29 ± 0.24 d | 2.10 ± 0.13 e | 3.33 ± 0.10 e | 0.24 ± 0.03 d | 0.30 ± 0.02 e |
| 60 | 11.19 ± 0.20 e | 10.47 ± 0.16 d | 2.69 ± 0.17 d | 4.13 ± 0.20 d | 0.25 ± 0.02 d | 0.37 ± 0.02 d |
| 90 | 12.40 ± 0.44 d | 11.21 ± 0.18 c | 3.21 ± 0.18 c | 4.33 ± 0.08 cd | 0.28 ± 0.02 cd | 0.39 ± 0.02 cd |
| 120 | 13.33 ± 0.40 c | 11.35 ± 0.34 c | 3.62 ± 0.09 b | 4.46 ± 0.04 c | 0.32 ± 0.02 c | 0.42 ± 0.02 c |
| 150 | 14.15 ± 0.15 b | 13.29 ± 0.26 b | 4.06 ± 0.08 a | 5.29 ± 0.26 b | 0.40 ± 0.02 b | 0.53 ± 0.02 b |
| M. phaseolina | ||||||
| C (uninfected) | 15.94 ± 0.76 a | 15.33 ± 0.33 a | 3.38 ± 0.23 a | 7.25 ± 0.31 a | 0.52 ± 0.05 a | 0.63 ± 0.04 a |
| C (Infected) | 7.39 ± 0.43 h | 6.17 ± 0.17 h | 1.02 ± 0.09 g | 1.19 ± 0.08 h | 0.14 ± 0.03 g | 0.25 ± 0.03 f |
| 5 | 8.35 ± 0.43 g | 7.24 ± 0.21 g | 1.45 ± 0.08 f | 2.06 ± 0.07 g | 0.18 ± 0.03 fg | 0.28 ± 0.02 ef |
| 15 | 9.39 ± 0.35 f | 8.12 ± 0.10 f | 2.13 ± 0.14 e | 3.09 ± 0.08 f | 0.17 ± 0.02 fg | 0.30 ± 0.02 e |
| 30 | 10.37 ± 0.42 e | 9.37 ± 0.42 e | 2.65 ± 0.19 d | 3.37 ± 0.04 e | 0.21 ± 0.02 ef | 0.33 ± 0.03 de |
| 60 | 12.38 ± 0.34 d | 11.37 ± 0.42 d | 2.89 ± 0.08 c | 3.68 ± 0.05 d | 0.25 ± 0.02 de | 0.36 ± 0.03 d |
| 90 | 13.18 ± 0.16 c | 12.28 ± 0.25 c | 3.10 ± 0.13 bc | 4.11 ± 0.11 c | 0.29 ± 0.02 d | 0.37 ± 0.02 d |
| 120 | 13.22 ± 0.25 c | 12.53 ± 0.23 c | 3.16 ± 0.09 ab | 4.35 ± 0.07 c | 0.35 ± 0.02 c | 0.42 ± 0.03 c |
| 150 | 14.32 ± 0.34 b | 13.59 ± 0.27 b | 3.26 ± 0.07 ab | 5.76 ± 0.22 b | 0.43 ± 0.03 b | 0.58 ± 0.03 b |
| P. carotovorum | ||||||
| C (uninfected) | 15.94 ± 0.76 a | 15.33 ± 0.33 a | 3.38 ± 0.23 a | 7.25 ± 0.31 a | 0.52 ± 0.05 a | 0.63 ± 0.04 a |
| C (Infected) | 6.73 ± 0.09 g | 6.63 ± 0.17 h | 0.70 ± 0.04 f | 1.27 ± 0.05 g | 0.14 ± 0.03 e | 0.26 ± 0.04 g |
| 5 | 7.28 ± 0.26 g | 8.10 ± 0.10 g | 1.15 ± 0.04 e | 2.51 ± 0.03 f | 0.13 ± 0.02 e | 0.28 ± 0.02 fg |
| 15 | 8.21 ± 0.20 f | 9.19 ± 0.18 f | 1.37 ± 0.06 d | 3.00 ± 0.08 e | 0.19 ± 0.02 d | 0.30 ± 0.02 fg |
| 30 | 9.39 ± 0.35 e | 10.32 ± 0.34 e | 1.84 ± 0.10 c | 3.19 ± 0.03 e | 0.19 ± 0.01 d | 0.32 ± 0.03 ef |
| 60 | 11.29 ± 0.35 d | 11.45 ± 0.49 d | 1.86 ± 0.07 c | 3.99 ± 0.09 d | 0.22 ± 0.02 d | 0.36 ± 0.03 de |
| 90 | 12.20 ± 0.19 c | 12.23 ± 0.24 c | 1.85 ± 0.03 c | 4.07 ± 0.14 d | 0.30 ± 0.02 c | 0.37 ± 0.02 d |
| 120 | 12.60 ± 0.38 c | 12.30 ± 0.28 c | 1.92 ± 0.04 c | 4.46 ± 0.05 c | 0.33 ± 0.03 c | 0.44 ± 0.03 c |
