Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concept of the CO2–Water–Rock Interaction
2.2. Materials
2.2.1. Core Samples
2.2.2. Gases
2.2.3. Brine Preparation
2.3. Method
2.3.1. Porosity and Permeability Measurement
2.3.2. Core Flooding Procedure
3. Results and Discussion
3.1. Investigation of CO2–Brine Flow Behaviour for Different Salt Types and Concentrations
3.2. Pressure Decay Test
3.3. Effect of Different Salt Types and Concentration on CO2 Storage
3.4. Porosity Reduction and Permeability Variation
4. Conclusions
- The optimum range for CO2 sequestration in deep saline aquifers is within the range of 10 wt.% to 20 wt.% concentration (salinity). A substantial volume of CO2 was found to be stored at this range of brine concentration;
- An increase in the brine density because of an increase in salinity reduces the free flow path of gas, as well as reduces the CO2 storage capacity;
- Breakthrough times of CO2 from the core sample at lower brine concentrations are longer than those of the higher brine concentrations. This implies that the CO2–brine–rock interaction is predominant at lower brine concentrations;
- A higher pressure decay rate indicates high solubility of CO2 in different brine solutions in a porous media. The solubility of CO2 is clearly dependent on the salt type as well as the concentration of the brine;
- The high fluctuation of differential pressure (dp) indicates that there is a slug-type flow in the core sample as the CO2 is injected into the simulated deep saline aquifer;
- The salting-out effect at the highest brine concentration is greater in MgCl2 and CaCl2 brine as compared to NaCl and KCl brine;
- An increase in brine concentration leads to a reduction in both the porosity and permeability of the core sample. Thus, the decrease in permeability is generally observed due to salt deposition, which was influenced by the concentration of the brines tested;
- Salt solubility in water/aqueous phase also affects the storability of CO2 in deep saline aquifers with highly insoluble salts precipitating out more easily than their higher solubility counterparts. The drying-out effect was observed to be more noticeable in the divalent brine scenarios compared to the monovalent scenarios. However, KCl showed a lower tolerance in terms of CO2 storability compared to its monovalent counterpart, NaCl.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | cross sectional area of the flow (cm2) |
| Q | flow rate (cm3/s) |
| ΔV | volume of the gas passing through the core sample (cm3) |
| ΔT | time (s) |
| K | permeability of the sample (millidarcies, md) |
| L | length of the core sample (cm) |
| Pa | absolute atmospheric pressure (atm) |
| P1 | upstream pressure (atm) |
| P2 | downstream pressure (atm) |
| ΔP | differential pressure (psig) |
| V | flow volume (mL) |
| Vc | volume of sample chamber (mL) |
| Vg | grain volume (mL) |
| Vr | volume of reference chamber (mL) |
| Vv | volume of valve displacement (mL) |
| µ | fluid viscosity (cP) |
References
- Raza, A.; Gholami, R.; Meiyu, G.; Rasouli, V.; Bhatti, A.A.; Rezaee, R. A review on the natural gas potential of Pakistan for the transition to a low-carbon future. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1149–1159. [Google Scholar] [CrossRef]
- Raza, A.; Meiyu, G.; Gholami, R.; Rezaee, R.; Rasouli, V.; Sarmadivaleh, M.; Bhatti, A.A. Shale gas: A solution for energy crisis and lower CO2 emission in Pakistan. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1647–1656. [Google Scholar] [CrossRef]
- Ahmed Bhuiyan, M.; Rashid Khan, H.U.; Zaman, K.; Hishan, S.S. Measuring the impact of global tropospheric ozone, carbon dioxide and sulfur dioxide concentrations on biodiversity loss. Environ. Res. 2018, 160, 398–411. [Google Scholar] [CrossRef]
