Predicting Infection Positivity, Risk Estimation, and Disease Prognosis in Dengue Infected Patients by ML Expert System
Abstract
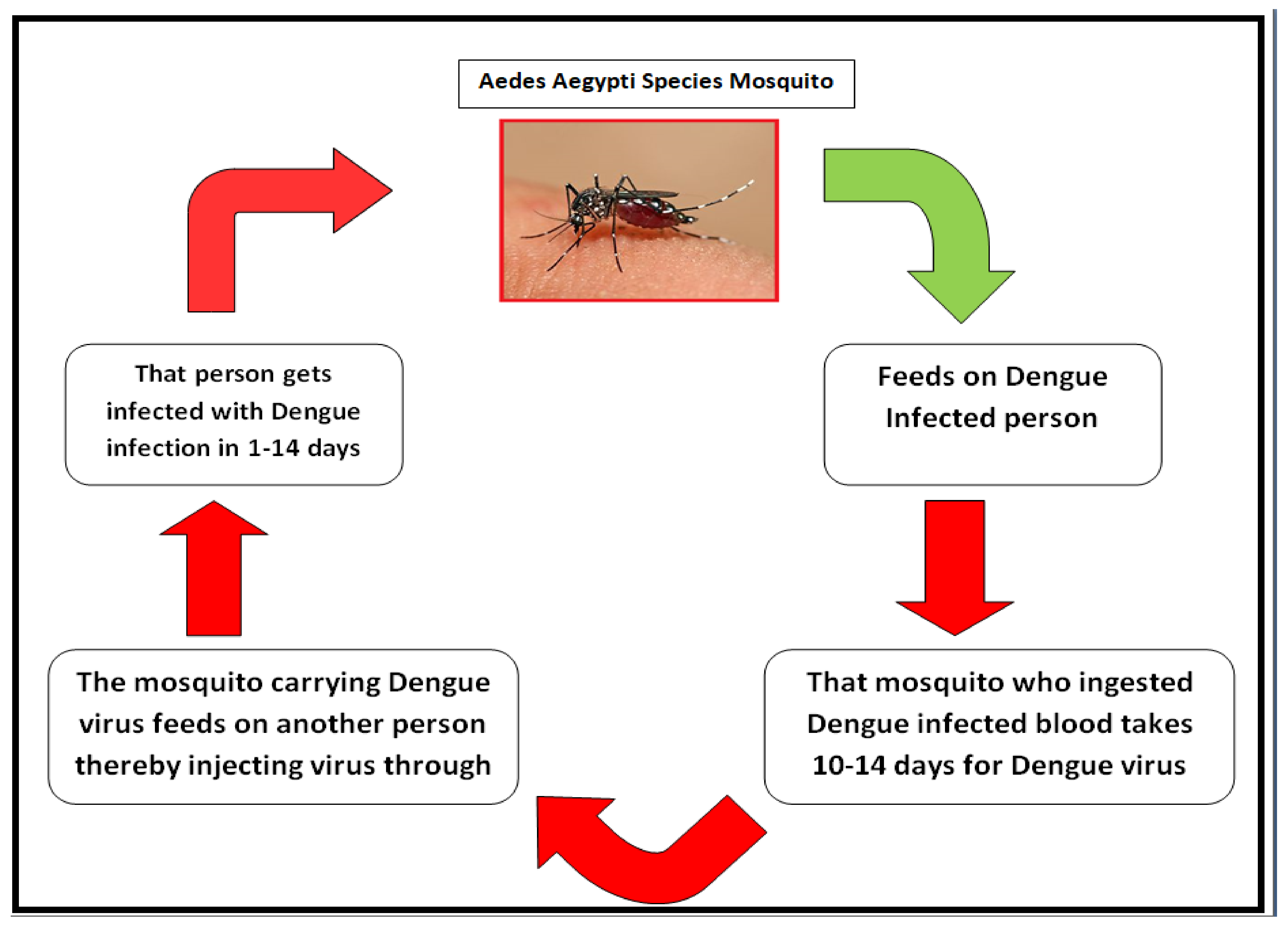
:1. Introduction
- To emphasize the need to predict dengue infection effectively and precisely.
