Graphene–Silver Hybrid Nanoparticle based Organic Phase Change Materials for Enhanced Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methodology
2.1. Material Specifications
2.2. Preparation of Nanocomposite PCM
2.3. Instruments and Characterizations
3. Results and Discussion
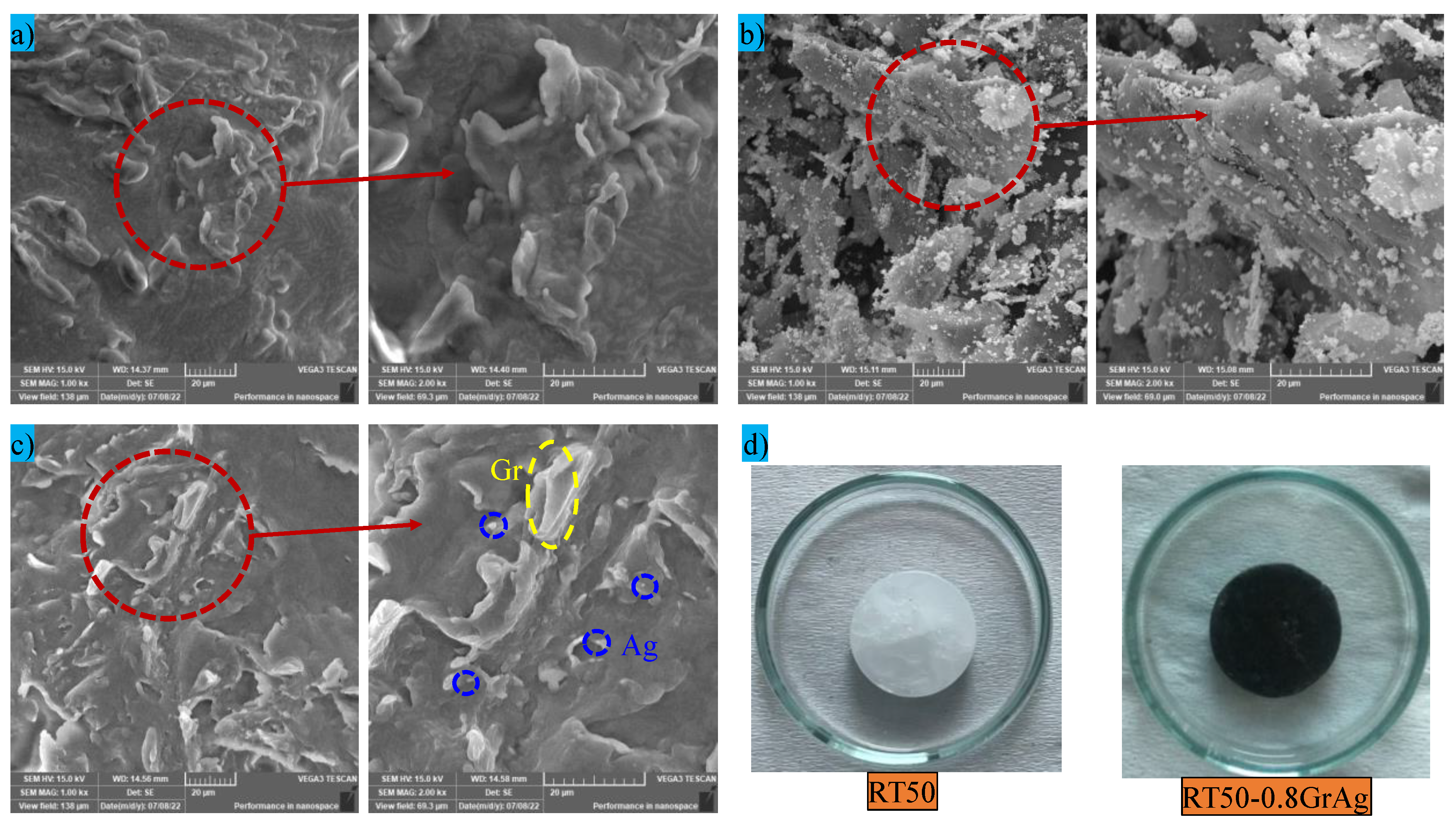