- Bensouilah, R.; Knani, S.; Mansour, S.; Ksibi, Z. Chapter 2—Air pollution (volatile organic compound, etc.) and climate change. In Current Trends and Future Developments on (Bio-) Membranes; Figoli, A., Li, Y., Basile, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–46. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Comprehensive energy systems: Part A—Section 1.3: Environmental Dimensions of Energy; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Liu, Y.; Hou, M.; Yang, G.; Han, B. Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. J. Supercrit. Fluids 2011, 56, 125–129. [Google Scholar] [CrossRef]
- Keong, C.Y. Chapter 3—The United Nations’ journey to global environmental sustainability since Stockholm: The paradox. In Global Environmental Sustainability; Keong, C.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 63–212. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Sokama-Neuyam, Y.A.; Ursin, J.R.; Boakye, P. Experimental Investigation of the Mechanisms of Salt Precipitation during CO2 Injection in Sandstone. C—J. Carbon Res. 2019, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Holloway, S. An overview of the underground disposal of carbon dioxide. Energy Convers. Manag. 1997, 38, S193–S198. [Google Scholar] [CrossRef]
- Holloway, S. Safety of the underground disposal of carbon dioxide. Energy Convers. Manag. 1997, 38, S241–S245. [Google Scholar] [CrossRef]
- Gunter, W.D.; Bachu, S.; Law, D.H.S.; Marwaha, V.; Drysdale, D.L.; Macdonald, D.E.; McCann, T.J. Technical and economic feasibility of CO2 disposal in aquifers within the Alberta sedimentary basin, Canada. Energy Convers. Manag. 1996, 37, 1135–1142. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1454. [Google Scholar]
- Spycher, N.; Pruess, K.; Ennis-King, J. CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 C and up to 600 bar. Geochim. Cosmochim. Acta 2003, 67, 3015–3031. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; p. 442. [Google Scholar]
- Zhao, H.; Liao, X.; Chen, Y.; Zhao, X. Sensitivity analysis of CO2 sequestration in saline aquifers. Pet. Sci. 2010, 7, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Grude, S.; Landrø, M.; Dvorkin, J. Pressure effects caused by CO2 injection in the Tubåen Fm., the Snøhvit field. Int. J. Greenh. Gas Control 2014, 27, 178–187. [Google Scholar] [CrossRef]
- Muller, N.; Qi, R.; Mackie, E.; Pruess, K.; Blunt, M.J. CO2 injection impairment due to halite precipitation. Energy Procedia 2009, 1, 3507–3514. [Google Scholar] [CrossRef] [Green Version]
- Ott, H.; Roels, S.; De Kloe, K. Salt precipitation due to supercritical gas injection: I. Capillary-driven flow in unimodal sandstone. Int. J. Greenh. Gas Control 2015, 43, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Peysson, Y.; André, L.; Azaroual, M. Well injectivity during CO2 storage operations in deep saline aquifers—Part 1: Experimental investigation of drying effects, salt precipitation and capillary forces. Int. J. Greenh. Gas Control 2014, 22, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Miri, R.; Hellevang, H. Salt precipitation during CO2 storage—A review. Int. J. Greenh. Gas Control 2016, 51, 136–147. [Google Scholar] [CrossRef]
- Cinar, Y.; Riaz, A. Carbon dioxide sequestration in saline formations: Part 2—Review of multiphase flow modeling. J. Pet. Sci. Eng. 2014, 124, 381–398. [Google Scholar] [CrossRef]
- Hurter, S.; Berge, J.G.; Labregere, D. Simulations for CO2 injection projects with compositional simulator. In Proceedings of the Offshore Europe, Scotland, UK, 4–7 September 2007. [Google Scholar]
- Baumann, G.; Henninges, J.; De Lucia, M. Monitoring of saturation changes and salt precipitation during CO2 injection using pulsed neutron-gamma logging at the Ketzin pilot site. Int. J. Greenh. Gas Control 2014, 28, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Sminchak, J.; Zeller, E.; Bhattacharya, I. Analysis of unusual scale build-up in a CO2 injection well for a pilot-scale CO2 storage demonstration project. Greenh. Gases Sci. Technol. 2014, 4, 357–366. [Google Scholar] [CrossRef]