- To propose a tool for data collection and analysis for dengue patients.
- To provide a real-time patient diagnosis of dengue infection present or not by analyzing more than 17 data attributes.
- To further predict if the identified dengue-infected patient has a pattern of a possible internal hemorrhagic manifestation that may threaten life as a severe case of thrombocytopenia.
- To examine the proposed model’s efficiency in identifying which stage of dengue infection level the positive dengue patient may be suffering i.e., DHF or DSS.
- To provide alerts to concerned stakeholders for providing timely medical aid.
2. Related Work
2.1. Dengue Fever Infection
2.2. Cloud Computing and IoT-Based Dengue Healthcare
3. The Proposed Model
3.1. Model Architecture
- Data Collection: The dengue patient information was screened and retrieved for those admitted/referred to the government medical college in Amritsar for dengue monitoring as well as those who were dengue positive but hospitalization was not required. The positive patients were confirmed to have dengue infection through NS1 antigen or IgM/IgG tests. The vital signs and clinical test parameters were then recorded in a Microsoft Excel sheet for further preprocessing, in which missing value records were dropped. This study works with 19 data attributes that, to the best of the author’s knowledge, have not been included in only the symptomatic dengue infection model.
- Prediction Model: The random forest tree algorithm in the machine learning library of Python was trained by using training data, but before that, the stratified k-fold cross-validation for the value of k = 6 was applied to the pre-processed dataset generated in the previous phase to obtain quality random training and testing dataset. The predicting algorithms were saved in the Jupyter Notebook for further work.
- Validation and Improvement: The predictive models were tested using a testing dataset generated in Phase 2, and the accuracy for each was calculated. The confusion matrix was then made to check how well the models work, and the values of precision, F1 score, sensitivity, and specificity were calculated so that the models can be compared.
- Data Collection: The dengue patient’s positive information was screened based on NS1antigen or IgM/IgG test and retrieved from the government medical college in Amritsar. The clinical test parameters were extracted from their file and then were recorded in a Microsoft Excel sheet for further preprocessing in which missing value rows were dropped even if one column was null.
- Prediction Model: The stratified k-fold cross-validation for the value of k = 8 was applied to the dataset generated, which was further inputted to the random forest tree classifier. The predicting algorithms were saved in Jupyter Notebook for further work.
- Validation and Improvement: The predictive model was tested using a testing dataset, and the model’s accuracy was calculated. The confusion matrix is then made to check the performance of the models based on the values for precision, F1 score, sensitivity, and specificity.
3.2. Proposed Dengue Diagnosis System’s Workflow
3.3. Data Collection
3.4. Train-Test Dataset
3.5. Classification
3.6. Alert Generation
4. Experimental Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DF | Dengue fever |
| DFNB | Dengue fever with no bleeding |
| DHF | Dengue hemorrhagic fever |
| DSS | Dengue shock syndrome |
| DFW | Dengue fever with warning signs |
| IOT | Internet of Things |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
References
- Dengue. Available online: https://www.cdc.gov/dengue/index.html (accessed on 12 February 2022).
- World Health Organization. Thailand Dengue and Severe Dengue. Available online: http://www.searo.who.int/thailand/factsheets/fs0008/en/ (accessed on 28 April 2018).
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 12 February 2022).
- Lab Tests Online. Dengue Fever Testing. Available online: https://labtestsonline.org/tests/Dengue-fever-testing (accessed on 26 April 2018).