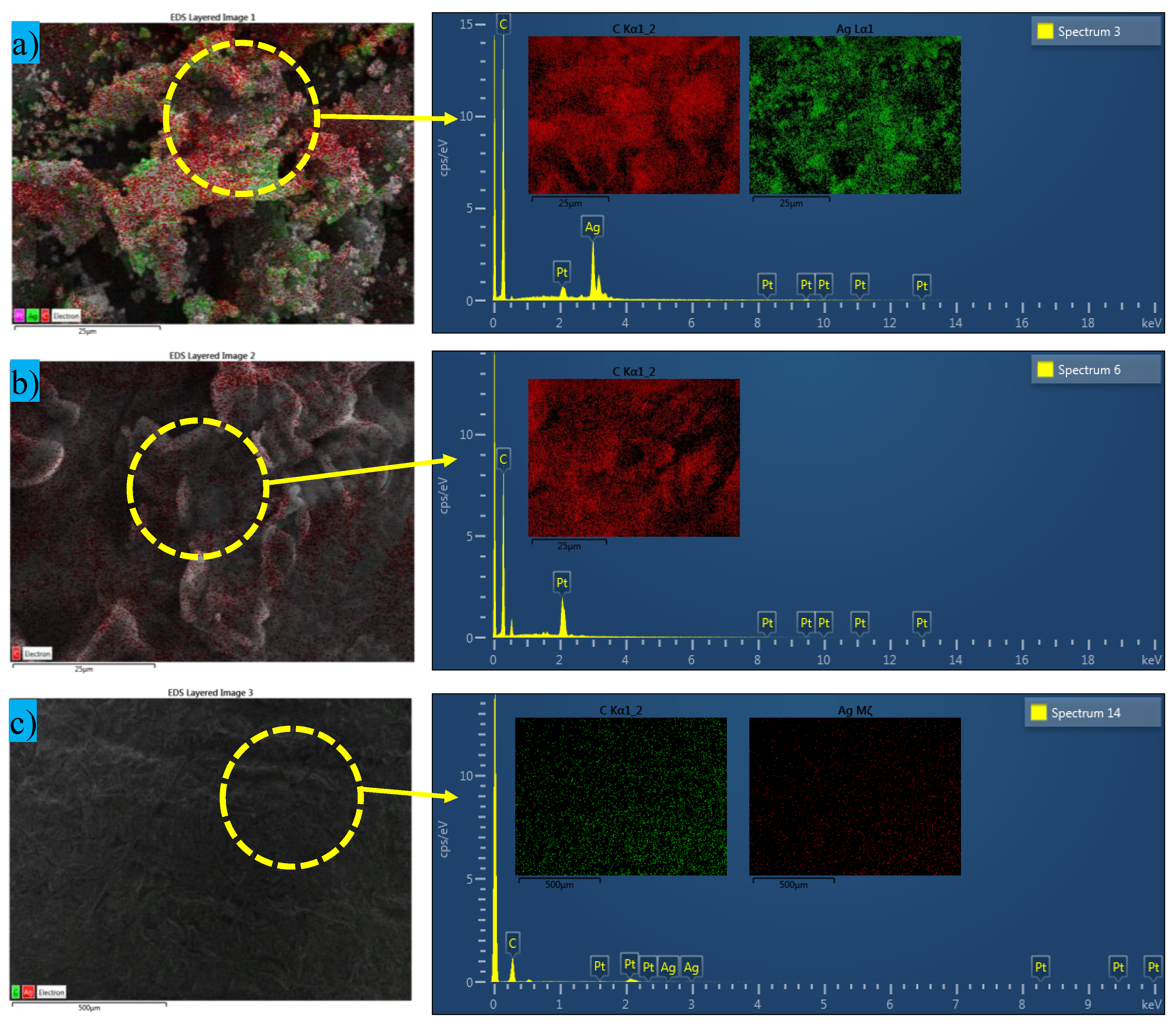
3.1. Microstructure and Composition Analysis Using SEM and EDX
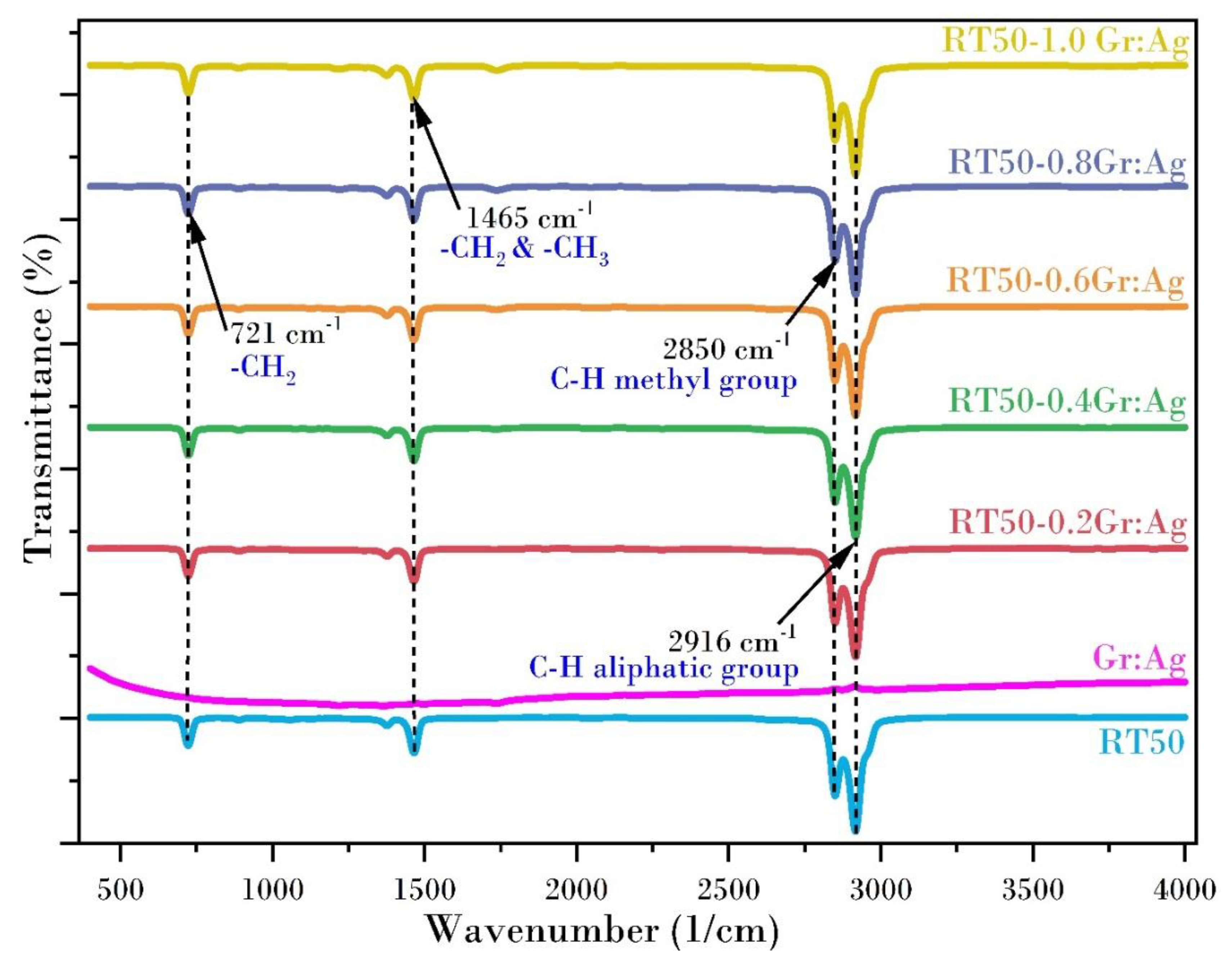
3.2. Chemical Stability Investigation Using FTIR
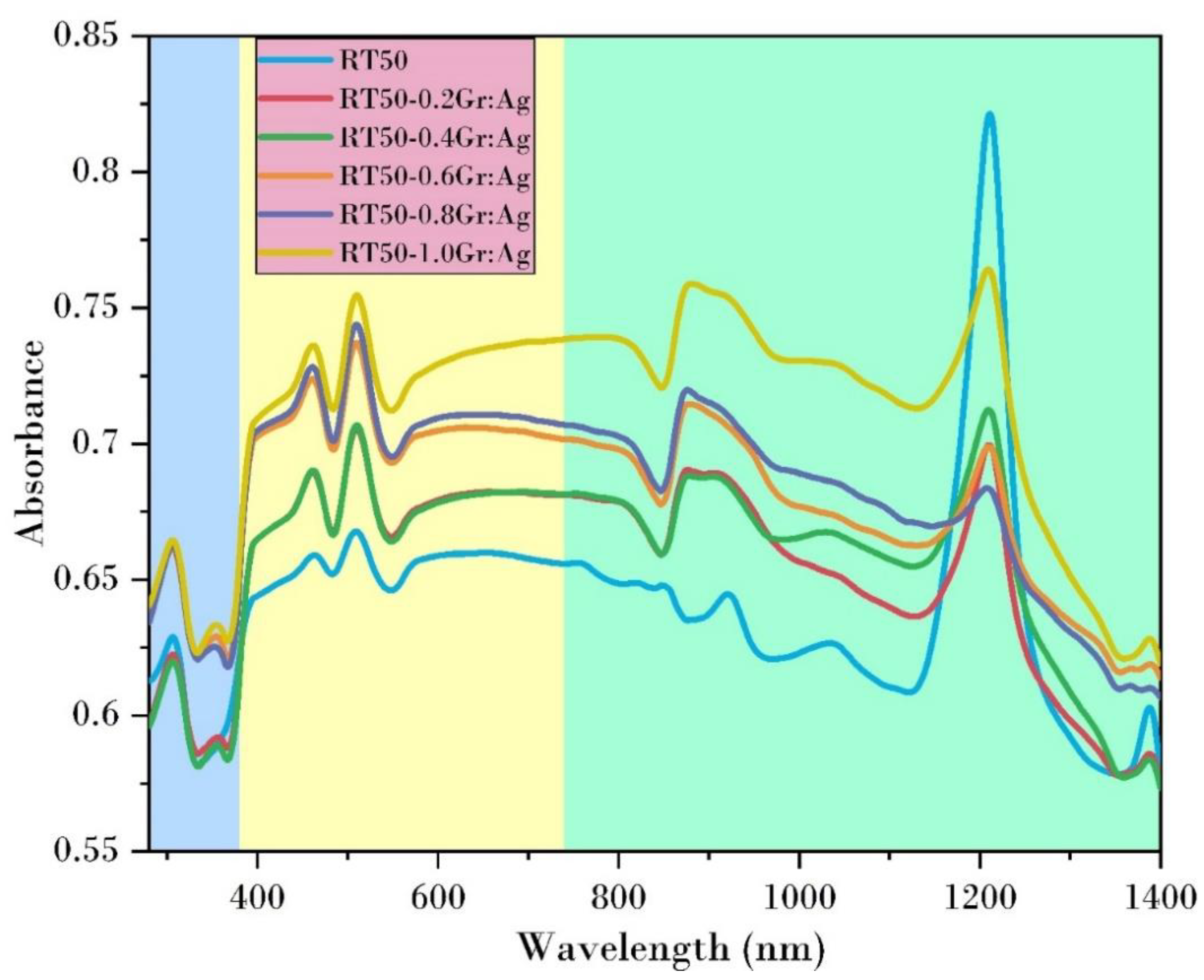
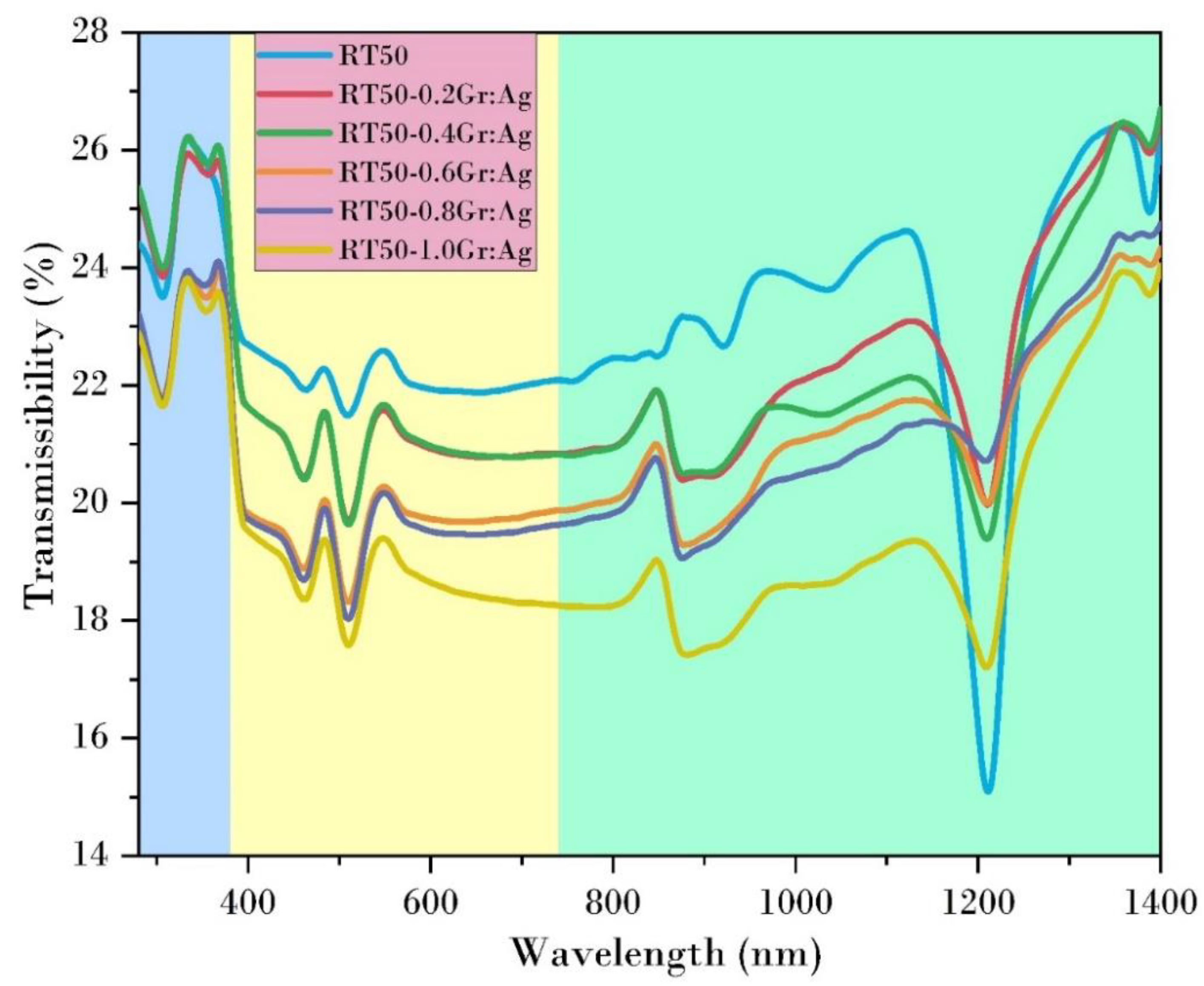
3.3. UV-Vis Analysis
3.4. Thermal Conductivity