| 150 | 14.44 ± 0.37 b | 13.62 ± 0.36 b | 2.47 ± 0.18 b | 5.79 ± 0.19 b | 0.38 ± 0.02 b | 0.55 ± 0.04 b |
| Treatment | Ch a (mg g−1 FW) | Ch b (mg g−1 FW) | T. Ch (mg g−1 FW) | Carotenoids (µg g−1 FW) | TSS (µg g−1 FW) |
|---|---|---|---|---|---|
| F. oxysporum | |||||
| T1 | 4.21 ± 0.19 a | 2.45 ± 0.37 a | 6.67 ± 0.18 a | 1.08 ± 0.12 a | 5.67 ± 0.14 bc |
| T2 | 0.90 ± 0.25 d | 0.70 ± 0.21 b | 1.61 ± 0.04 e | 0.20 ± 0.07 e | 4.42 ± 0.07 e |
| T3 | 3.41 ± 0.27 b | 2.29 ± 0.41 a | 5.70 ± 0.15 b | 0.84 ± 0.13 bc | 4.75 ± 0.25 d |
| T4 | 1.86 ± 0.33 c | 1.15 ± 0.26 b | 3.01 ± 0.34 d | 0.55 ± 0.06 d | 5.50 ± 0.13 c |
| T5 | 3.05 ± 0.30 b | 2.08 ± 0.24 a | 5.13 ± 0.23 c | 0.71 ± 0.07 cd | 5.85 ± 0.04 ab |
| T6 | 3.25 ± 0.36 b | 2.33 ± 0.19 a | 5.58 ± 0.20 b | 0.93 ± 0.13 ab | 5.96 ± 0.07 a |
| LSD 0.05 | 0.511 | 0.517 | 0.376 | 0.178 | 0.241 |
| M. phaseolina | |||||
| T1 | 4.21 ± 0.19 a | 2.45 ± 0.37 a | 6.67 ± 0.18 a | 1.08 ± 0.12 bc | 5.67 ± 0.14 c |
| T2 | 1.03 ± 0.31 d | 0.45 ± 0.15 d | 1.48 ± 0.45 e | 0.24 ± 0.12 d | 4.46 ± 0.07 d |
| T3 | 3.26 ± 0.67 b | 2.23 ± 0.36 ab | 5.50 ± 0.59 bc | 0.91 ± 0.15 c | 5.96 ± 0.07 ab |
| T4 | 1.97 ± 0.27 c | 1.07 ± 0.18 c | 3.04 ± 0.35 d | 1.00 ± 0.06 c | 5.54 ± 0.07 c |
| T5 | 2.91 ± 0.37 b | 1.88 ± 0.09 b | 4.78 ± 0.46 c | 1.13 ± 0.08 ab | 5.88 ± 0.13 b |
| T6 | 3.44 ± 0.16 b | 2.43 ± 0.19 a | 5.87 ± 0.25 b | 1.27 ± 0.01 a | 6.08 ± 0.07 a |
| LSD 0.05 | 0.654 | 0.437 | 0.719 | 0.180 | 0.173 |
| P. carotovorum | |||||
| T1 | 4.21 ± 0.19 a | 2.45 ± 0.37 a | 6.67 ± 0.18 a | 1.08 ± 0.12 a | 5.67 ± 0.14 c |
| T2 | 0.99 ± 0.07 e | 1.21 ± 0.10 d | 2.20 ± 0.04 e | 0.51 ± 0.14 d | 4.42 ± 0.31 d |
| T3 | 3.17 ± 0.13 b | 2.29 ± 0.31 ab | 5.47 ± 0.25 b | 0.98 ± 0.15 ab | 5.95 ± 0.06 ab |
| T4 | 1.84 ± 0.16 d | 1.55 ± 0.17 cd | 3.38 ± 0.32 d | 0.49 ± 0.03 d | 5.71 ± 0.07 bc |
| T5 | 2.59 ± 0.11 c | 1.95 ± 0.12 bc | 4.54 ± 0.21 c | 0.59 ± 0.11 cd | 5.94 ± 0.12 ab |
| T6 | 3.12 ± 0.32 b | 2.36 ± 0.30 ab | 5.48 ± 0.18 b | 0.79 ± 0.15 bc | 6.05 ± 0.07 a |
| LSD 0.05 | 0.320 | 0.444 | 0.377 | 0.219 | 0.276 |
| Treatment | N | P | K | Cu |
|---|---|---|---|---|
| F. oxysporum | ||||
| T1 | 1.87 ± 0.12 a | 0.149 ± 0.005 a | 3.60 ± 0.13 a | 0.012 ± 0.001 c |
| T2 | 0.59 ± 0.05 e | 0.047 ± 0.004 d | 1.15 ± 0.41 c | 0.012 ± 0.002 c |
| T3 | 1.56 ± 0.10 b | 0.144 ± 0.003 a | 3.29 ± 0.11 ab | 0.011 ± 0.002 c |
| T4 | 0.80 ± 0.08 d | 0.082 ± 0.001 c | 3.16 ± 0.92 ab | 0.024 ± 0.003 b |