- Hashi, E.K.; Zaman, M.S.U.; Hasan, M.R. An expert clinical decision support system to predict disease using classification techniques. In Proceedings of the International Conference on Electrical, Computer and Communication Engineering (ECCE), IEEE, Cox’s Bazar, Bangladesh, 16–18 February 2017; pp. 396–400. [Google Scholar]
- Tufail, A.B.; Anwar, N.; Othman, M.T.B.; Ullah, I.; Khan, R.A.; Ma, Y.-K.; Adhikari, D.; Rehman, A.U.; Shafiq, M.; Hamam, H. Early-Stage Alzheimer’s Disease Categorization Using PET Neuroimaging Modality and Convolutional Neural Networks in the 2D and 3D Domains. Sensors 2022, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Zainee, N.B.M.; Chellappan, K. A preliminary dengue fever prediction model based on vital signs and blood profile. In Proceedings of the IECBES 2016—IEEE-EMBS Conference on Biomedical Engineering and Sciences, Kuala Lumpur, Malaysia, 4–8 December 2016; pp. 652–656. [Google Scholar] [CrossRef]
- Anggraeni, W.; Abdillah, A.; Pujiadi; Trikoratno, L.T.; Wibowo, R.P.; Purnomo, M.H.; Sudiarti, Y. Modelling and Forecasting the Dengue Hemorrhagic Fever Cases Number Using Hybrid Fuzzy-ARIMA. In Proceedings of the 2019 IEEE 7th International Conference on Serious Games and Applications for Health, SeGAH 2019, Kyoto, Japan, 5–7 August 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Gambhir, S.; Malik, S.K.; Kumar, Y. PSO-ANN based diagnostic model for the early detection of dengue disease. New Horiz. Transl. Med. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Gambhir, S.; Malik, S.K.; Kumar, Y. The Diagnosis of Dengue Disease: An Evaluation of Three Machine Learning Approaches. Int. J. Healthc. Inf. Syst. Inform. 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Hair, G.M.E.; Nobre, F.F.; Brasil, P. Characterization of clinical patterns of dengue patients using an unsupervised machine learning approach. BMC Infect. Dis. 2019, 19, 649. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.D.P.; Narayan, K.A. Forecasting Dengue Fever Incidence Using ARIMA Analysis. Int. J. Collab. Res. Intern. Med. Public Health 2019, 11, 924–932. Available online: https://www.iomcworld.org/abstract/forecasting-dengue-fever-incidence-using-arima-analysis-44475.html (accessed on 12 February 2022).
- Pandiyarajan, P.; Thangairulappan, K. Classification of dengue serotypes using protein sequence based on rule extraction from neural network. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2018; Volume 11308, pp. 127–137. [Google Scholar] [CrossRef]
- Potts, J.A.; Gibbons, R.V.; Rothman, A.L.; Srikiatkhachorn, A.; Thomas, S.J.; Supradish, P.-O.; Lemon, S.C.; Libraty, D.H.; Green, S.; Kalayanarooj, S. Prediction of dengue disease severity among pediatric Thai patients using early clinical laboratory indicators. PLoS Negl. Trop. Dis. 2010, 4, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Saikia, D.; Dutta, J.C. Early diagnosis of dengue disease using fuzzy inference system. In Proceedings of the International Conference on Microelectronics, Computing and Communication, MicroCom 2016, Durgapur, India, 23–25 January 2016. [Google Scholar] [CrossRef]
- Sreenivasan, P.; Geetha, S.; Sasikala, K. Development of a Prognostic Prediction Model to Determine Severe Dengue in Children. Indian J. Pediatr. 2018, 85, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, C.; Rautray, S.S.; Pandey, M. Prevention of infectious disease based on big data analytics and map-reduce. In Proceedings of the Second International Conference on Electrical, Computer and Communication Technologies (ICECCT), IEEE, Coimbatore, India, 22–24 February 2017; pp. 1–4. [Google Scholar]