- Bacci, G.; Korre, A.; Durucan, S. Experimental investigation into salt precipitation during CO2 injection in saline aquifers. Energy Procedia 2011, 4, 4450–4456. [Google Scholar] [CrossRef] [Green Version]
- Pruess, K. Formation dry-out from CO2 injection into saline aquifers: 2. Analytical model for salt precipitation. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef] [Green Version]
- Kleinitz, W.; Koehler, M.; Dietzsch, G. The precipitation of salt in gas producing wells. In Proceedings of the SPE European formation damage conference, The Hague, The Netherlands, 21–22 May 2001. [Google Scholar]
- Pruess, K.; Müller, N. Formation dry-out from CO2 injection into saline aquifers: 1. Effects of solids precipitation and their mitigation. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef] [Green Version]
- Görgényi, M.; Dewulf, J.; Van Langenhove, H.; Héberger, K. Aqueous salting-out effect of inorganic cations and anions on non-electrolytes. Chemosphere 2006, 65, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Hasseine, A.; Meniai, A.-H.; Korichi, M. Salting-out effect of single salts NaCl and KCl on the LLE of the systems (water+toluene+acetone),(water+cyclohexane+2-propanol) and (water+xylene+methanol). Desalination 2009, 242, 264–276. [Google Scholar] [CrossRef]
- Hey, M.J.; Jackson, D.P.; Yan, H. The salting-out effect and phase separation in aqueous solutions of electrolytes and poly (ethylene glycol). Polymer 2005, 46, 2567–2572. [Google Scholar] [CrossRef]
- Millero, F.J.; Huang, F.; Laferiere, A.L. Solubility of oxygen in the major sea salts as a function of concentration and temperature. Mar. Chem. 2002, 78, 217–230. [Google Scholar] [CrossRef]
- Miri, R.; van Noort, R.; Aagaard, P.; Hellevang, H. New insights on the physics of salt precipitation during injection of CO2 into saline aquifers. Int. J. Greenh. Gas Control 2015, 43, 10–21. [Google Scholar] [CrossRef]
- Jacob, R.; Saylor, B.Z. CO2 solubility in multi-component brines containing NaCl, KCl, CaCl2 and MgCl2 at 297 K and 1–14 MPa. Chem. Geol. 2016, 424, 86–95. [Google Scholar] [CrossRef]
- Tong, D.; Trusler, J.M.; Vega-Maza, D. Solubility of CO2 in aqueous solutions of CaCl2 or MgCl2 and in a synthetic formation brine at temperatures up to 423 K and pressures up to 40 MPa. J. Chem. Eng. Data 2013, 58, 2116–2124. [Google Scholar] [CrossRef]
- Boström, M.; Ninham, B. Contributions from dispersion and Born self-free energies to the solvation energies of salt solutions. J. Phys. Chem. B 2004, 108, 12593–12595. [Google Scholar] [CrossRef]
- Li, Z.; Dong, M.; Li, S.; Dai, L. Densities and solubilities for binary systems of carbon dioxide+ water and carbon dioxide+brine at 59 C and pressures to 29 MPa. J. Chem. Eng. Data 2004, 49, 1026–1031. [Google Scholar] [CrossRef]
- Austegard, A.; Solbraa, E.; De Koeijer, G.; Mølnvik, M. Thermodynamic models for calculating mutual solubilities in H2O–CO2–CH4 mixtures. Chem. Eng. Res. Des. 2006, 84, 781–794. [Google Scholar] [CrossRef]
- Chapoy, A.; Mohammadi, A.; Chareton, A.; Tohidi, B.; Richon, D. Measurement and modeling of gas solubility and literature review of the properties for the carbon dioxide—Water system. Ind. Eng. Chem. Res. 2004, 43, 1794–1802. [Google Scholar] [CrossRef]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Veiga, B.A.; dos Santos, J.T.F.; Junior, L.F.d.L.L.; Corazza, M.L. Phase equilibrium measurements and thermodynamic modelling for the systems involving valeric acid, ethanol, ethyl valerate and water plus CO2. J. Chem. Thermodyn. 2017, 112, 240–248. [Google Scholar] [CrossRef]
- Nighswander, J.A.; Kalogerakis, N.; Mehrotra, A.K. Solubilities of carbon dioxide in water and 1 wt.% sodium chloride solution at pressures up to 10 MPa and temperatures from 80 to 200. degree. C. J. Chem. Eng. Data 1989, 34, 355–360. [Google Scholar] [CrossRef]