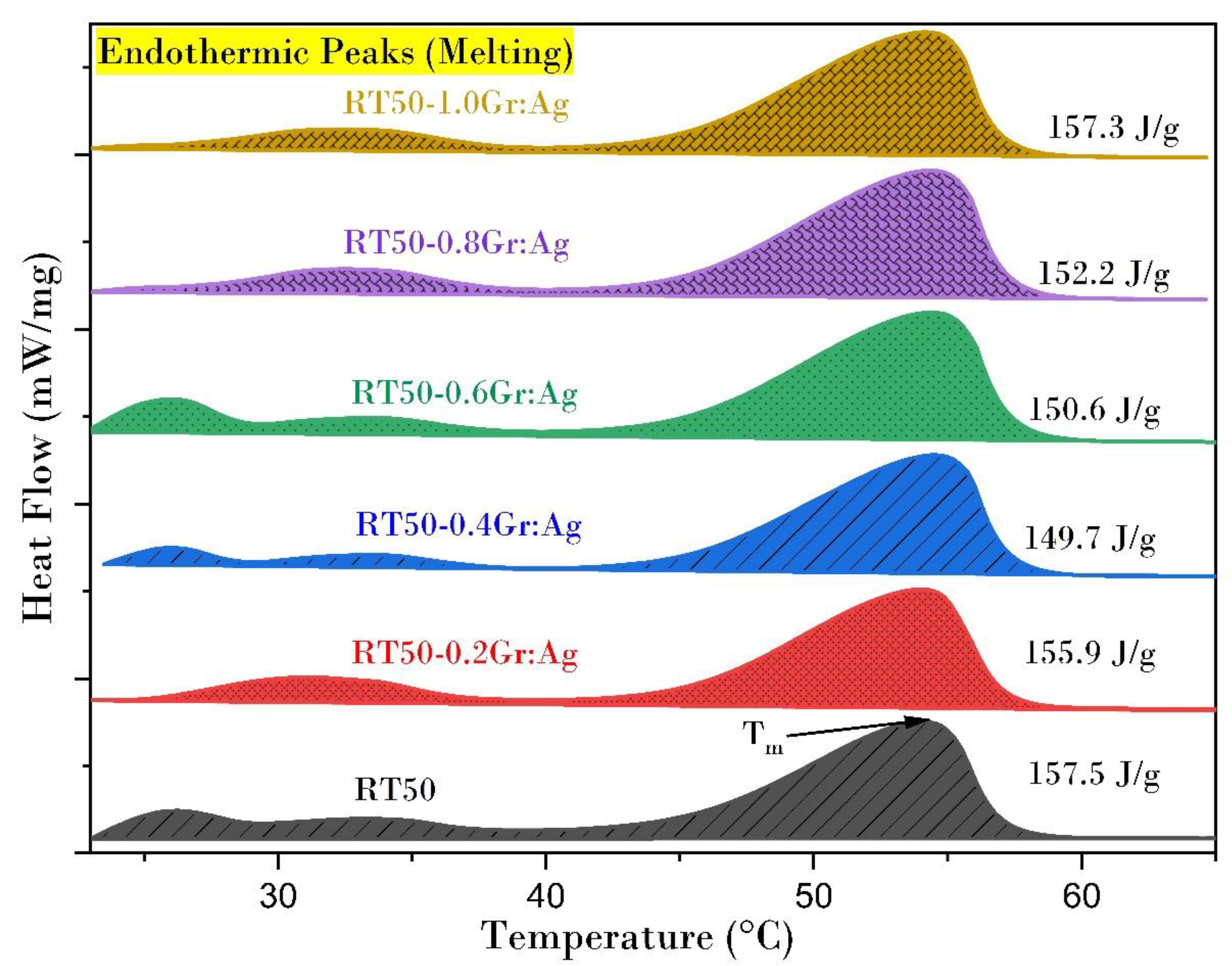
3.5. Heating Enthalpy
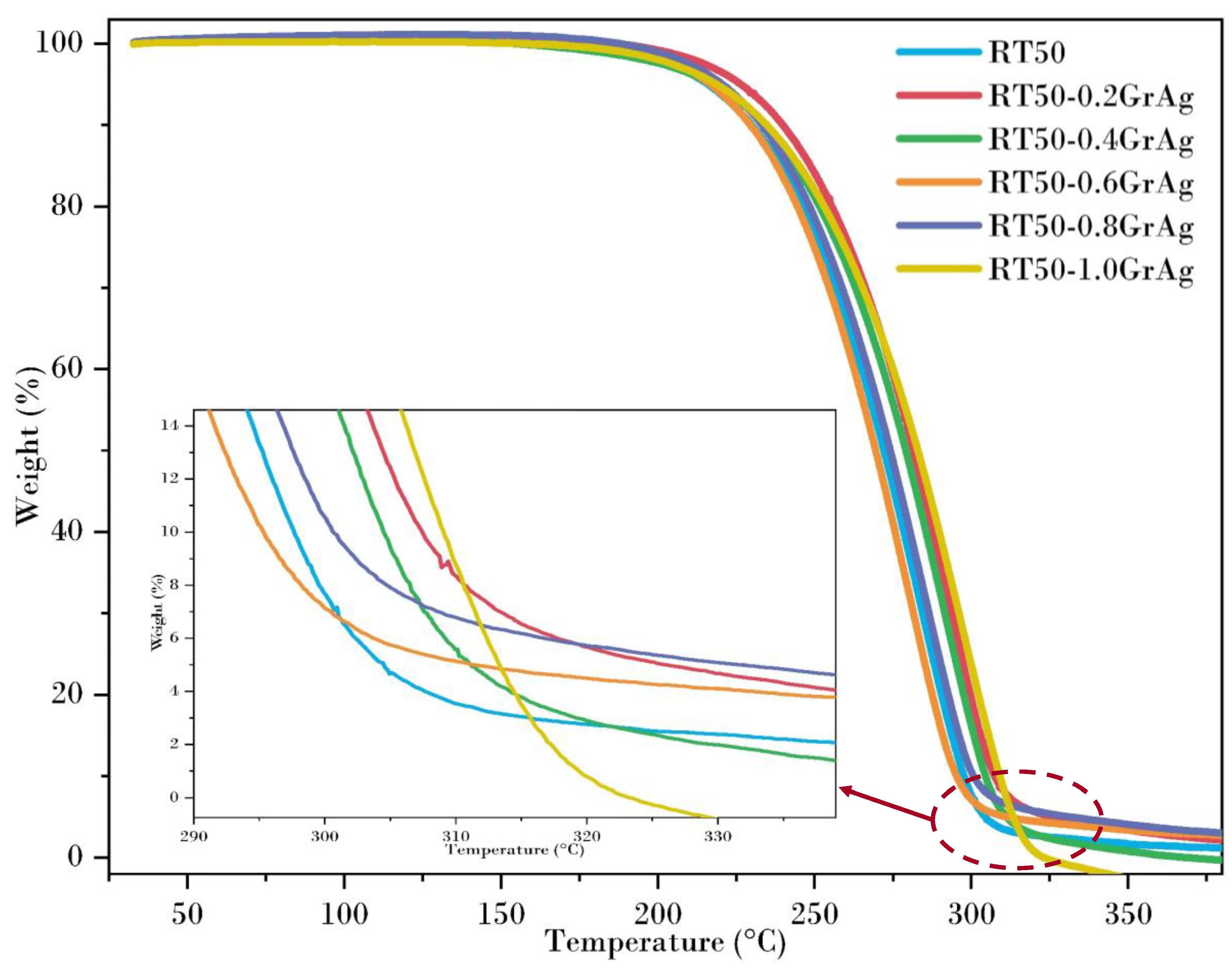
3.6. Thermal Degradation Evaluation
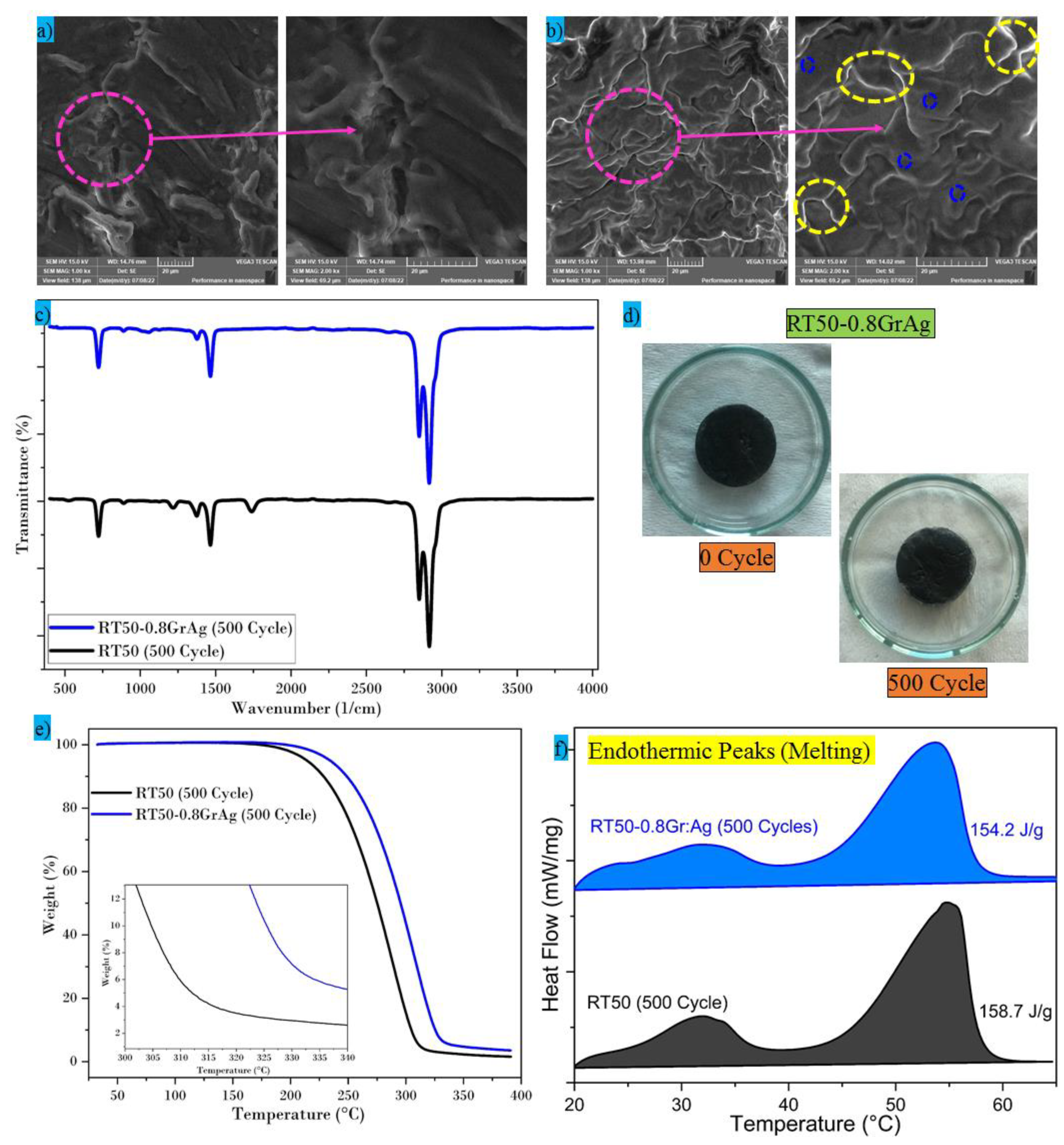
3.7. Thermal Cycling Analysis
4. Conclusions
- SEM images ensure the uniform dispersion of hybrid nanoparticles within the PCM matrix, which provides evidence that the thermal networks facilitated effective heat transfer.
- The addition of the hybrid nanoparticle Gr:Ag leads to an increase in thermal conductivity of the base organic PCM from 0.212 to 0.326, which is considerably about 53.77% higher due to the well-developed thermal networks and phonon–phonon interaction.
- The latent heat value of the composite PCM is slightly reduced when hybrid nanoparticles form a fraction of the PCM volume that is occupied by the nanoparticles. The drop in latent heat value is considerably lower. Thermal decomposition temperature of the PCMs is slightly enhanced by nanoparticles.
- The thermal decomposition of the composite samples was analyzed using TGA, where the decomposition temperature of the hybrid nanoparticle dispersed PCM were substantially increased compared to the base PCMs. With higher dispersion of the nanoparticles, there was a reduction in the decomposition temperature.