- Ooi, J.Y.L.; Thomas, J.J. DengueViz: A Knowledge-Based Expert System Integrated with Parallel Coordinates Visualization in the Dengue Diagnosis. In Advances in Visual Informatics, Proceedings of the International Visual Informatics Conference, Bangi, Malaysia, 28–30 November 2017; Springer: Cham, Switzerland, 2017; pp. 50–61. [Google Scholar]
- Shahwar, T.; Zafar, J.; Almogren, A.; Zafar, H.; Rehman, A.U.; Shafiq, M.; Hamam, H. Automated Detection of Alzheimer’s via Hybrid Classical Quantum Neural Networks. Electronics 2022, 11, 721. [Google Scholar] [CrossRef]
- Mondal, M.R.H.; Bharati, S.; Podder, P.; Podder, P. Data analytics for novel coronavirus disease. Inform. Med. Unlocked 2020, 20, 100374. [Google Scholar] [CrossRef]
- Srivastava, R.; Bhardwaj, V.P.; Othman, M.T.B.; Pushkarna, M.; Anushree; Mangla, A.; Bajaj, M.; Rehman, A.U.; Shafiq, M.; Hamam, H. Match-Level Fusion of Finger-Knuckle Print and Iris for Human Identity Validation Using Neuro-Fuzzy Classifier. Sensors 2022, 22, 3620. [Google Scholar] [CrossRef]
- Alto, B.W.; Bettinardi, D. Temperature and dengue virus infection in mosquitoes: Independent effects on the immature and adult stages. Am. J. Trop. Med. Hyg. 2013, 88, 497–505. [Google Scholar] [CrossRef]
- Jayaraj, V.J.; Avoi, R.; Gopalakrishnan, N.; Raja, D.B.; Umasa, Y. Developing a dengue prediction model based on climate in Tawau, Malaysia. Acta Trop. 2019, 197, 105055. [Google Scholar] [CrossRef]
- Tian, H.; Sun, Z.; Faria, N.R.; Yang, J.; Cazelles, B.; Huang, S.; Xu, B.; Yang, Q.; Pybus, O.G.; Xu, B. Increasing airline travel may facilitate co-circulation of multiple dengue virus serotypes in Asia. PLoS Negl. Trop. Dis. 2017, 11, e0005694. [Google Scholar] [CrossRef] [Green Version]
- Shafique, M.; Lopes, S.; Doum, D.; Keo, V.; Sokha, L.; Sam, B.; Vibol, C.; Alexander, N.; Bradley, J.; Liverani, M.; et al. Implementation of guppy fish (Poecilia reticulata), and a novel larvicide (Pyriproxyfen) product (Sumilarv 2MR) for dengue control in Cambodia: A qualitative study of acceptability, sustainability and community engagement. PLoS Negl. Trop. Dis. 2019, 13, e0007907. [Google Scholar] [CrossRef]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-insect methods for control of mosquito-borne diseases: An analysis. Vector-Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef]
- Buczak, A.L.; Baugher, B.; Babin, S.M.; Ramac-Thomas, L.C.; Guven, E.; Elbert, Y.; Koshute, P.T.; Velasco, J.M.S.; Roque, V.G., Jr.; Tayag, E.A.; et al. Prediction of high incidence of Dengue in the Philippines. PLoS Negl. Trop. Dis. 2014, 8, e2771. [Google Scholar] [CrossRef] [Green Version]
- Phung, D.; Huang, C.; Rutherford, S.; Chu, C.; Wang, X.; Nguyen, M.; Nguyen, N.H.; Manh, C.D. Identification of the prediction model for dengue incidence in Can Tho city, a Mekong Delta area in Vietnam. Acta Trop. 2015, 141, 88–96. [Google Scholar] [CrossRef]
- Othman, M.K.; Danuri, M.S.N.M. Proposed conceptual framework of Dengue Active Surveillance System (DASS) in Malaysia. In Proceedings of the International Conference on Information and Communication Technology (ICICTM), IEEE, Kuala Lumpur, Malaysia, 16–17 May 2016; pp. 90–96. [Google Scholar]