| T5 | 1.33 ± 0.12 c | 0.118 ± 0.001 b | 2.53 ± 0.30 b | 0.026 ± 0.002 a |
| T6 | 1.62 ± 0.05 b | 0.147 ± 0.002 a | 3.10 ± 0.65 ab | 0.027 ± 0.001 a |
| LSD 0.05 | 0.161 | 0.031 | 0.899 | 0.007 |
| M. phaseolina | ||||
| T1 | 1.87 ± 0.12 a | 0.149 ± 0.005 a | 3.60 ± 0.13 a | 0.012 ± 0.002 c |
| T2 | 0.57 ± 0.04 d | 0.068 ± 0.001 d | 1.28 ± 0.13 d | 0.011 ± 0.001 c |
| T3 | 1.58 ± 0.02 b | 0.147 ±0.003 a | 3.06 ± 0.20 b | 0.010 ± 0.001 c |
| T4 | 1.03 ± 0.08 c | 0.117 ±0.002 c | 2.35 ± 0.07 c | 0.022 ± 0.003 b |
| T5 | 1.56 ± 0.03 b | 0.132 ±0.005 b | 3.05 ± 0.20 b | 0.024 ± 0.001 a |
| T6 | 1.61 ± 0.02 b | 0.153 ±0.007 a | 3.46 ± 0.04 a | 0.025 ± 0.003 a |
| LSD 0.05 | 0.109 | 0.007 | 0.240 | 0.006 |
| P. carotovorum | ||||
| T1 | 1.87 ± 0.12 a | 0.149 ± 0.005 a | 3.60 ± 0.13 a | 0.012 ± 0.002 c |
| T2 | 0.52 ± 0.10 d | 0.052 ± 0.009 e | 0.93 ± 0.12 e | 0.012 ± 0.001 c |
| T3 | 1.46 ± 0.09 b | 0.136 ± 0.003 b | 2.95 ± 0.26 bc | 0.013 ± 0.001 c |
| T4 | 0.88 ± 0.05 c | 0.087 ± 0.004 d | 2.06 ± 0.10 d | 0.025 ± 0.003 b |
| T5 | 1.42 ± 0.12 b | 0.114 ± 0.006 c | 2.79 ± 0.12 c | 0.026 ± 0.001 a |
| T6 | 1.70 ± 0.08 b | 0.129 ± 0.004 b | 3.10 ± 0.10 b | 0.029 ± 0.003 a |
| LSD 0.05 | 0.171 | 0.012 | 0.243 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Salem, E.; Ahmed, A.R.; Elbagory, M.; Omara, A.E.-D. Efficacy of Biological Copper Oxide Nanoparticles on Controlling Damping-Off Disease and Growth Dynamics of Sugar Beet (Beta vulgaris L.) Plants. Sustainability 2022, 14, 12871. https://doi.org/10.3390/su141912871
Abou-Salem E, Ahmed AR, Elbagory M, Omara AE-D. Efficacy of Biological Copper Oxide Nanoparticles on Controlling Damping-Off Disease and Growth Dynamics of Sugar Beet (Beta vulgaris L.) Plants. Sustainability. 2022; 14(19):12871. https://doi.org/10.3390/su141912871
Chicago/Turabian StyleAbou-Salem, Eman, Abdulmageed R. Ahmed, Mohssen Elbagory, and Alaa El-Dein Omara. 2022. "Efficacy of Biological Copper Oxide Nanoparticles on Controlling Damping-Off Disease and Growth Dynamics of Sugar Beet (Beta vulgaris L.) Plants" Sustainability 14, no. 19: 12871. https://doi.org/10.3390/su141912871
APA StyleAbou-Salem, E., Ahmed, A. R., Elbagory, M., & Omara, A. E.-D. (2022). Efficacy of Biological Copper Oxide Nanoparticles on Controlling Damping-Off Disease and Growth Dynamics of Sugar Beet (Beta vulgaris L.) Plants. Sustainability, 14(19), 12871. https://doi.org/10.3390/su141912871