- Rumpf, B.; Nicolaisen, H.; Öcal, C.; Maurer, G. Solubility of carbon dioxide in aqueous solutions of sodium chloride: Experimental results and correlation. J. Solut. Chem. 1994, 23, 431–448. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K. A phase-partitioning model for CO2–brine mixtures at elevated temperatures and pressures: Application to CO2-enhanced geothermal systems. Transp. Porous Media 2010, 82, 173–196. [Google Scholar] [CrossRef] [Green Version]
- Kiepe, J.; Horstmann, S.; Fischer, K.; Gmehling, J. Experimental determination and prediction of gas solubility data for CO2+H2O mixtures containing NaCl or KCl at temperatures between 313 and 393 K and pressures up to 10 MPa. Ind. Eng. Chem. Res. 2002, 41, 4393–4398. [Google Scholar] [CrossRef]
- Izgec, O.; Demiral, B.; Bertin, H.J.; Akin, S. Experimental and numerical investigation of carbon sequestration in saline aquifers. In SPE/EPA/DOE Exploration and Production Environmental Conference; Society of Petroleum Engineers: Dallas, TX, USA, 2005. [Google Scholar]
- Liu, H.-H.; Zhang, G.; Yi, Z.; Wang, Y. A permeability-change relationship in the dryout zone for CO2 injection into saline aquifers. Int. J. Greenh. Gas Control 2013, 15, 42–47. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.-H. Porosity–permeability relationships in modeling salt precipitation during CO2 sequestration: Review of conceptual models and implementation in numerical simulations. Int. J. Greenh. Gas Control 2016, 52, 24–31. [Google Scholar] [CrossRef]
- Yang, F.; Bai, B.; Tang, D.; Shari, D.-N.; David, W. Characteristics of CO2 sequestration in saline aquifers. Pet. Sci. 2010, 7, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Belgodere, C.; Dubessy, J.; Vautrin, D.; Caumon, M.-C.; Sterpenich, J.; Pironon, J.; Robert, P.; Randi, A.; Birat, J.-P. Experimental determination of CO2 diffusion coefficient in aqueous solutions under pressure at room temperature via Raman spectroscopy: Impact of salinity (NaCl). J. Raman Spectrosc. 2015, 46, 1025–1032. [Google Scholar] [CrossRef]
- Uelker, B.; Alkan, H.; Pusch, G. Implications of the phase-solubility behaviour on the performance predictions of the CO2 trapping in depleted gas reservoir and aquifers. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, London, UK, 11–14 June 2007. [Google Scholar]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Balashov, V.N.; Guthrie, G.D.; Lopano, C.L.; Hakala, J.A.; Brantley, S.L. Reaction and diffusion at the reservoir/shale interface during CO2 storage: Impact of geochemical kinetics. Appl. Geochem. 2015, 61, 119–131. [Google Scholar] [CrossRef]
- Koukouzas, N.; Kypritidou, Z.; Purser, G.; Rochelle, C.A.; Vasilatos, C.; Tsoukalas, N. Assessment of the impact of CO2 storage in sandstone formations by experimental studies and geochemical modeling: The case of the Mesohellenic Trough, NW Greece. Int. J. Greenh. Gas Control 2018, 71, 116–132. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2–brine–caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Dilmore, R.; Lu, P.; Allen, D.; Soong, Y.; Hedges, S.; Fu, J.K.; Dobbs, C.L.; Degalbo, A.; Zhu, C. Sequestration of CO2 in mixtures of bauxite residue and saline wastewater. Energy Fuels 2008, 22, 343–353. [Google Scholar] [CrossRef]
- Pruess, K.; Garcia, J. Multiphase flow dynamics during CO2 disposal into saline aquifers. Environ. Geol. 2002, 42, 282–295. [Google Scholar] [CrossRef]
- Rochelle, C.A.; Czernichowski-Lauriol, I.; Milodowski, A. The impact of chemical reactions on CO2 storage in geological formations: A brief review. Geol. Soc. Lond. Spec. Publ. 2004, 233, 87–106. [Google Scholar] [CrossRef]
- INC, K.I. Details for Our Sandstone. Available online: https://kocurekindustries.com/sandstone-cores (accessed on 23 March 2019).