- Kerdprasop, N.; Kerdprasop, K. Remote sensing based modeling of Dengue outbreak with regression and binning classification. In Proceedings of the 2016 2nd IEEE International Conference on Computer and Communications (ICCC), Chengdu, China, 14–17 October 2016; pp. 46–49. [Google Scholar]
- Idris, M.F.I.M.; Abdullah, A.; Fauzi, S.S.M. Prediction of Dengue Outbreak in Selangor Using Fuzzy Logic. In Proceedings of the Second International Conference on the Future of ASEAN (ICoFA) 2017, Perlis, Malaysia, 16–17 August 2017; Springer: Singapore, 2018; Volume 2, pp. 593–603. [Google Scholar]
- Pravin, A.; Jacob, T.P.; Nagarajan, G. An intelligent and secure healthcare framework for the prediction and prevention of Dengue virus outbreak using fog computing. Health Technol. 2019, 10, 303–311. [Google Scholar] [CrossRef]
- Bharati, S.; Podder, P.; Mondal, M.; Prasath, V.B. CO-ResNet: Optimized ResNet model for COVID-19 diagnosis from X-ray images. Int. J. Hybrid Intell. Syst. 2021, 17, 71–85. [Google Scholar] [CrossRef]
- Chatterjee, P.; Cymberknop, L.J.; Armentano, R.L. IoT-based decision support system for intelligent healthcare—Applied to cardiovascular diseases. In Proceedings of the 2017 7th International Conference on Communication Systems and Network Technologies (CSNT), IEEE, Nagpur, India, 11–13 November 2017; pp. 362–366. [Google Scholar]
- Kumar, P.M.; Lokesh, S.; Varatharajan, R.; Babu, G.C.; Parthasarathy, P. Cloud and IoT based disease prediction and diagnosis system for healthcare using Fuzzy neural classifier. Future Gener. Comput. Syst. 2018, 86, 527–534. [Google Scholar] [CrossRef]
- Muthu, B.; Sivaparthipan, C.B.; Manogaran, G.; Sundarasekar, R.; Kadry, S.; Shanthini, A.; Dasel, A. IOT based wearable sensor for diseases prediction and symptom analysis in healthcare sector. Peer-Peer Netw. Appl. 2020, 13, 2123–2134. [Google Scholar] [CrossRef]
- Onasanya, A.; Elshakankiri, M. Smart integrated IoT healthcare system for cancer care. Wirel. Netw. 2019, 27, 4297–4312. [Google Scholar] [CrossRef]
- Gope, P.; Gheraibia, Y.; Kabir, S.; Sikdar, B. A secure IoT-based modern healthcare system with fault-tolerant decision making process. IEEE J. Biomed. Health Inform. 2020, 25, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Saha, S. Combined committee machine for classifying Dengue fever. In Proceedings of the 2016 International Conference on Microelectronics, Computing and Communications (MicroCom), IEEE, Durgapur, India, 23–25 January 2016; pp. 1–6. [Google Scholar]
- Jiji, G.W.; Lakshmi, V.S.; Lakshmi, K.V.; Priya, S.S. Diagnosis and Prognosis of the Arbovirus-Dengue using Intelligent Algorithm. J. Inst. Eng. Ser. B 2016, 97, 115–120. [Google Scholar] [CrossRef]
- Fuad, M.A.M.; Ab Ghani, M.R.; Ghazali, R.; Izzuddin, T.A.; Sulaima, M.F.; Jano, Z.; Sutikno, T. Detection of Aedes aegypti larvae using single shot multibox detector with transfer learning. Bull. Electr. Eng. Inform. 2019, 8, 514–518. [Google Scholar] [CrossRef]
- Babu, A.N.; Niehaus, E.; Shah, S.; Unnithan, C.; Ramkumar, P.S.; Shah, J.; Binoy, V.V.; Soman, B.; Arunan, M.C.; Jose, C.P. Smartphone geospatial apps for dengue control, prevention, prediction, and education: MOSapp, DISapp, and the mosquito perception index (MPI). Environ. Monit. Assess. 2019, 191, 393. [Google Scholar] [CrossRef]