- Gr:Ag dispersed at a 0.8 wt% with PCM exhibits decent chemical and thermal stability after 500 cycles. Spectral peaks are constant before and after the thermal cycles, the decomposition points are further increased after the thermal cycling tests, and the latent heat value is almost the same before and after the thermal cycles, which indicates the PCMs suitability for energy storage applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Composite Code | |
| RT50 | Rubitherm organic PCM with melting point 50 °C |
| Gr:Ag | Graphene silver nanopowder |
| RT50-0.2Gr:Ag | RT50 with 0.2% Gr:Ag nanopowder |
| RT50-0.4Gr:Ag | RT50 with 0.4% Gr:Ag nanopowder |
| RT50-0.6Gr:Ag | RT50 with 0.6% Gr:Ag nanopowder |
| RT50-0.8Gr:Ag | RT50 with 0.8% Gr:Ag nanopowder |
| RT50-1.0Gr:Ag | RT50 with 1.0% Gr:Ag nanopowder |
References
- Smith, J.B.; Schneider, S.H.; Oppenheimer, M.; Yohe, G.W.; Hare, W.; Mastrandrea, M.D.; Patwardhan, A.; Burton, I.; Corfee-Morlot, J.; Magadza, C.H. Assessing dangerous climate change through an update of the Intergovernmental Panel on Climate Change (IPCC) “reasons for concern”. Proc. Natl. Acad. Sci. USA 2009, 106, 4133–4137. [Google Scholar] [CrossRef] [PubMed]
- Laghari, I.A.; Samykano, M.; Pandey, A.; Kadirgama, K.; Mishra, Y.N. Binary composite (TiO2-Gr) based nano-enhanced organic phase change material: Effect on thermophysical properties. J. Energy Storage 2022, 51, 104526. [Google Scholar] [CrossRef]
- Tariq, S.L.; Ali, H.M.; Akram, M.A.; Janjua, M.M.; Ahmadlouydarab, M. Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 2020, 176, 115305. [Google Scholar] [CrossRef]
- Sun, K.; Kou, Y.; Zhang, Y.; Liu, T.; Shi, Q. Photo-triggered hierarchical porous carbon-based composite phase-change materials with superior thermal energy conversion capacity. ACS Sustain. Chem. Eng. 2020, 8, 3445–3453. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, J.; Ma, C.; Guo, L.; Meng, X. Solar-driven phase change microencapsulation with efficient Ti4O7 nanoconverter for latent heat storage. Nano Energy 2018, 53, 579–586. [Google Scholar] [CrossRef]
- Chen, R.; Yao, R.; Xia, W.; Zou, R. Electro/photo to heat conversion system based on polyurethane embedded graphite foam. Appl. Energy 2015, 152, 183–188. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Liu, Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Hybrid graphene aerogels/phase change material composites: Thermal conductivity, shape-stabilization and light-to-thermal energy storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro-and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef]
- Li, Y.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. From biomass to high performance solar–thermal and electric–thermal energy conversion and storage materials. J. Mater. Chem. A 2014, 2, 7759–7765. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Zhang, S. Visible light-driven organic form-stable phase change materials for solar energy storage. RSC Adv. 2012, 2, 5964–5967. [Google Scholar] [CrossRef]
- Awad, S.A.; Khalaf, E.M. Improvement of the chemical, thermal, mechanical and morphological properties of polyethylene terephthalate–graphene particle composites. Bull. Mater. Sci. 2018, 41, 67. [Google Scholar] [CrossRef]
- Nan, H.Y.; Ni, Z.H.; Wang, J.; Zafar, Z.; Shi, Z.X.; Wang, Y.Y. The thermal stability of graphene in air investigated by Raman spectroscopy. J. Raman Spectrosc. 2013, 44, 1018–1021. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hao, P. Mechanical properties of monolayer graphene under tensile and compressive loading. Phys. E: Low-Dimens. Syst. Nanostruct. 2009, 41, 1561–1566. [Google Scholar] [CrossRef]
- Jamil, N.; Kaur, J.; Pandey, A.; Shahabuddin, S.; Hassani, S.; Saidur, R.; Ali, R.R.; Sidik, N.A.C.; Naim, M. A review on nano enhanced phase change materials: An enhancement in thermal properties and specific heat capacity. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 57, 110–120. [Google Scholar]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5, 100039. [Google Scholar] [CrossRef]
- Li, W.; Guo, S.; Tan, L.; Liu, L.; Ao, W. Heat transfer enhancement of nano-encapsulated phase change material (NEPCM) using metal foam for thermal energy storage. Int. J. Heat Mass Transf. 2021, 166, 120737. [Google Scholar] [CrossRef]
- Abdelrazik, A.; Saidur, R.; Al-Sulaiman, F.; Al-Ahmed, A.; Ben-Mansour, R. Multiwalled CNT and graphene nanoplatelets based nano-enhanced PCMs: Evaluation for the thermal performance and its implications on the performance of hybrid PV/thermal systems. Mater. Today Commun. 2022, 31, 103618. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Kou, Y.; Liu, H.; Wang, L.; Yin, N.; Dong, H.; Shi, Q. One-step synthesis of graphene-based composite phase change materials with high solar-thermal conversion efficiency. Chem. Eng. J. 2022, 429, 132439. [Google Scholar] [CrossRef]
- Vivekananthan, M.; Amirtham, V.A. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, R.; Tian, T.; Li, J. Characteristics of thermal storage heat pipe charged with graphene nanoplatelets enhanced organic phase change material. Energy Convers. Manag. 2022, 267, 115902. [Google Scholar] [CrossRef]