- Huang, Y.-H.; Yang, S.-Q.; Hall, M.R.; Zhang, Y.-C. The effects of NaCl concentration and confining pressure on mechanical and acoustic behaviors of brine-saturated sandstone. Energies 2018, 11, 385. [Google Scholar] [CrossRef] [Green Version]
- Bachu, S.; Bennion, B. Effects of in-situ conditions on relative permeability characteristics of CO2-brine systems. Environ. Geol. 2008, 54, 1707–1722. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Kaldi, J.G.; Gibson-Poole, C.M. Storage Capacity Estimation, Site Selection and Characterisation for CO2 Storage Projects; CO2CRC: Carlton, Australia, 2008. [Google Scholar]
- Ott, H.; de Kloe, K.; Taberner, C.; Marcelis, F.; Wang, Y.; Makurat, A. Rock/fluid interaction by injection of supercritical CO2/H2S: Investigation of dry-zone formation near the injection well. In Proceedings of the International Symposium of the Society of Core Analysts, Halifax, NS, Canada, 4–7 October 2010. [Google Scholar]
- Calabrese, M.; Masserano, F.; Blunt, M.J. Simulation of physical-chemical processes during carbon dioxide sequestration in geological structures. In SPE Annual Technical Conference and Exhibition; OnePetro: Dallas, TX, USA, 2005. [Google Scholar]