- Abeyrathna, M.P.A.R.; Abeygunawrdane, D.A.; Wijesundara, R.A.A.V.; Mudalige, V.B.; Bandara, M.; Perera, S.; Maldeniya, D.; Madhawa, K.; Locknathan, S. Dengue propagation prediction using human mobility. In Proceedings of the Moratuwa Engineering Research Conference (MERCon), IEEE, Moratuwa, Sri Lanka, 5–6 April 2016; pp. 156–161. [Google Scholar]
- Bal, S.; Sodoudi, S. Modeling and prediction of dengue occurrences in Kolkata, India, based on climate factors. Int. J. Biometeorol. 2020, 64, 1379–1391. [Google Scholar] [CrossRef]
- Yang, X.; Tong, Y.; Xiangfeng, M.; Shuai, Z.; Zhi, X.; Yanjun, L.; Guozhen, L.; Shaohua, T. Online adaptive method for disease prediction based on big data of clinical laboratory test. In Proceedings of the 2016 7th IEEE International Conference on Software Engineering and Service Science (ICSESS), IEEE, Beijing, China, 26–28 August 2016; pp. 889–892. [Google Scholar]
- Zhu, G.; Hunter, J.; Jiang, Y. Improved Prediction of Dengue Outbreak Using the Delay Permutation Entropy. In Proceedings of the 2016 IEEE International Conference on Internet of Things (iThings) and IEEE Green Computing and Communications (GreenCom) and IEEE Cyber, Physical and Social Computing (CPSCom) and IEEE Smart Data (SmartData), IEEE, Chengdu, China, 15–18 December 2016; pp. 828–832. [Google Scholar]
- Rehman, A.U.; Naqvi, R.A.; Rehman, A.; Paul, A.; Sadiq, M.T.; Hussain, D. A Trustworthy SIoT Aware Mechanism as an Enabler for Citizen Services in Smart Cities. Electronics 2020, 9, 918. [Google Scholar] [CrossRef]
- Sigera, P.C.; Amarasekara, R.; Rodrigo, C.; Rajapakse, S.; Weeratunga, P.; De Silva, N.L.; Huang, C.H.; Sahoo, M.K.; Pinsky, B.A.; Pillai, D.R.; et al. Risk prediction for severe disease and better diagnostic accuracy in early dengue infection; the Colombo dengue study. BMC Infect. Dis. 2019, 19, 680. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Islam, M. Machine learning for dengue outbreak prediction: A performance evaluation of different prominent classifiers. Informatica 2019, 43, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Chovatiya, M.; Dhameliya, A.; Deokar, J.; Gonsalves, J.; Mathur, A. Prediction of dengue using recurrent neural network. In Proceedings of the 2019 3rd International Conference on Trends in Electronics and Informatics (ICOEI), IEEE, Tirunelveli, India, 23–25 April 2019; pp. 926–929. [Google Scholar]
- Ahmad, H.; Ali, A.; Fatima, S.H.; Zaidi, F.; Khisroon, M.; Rasheed, S.B.; Ullah, I.; Ullah, S.; Shakir, M. Spatial modeling of dengue prevalence and kriging prediction of dengue outbreak in Khyber Pakhtunkhwa (Pakistan) using presence only data. Stoch. Environ. Res. Risk Assess. 2020, 34, 1023–1036. [Google Scholar] [CrossRef]
- Sarma, D.; Hossain, S.; Mittra, T.; Bhuiya, M.A.M.; Saha, I.; Chakma, R. Dengue Prediction using Machine Learning Algorithms. In Proceedings of the 2020 IEEE 8th R10 Humanitarian Technology Conference (R10-HTC), Kuching, Malaysia, 1–3 December 2020; pp. 1–6. [Google Scholar]
- Mishra, V.K.; Tiwari, N.; Ajaymon, S.L. Dengue disease spread prediction using twofold linear regression. In Proceedings of the 2019 IEEE 9th International Conference on Advanced Computing (IACC), IEEE, Tiruchirappalli, India, 13–14 December 2019; pp. 182–187. [Google Scholar]
- Chakraborty, T.; Chattopadhyay, S.; Ghosh, I. Forecasting dengue epidemics using a hybrid methodology. Phys. A Stat. Mech. Its Appl. 2019, 527, 121266. [Google Scholar] [CrossRef]