- Tariq, S.L.; Ali, H.M.; Akram, M.A.; Janjua, M.M. Experimental investigation on graphene based nanoparticles enhanced phase change materials (GbNePCMs) for thermal management of electronic equipment. J. Energy Storage 2020, 30, 101497. [Google Scholar] [CrossRef]
- Pasupathi, M.K.; Alagar, K.; Mm, M.; Aritra, G. Characterization of hybrid-nano/paraffin organic phase change material for thermal energy storage applications in solar thermal systems. Energies 2020, 13, 5079. [Google Scholar] [CrossRef]
- Pradeep, N.; Paramasivam, K.; Rajesh, T.; Purusothamanan, V.S.; Iyahraja, S. Silver nanoparticles for enhanced thermal energy storage of phase change materials. Mater. Today: Proc. 2021, 45, 607–611. [Google Scholar] [CrossRef]
- Prabhu, B.; ValanArasu, A. Stability analysis of TiO2-Ag nanocomposite particles dispersed paraffin wax as energy storage material for solar thermal systems. Renew. Energy 2020, 152, 358–367. [Google Scholar]
- Zhan, W.; Zhao, Y.; Yuan, Y.; Yi, H.; Song, S. Development of 2D-Mt/SA/AgNPs microencapsulation phase change materials for solar energy storage with enhancement of thermal conductivity and latent heat capacity. Sol. Energy Mater. Sol. Cells 2019, 201, 110090. [Google Scholar] [CrossRef]
- Wen, R.; Zhu, S.; Wu, M.; Chen, W. Design and preparation of Ag modified expanded graphite based composite phase change materials with enhanced thermal conductivity and light-to-thermal properties. J. Energy Storage 2021, 41, 102936. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Luo, W.; Hu, X.; Che, Y.; Zu, S.; Li, Q.; Jiang, X.; Liu, D. Form-stable phase change materials enhanced photothermic conversion and thermal conductivity by Ag-expanded graphite. J. Energy Storage 2022, 52, 105060. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Huang, X.; Zheng, H.; Lu, G.; Xi, Z.; Wang, G. Graphene-CoO/PEG composite phase change materials with enhanced solar-to-thermal energy conversion and storage capacity. Compos. Sci. Technol. 2020, 195, 108197. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.; Shahabuddin, S.; George, M.; Sharma, K.; Samykano, M.; Tyagi, V.; Saidur, R. Synthesis and characterization of conducting Polyaniline@ cobalt-Paraffin wax nanocomposite as nano-phase change material: Enhanced thermophysical properties. Renew. Energy 2021, 173, 1057–1069. [Google Scholar]
- Fikri, M.A.; Pandey, A.; Samykano, M.; Kadirgama, K.; George, M.; Saidur, R.; Selvaraj, J.; Abd Rahim, N.; Sharma, K.; Tyagi, V. Thermal conductivity, reliability, and stability assessment of phase change material (PCM) doped with functionalized multi-wall carbon nanotubes (FMWCNTs). J. Energy Storage 2022, 50, 104676. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.; Saidur, R.; Samykano, M.; Tyagi, V. Nano additive enhanced salt hydrate phase change materials for thermal energy storage. Int. Mater. Rev. 2022, 1–44. [Google Scholar] [CrossRef]
- Yousefi, A.; Tang, W.; Khavarian, M.; Fang, C. Development of novel form-stable phase change material (PCM) composite using recycled expanded glass for thermal energy storage in cementitious composite. Renew. Energy 2021, 175, 14–28. [Google Scholar] [CrossRef]
- Balaji, A.; Saravanan, R.; Purushothaman, R.; Vijayaraj, S.; Balasubramanian, P. Investigation of Thermal Energy Storage (TES) with lotus stem biocomposite block using PCM. Clean. Eng. Technol. 2021, 4, 100146. [Google Scholar] [CrossRef]
- Anand, A.; Shukla, A.; Kumar, A.; Buddhi, D.; Sharma, A. Cycle test stability and corrosion evaluation of phase change materials used in thermal energy storage systems. J. Energy Storage 2021, 39, 102664. [Google Scholar] [CrossRef]


| Properties | PCM | Nano Powder | |
|---|---|---|---|
| RT 50 | Graphene (Gr) | Silver (Ag) | |
| Phase Transition Temperature | 50 °C | 3550 °C | 961.8 °C |
| Thermal Conductivity | 0.2 W/mK | 4000 W/mK | 429 W/mK |
| Heating Enthalpy | 160 kJ/kg | - | - |
| Density | 880 kg/m3 | 130 kg/m3 | 10,500 kg/m3 |
| Particle Size | - | 1– μm | 20 nm |
| Color | White | Black | Black |
| Purity | - | >99 wt% | 99.99% |
| Surface Area | - | 500–1200 m2/g | 18–22 m2/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalidasan, B.; Pandey, A.K.; Rahman, S.; Yadav, A.; Samykano, M.; Tyagi, V.V. Graphene–Silver Hybrid Nanoparticle based Organic Phase Change Materials for Enhanced Thermal Energy Storage. Sustainability 2022, 14, 13240. https://doi.org/10.3390/su142013240
Kalidasan B, Pandey AK, Rahman S, Yadav A, Samykano M, Tyagi VV. Graphene–Silver Hybrid Nanoparticle based Organic Phase Change Materials for Enhanced Thermal Energy Storage. Sustainability. 2022; 14(20):13240. https://doi.org/10.3390/su142013240
Chicago/Turabian StyleKalidasan, B., A. K. Pandey, Saidur Rahman, Aman Yadav, M. Samykano, and V. V. Tyagi. 2022. "Graphene–Silver Hybrid Nanoparticle based Organic Phase Change Materials for Enhanced Thermal Energy Storage" Sustainability 14, no. 20: 13240. https://doi.org/10.3390/su142013240
APA StyleKalidasan, B., Pandey, A. K., Rahman, S., Yadav, A., Samykano, M., & Tyagi, V. V. (2022). Graphene–Silver Hybrid Nanoparticle based Organic Phase Change Materials for Enhanced Thermal Energy Storage. Sustainability, 14(20), 13240. https://doi.org/10.3390/su142013240