- Edem, D.; Abba, M.; Nourian, A.; Babaie, M.; Mohammed, N. The Effects of Different Salt Types and Concentration on CO2 Injectivity During CO2 Sequestration in Deep Saline Aquifers. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Virtual, 11–13 August 2020. [Google Scholar]
- Edem, D.; Abba, M.; Nourian, A.; Babaie, M.; Naeem, Z. Experimental Investigation of the Extent of the Impact of Halite Precipitation on CO2 Injection in Deep Saline Aquifers. In Proceedings of the SPE Europec featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 18–21 October 2021. [Google Scholar]
- Abba, M.K.; Abbas, A.J.; Nasr, G.G.; Al-Otaibi, A.; Burby, M.; Saidu, B.; Suleiman, S.M. Solubility trapping as a potential secondary mechanism for CO2 sequestration during enhanced gas recovery by CO2 injection in conventional natural gas reservoirs: An experimental approach. J. Nat. Gas Sci. Eng. 2019, 71, 103002. [Google Scholar] [CrossRef]
- Abba, M.K. Enhanced gas recovery by CO2 injection: Influence of monovalent and divalent brines and their concentrations on CO2 dispersion in porous media. J. Nat. Gas Sci. Eng. 2020, 84, 103643. [Google Scholar] [CrossRef]