- Herath, H.M.M.T.B.; Udeshika, W.A.E.; Samarawickrama, S.S.M.; Yogendranathan, N.; Jayamali, W.D.; Kulatunga, A.; Rodrigo, C. Prediction of plasma leakage phase of dengue in resource limited settings. Clin. Epidemiol. Glob. Health 2019, 7, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Sangkaew, S.; Ming, D.; Boonyasiri, A.; Honeyford, K.; Kalayanarooj, S.; Yacoub, S.; Dorigatti, I.; Holmes, A.H. Enhancing risk prediction of progression to severe disease during the febrile phase of dengue: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 101, 237–238. [Google Scholar] [CrossRef]
- Kannimuthu, S.; Bhuvaneshwari, K.S.; Bhanu, D.; Vaishnavi, A.; Ahalya, S. Performance Evaluation of Machine Learning Algorithms for Dengue Disease Prediction. J. Comput. Theor. Nanosci. 2019, 16, 5105–5110. [Google Scholar] [CrossRef]
- Abdali-Mohammadi, F.; Meqdad, M.N.; Kadry, S. Development of an IoT-based and cloud-based disease prediction and diagnosis system for healthcare using machine learning algorithms. IAES Int. J. Artif. Intell. 2020, 9, 766. [Google Scholar] [CrossRef]
- Akkaş, M.A.; Sokullu, R.; Ertürk Çetin, H. Healthcare and patient monitoring using IoT. Internet Things 2020, 11, 100173. [Google Scholar] [CrossRef]
- Bharany, S.; Sharma, S.; Badotra, S.; Khalaf, O.I.; Alotaibi, Y.; Alghamdi, S.; Alassery, F. Energy-Efficient Clustering Scheme for Flying Ad-Hoc Networks Using an Optimized LEACH Protocol. Energies 2021, 14, 6016. [Google Scholar] [CrossRef]
- Li, Z.; Gurgel, H.; Xu, L.; Yang, L.; Dong, J. Improving Dengue Forecasts by Using Geospatial Big Data Analysis in Google Earth Engine and the Historical Dengue Information-Aided Long Short Term Memory Modeling. Biology 2022, 11, 169. [Google Scholar] [CrossRef]
- Bharany, S.; Sharma, S.; Frnda, J.; Shuaib, M.; Khalid, M.I.; Hussain, S.; Iqbal, J.; Ullah, S.S. Wildfire Monitoring Based on Energy Efficient Clustering Approach for FANETS. Drones 2022, 6, 193. [Google Scholar] [CrossRef]
- Laureano-Rosario, A.E.; Duncan, A.P.; Mendez-Lazaro, P.A.; Garcia-Rejon, J.E.; Gomez-Carro, S.; Farfan-Ale, J.; Savic, D.A.; Muller-Karger, F.E. Application of Artificial Neural Networks for Dengue Fever Outbreak Predictions in the Northwest Coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop. Med. Infect. Dis. 2018, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Bharany, S.; Sharma, S.; Bhatia, S.; Rahmani, M.K.I.; Shuaib, M.; Lashari, S.A. Energy Efficient Clustering Protocol for FANETS Using Moth Flame Optimization. Sustainability 2022, 14, 6159. [Google Scholar] [CrossRef]
- Mazhar, M.S.; Saleem, Y.; Almogren, A.; Arshad, J.; Jaffery, M.H.; Rehman, A.U.; Shafiq, M.; Hamam, H. Forensic Analysis on Internet of Things (IoT) Device Using Machine-to-Machine (M2M) Framework. Electronics 2022, 11, 1126. [Google Scholar] [CrossRef]
- Bharany, S.; Sharma, S.; Khalaf, O.I.; Abdulsahib, G.M.; Al Humaimeedy, A.S.; Aldhyani, T.H.H.; Maashi, M.; Alkahtani, H. A Systematic Survey on Energy-Efficient Techniques in Sustainable Cloud Computing. Sustainability 2022, 14, 6256. [Google Scholar] [CrossRef]
- Xu, J.; Xu, K.; Li, Z.; Meng, F.; Tu, T.; Xu, L.; Liu, Q. Forecast of Dengue Cases in 20 Chinese Cities Based on the Deep Learning Method. Int. J. Environ. Res. Public Health 2020, 17, 453. [Google Scholar] [CrossRef] [Green Version]