- Abba, M.K.; Al-Othaibi, A.; Abbas, A.J.; Nasr, G.G.; Mukhtar, A. Experimental investigation on the impact of connate water salinity on dispersion coefficient in consolidated rocks cores during Enhanced Gas Recovery by CO2 injection. J. Nat. Gas Sci. Eng. 2018, 60, 190–201. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]


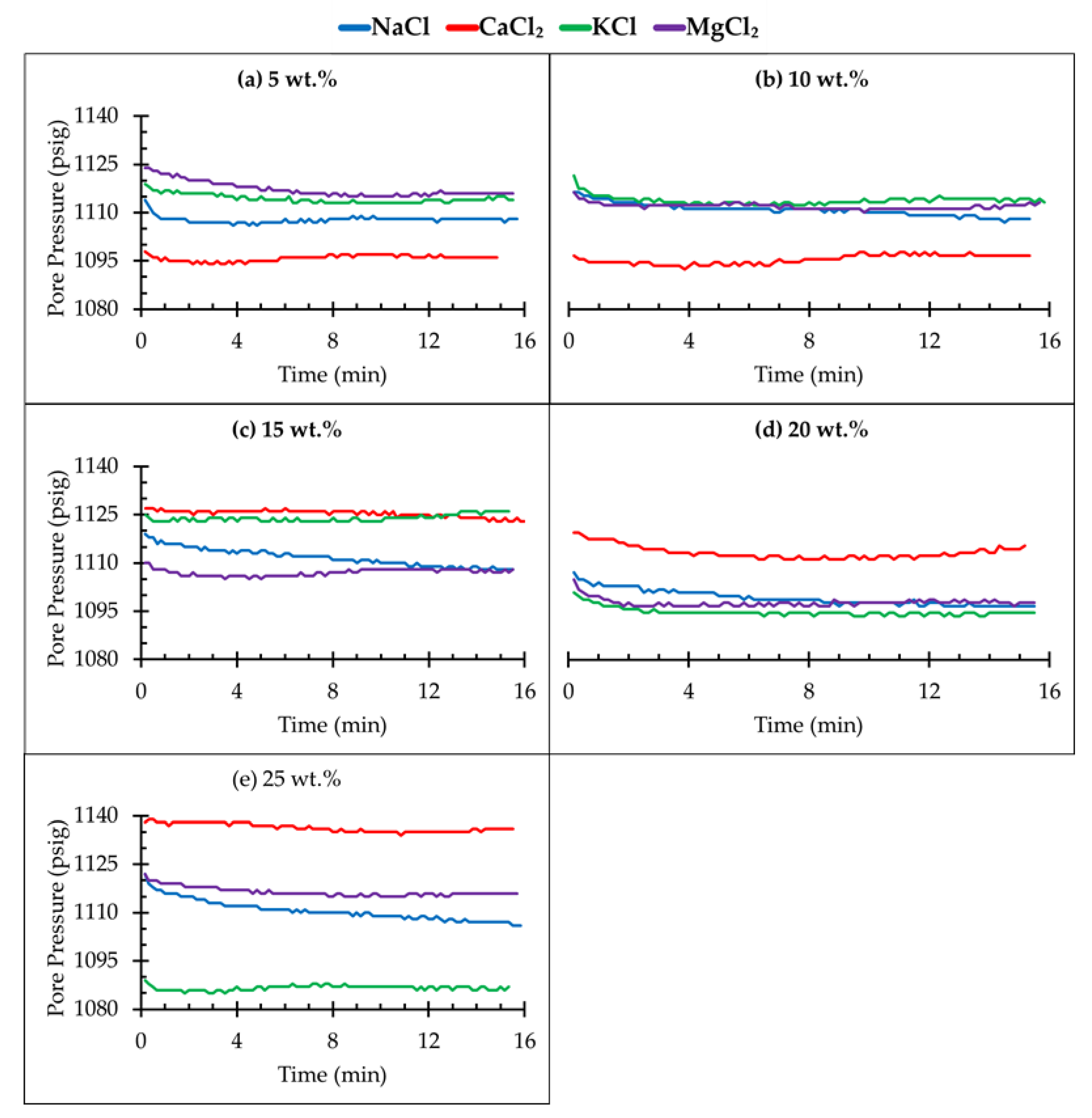

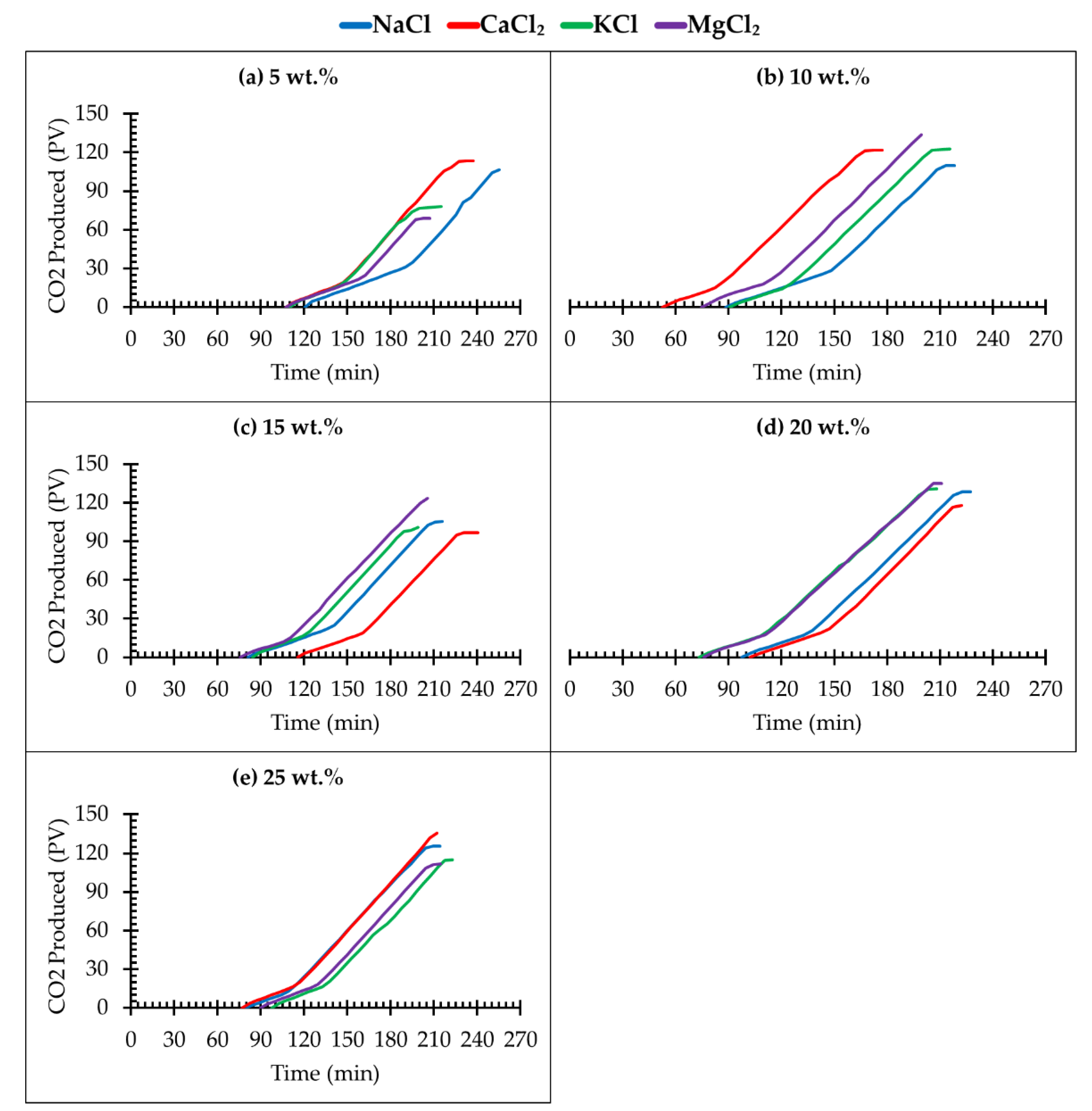
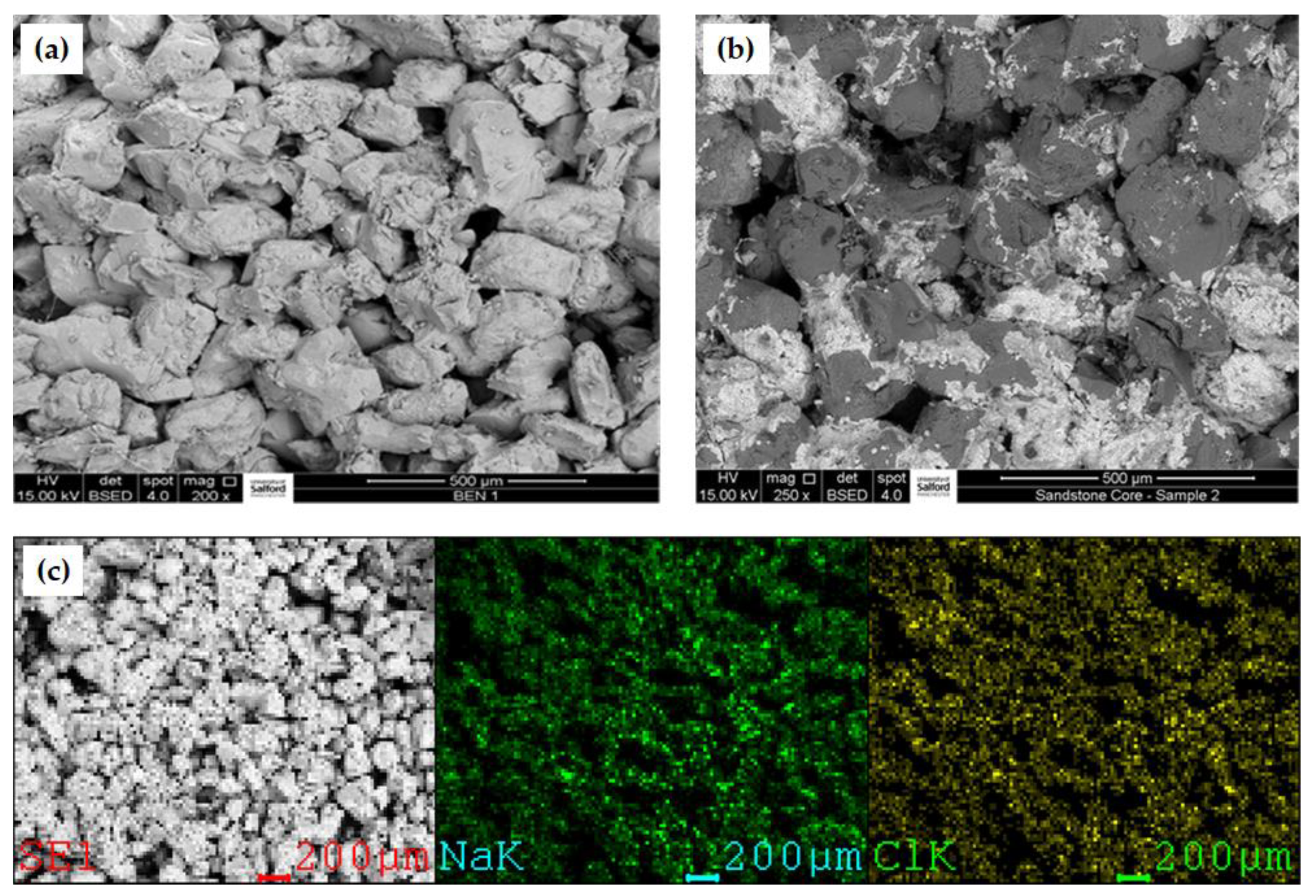
| Salt Type | Brine Concentration (wt.%) | Pressure Change (psi) | Pressure Decay Rate (psi/min) | Brine Saturation (%) |
|---|---|---|---|---|
| NaCl | 5% 10% 15% 20% 25% | 16 ± 0.3 8 ± 0.3 11 ± 0.3 10 ± 0.3 6 ± 0.4 | 1.06 0.53 0.73 0.60 0.40 | 89 87 91 85 91 |
| CaCl2 | 5% 10% 15% 20% 25% | 2 ± 0.2 4 ± 0.2 4 ± 0.2 5 ± 0.3 2 ± 0.1 | 0.13 0.23 0.26 0.30 0.13 | 87 96 85 97 89 |
| KCl | 5% 10% 15% 20% 25% | 5 ± 0.2 8 ± 0.4 2 ± 0.3 6 ± 0.4 3 ± 0.2 | 0.30 0.53 0.13 0.40 0.20 | 85 90 91 92 96 |
| MgCl2 | 5% 10% 15% 20% 25% | 8 ± 0.4 4 ± 0.3 3 ± 0.5 7 ± 0.8 6 ± 0.2 | 0.53 0.25 0.20 0.46 0.40 | 89 89 95 95 93 |
| Salt Type | Salt Concentration (wt.%) | % of Porosity Reduction | % of Permeability Reduction |