- Bharany, S.; Kaur, K.; Badotra, S.; Rani, S.; Kavita; Wozniak, M.; Shafi, J.; Ijaz, M.F. Efficient Middleware for the Portability of PaaS Services Consuming Applications among Heterogeneous Clouds. Sensors 2022, 22, 5013. [Google Scholar] [CrossRef]









| Step No. | Suggestions |
|---|---|
| Step 1 | Each user registers with the system by using a mobile phone or website. Identification health ID is automatically generated by the system and allotted to each user after registration (each health ID generated can further include five distinct health records, which may be family or friends). |
| Step 2 | During registration, the user needs to share information about their name, age, sex, mobile number, address, UID, and state, before proceeding to the next stage. |
| Step 3 | A form appears in which all possible dengue disease symptoms are mentioned and the user is urged to select symptoms applicable to their present condition, along with the duration they have experienced those symptoms (in days).
|
| Step 4 | The random forest algorithm is used to classify the user’s category into infected, not infected, or infected with warning signs. |
| Step 5 | Results are shown and based on the symptomatic period experienced. Dengue confirmation test is also advised for the infected user as follows:
|
| Step 6 | If the result shows dengue infection with warning signs, then based on symptom mining, an alert message is sent to advise additional clinical tests which may include:
|
| Step 7 | The infected user comes back and updates records to input all the test’s parametric values after undergoing tests recommended by the system, and this goes again into a random forest algorithm to generate a classification of dengue infection level. |
| Step 8 | The system classifies the user’s category as DFNH (dengue fever with no hemorrhage), DHF (dengue hemorrhage fever), or DSS (dengue shock syndrome), and messages alerting the user to seek medical aid are are sent accordingly. |
| Step 9 | Users can update information in their records anytime, collected from wearable sensors, test reports, etc. |
| Step 10 | The classification metrics are calculated to examine the accuracy of the proposed system and at each diagnosis, the user can print the complete decision, set i.e., the combination set of symptoms observed or percentage certainty of acquiring the disease according to the analysis, which can be shown to a doctor to further aid in receiving proper medical help. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, S.; Sharma, S.; Rehman, A.U.; Eldin, E.T.; Ghamry, N.A.; Shafiq, M.; Bharany, S. Predicting Infection Positivity, Risk Estimation, and Disease Prognosis in Dengue Infected Patients by ML Expert System. Sustainability 2022, 14, 13490. https://doi.org/10.3390/su142013490
Kaur S, Sharma S, Rehman AU, Eldin ET, Ghamry NA, Shafiq M, Bharany S. Predicting Infection Positivity, Risk Estimation, and Disease Prognosis in Dengue Infected Patients by ML Expert System. Sustainability. 2022; 14(20):13490. https://doi.org/10.3390/su142013490
Chicago/Turabian StyleKaur, Supreet, Sandeep Sharma, Ateeq Ur Rehman, Elsayed Tag Eldin, Nivin A. Ghamry, Muhammad Shafiq, and Salil Bharany. 2022. "Predicting Infection Positivity, Risk Estimation, and Disease Prognosis in Dengue Infected Patients by ML Expert System" Sustainability 14, no. 20: 13490. https://doi.org/10.3390/su142013490