|---|---|---|---|
| NaCl | 5 10 15 20 25 | 1.2 1.9 3.4 3.6 6.2 | 37.0 42.0 49.1 50.0 51.7 |
| CaCl2 | 5 10 15 20 25 | 3.5 24.6 25.3 27.4 35.3 | 19.4 27.5 31.0 37.0 43.1 |
| KCl | 5 10 15 20 25 | 20.8 21.7 24.8 33.6 33.8 | 17.1 23.7 31.0 40.8 44.2 |
| MgCl2 | 5 10 15 20 25 | 22.8 24.8 34.2 36.2 40.9 | 44.2 56.1 61.3 62.1 63.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edem, D.E.; Abba, M.K.; Nourian, A.; Babaie, M.; Naeem, Z. Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Sustainability 2022, 14, 986. https://doi.org/10.3390/su14020986
Edem DE, Abba MK, Nourian A, Babaie M, Naeem Z. Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Sustainability. 2022; 14(2):986. https://doi.org/10.3390/su14020986
Chicago/Turabian StyleEdem, Donatus Ephraim, Muhammad Kabir Abba, Amir Nourian, Meisam Babaie, and Zainab Naeem. 2022. "Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers" Sustainability 14, no. 2: 986. https://doi.org/10.3390/su14020986
APA StyleEdem, D. E., Abba, M. K., Nourian, A., Babaie, M., & Naeem, Z. (2022). Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Sustainability, 14(2), 986. https://doi.org/10.3390/su14020986








