Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Areas and Banana Farms
2.2. Soil Sampling
2.3. Statistical Analysis
3. Results and Discussion
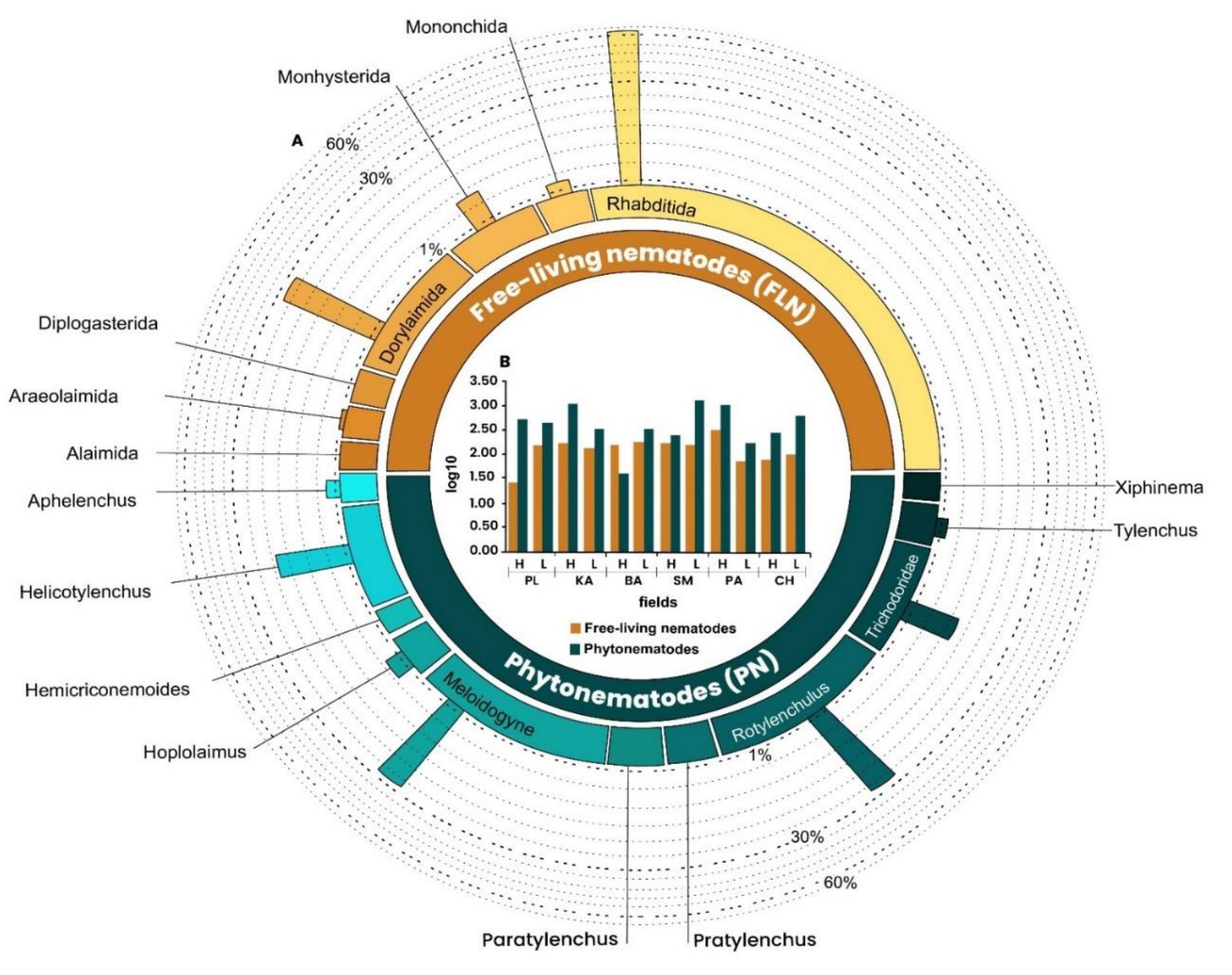
3.1. Proportion of Free-living Nematodes (FLN) and Phytonematodes (PN) in Banana Sites
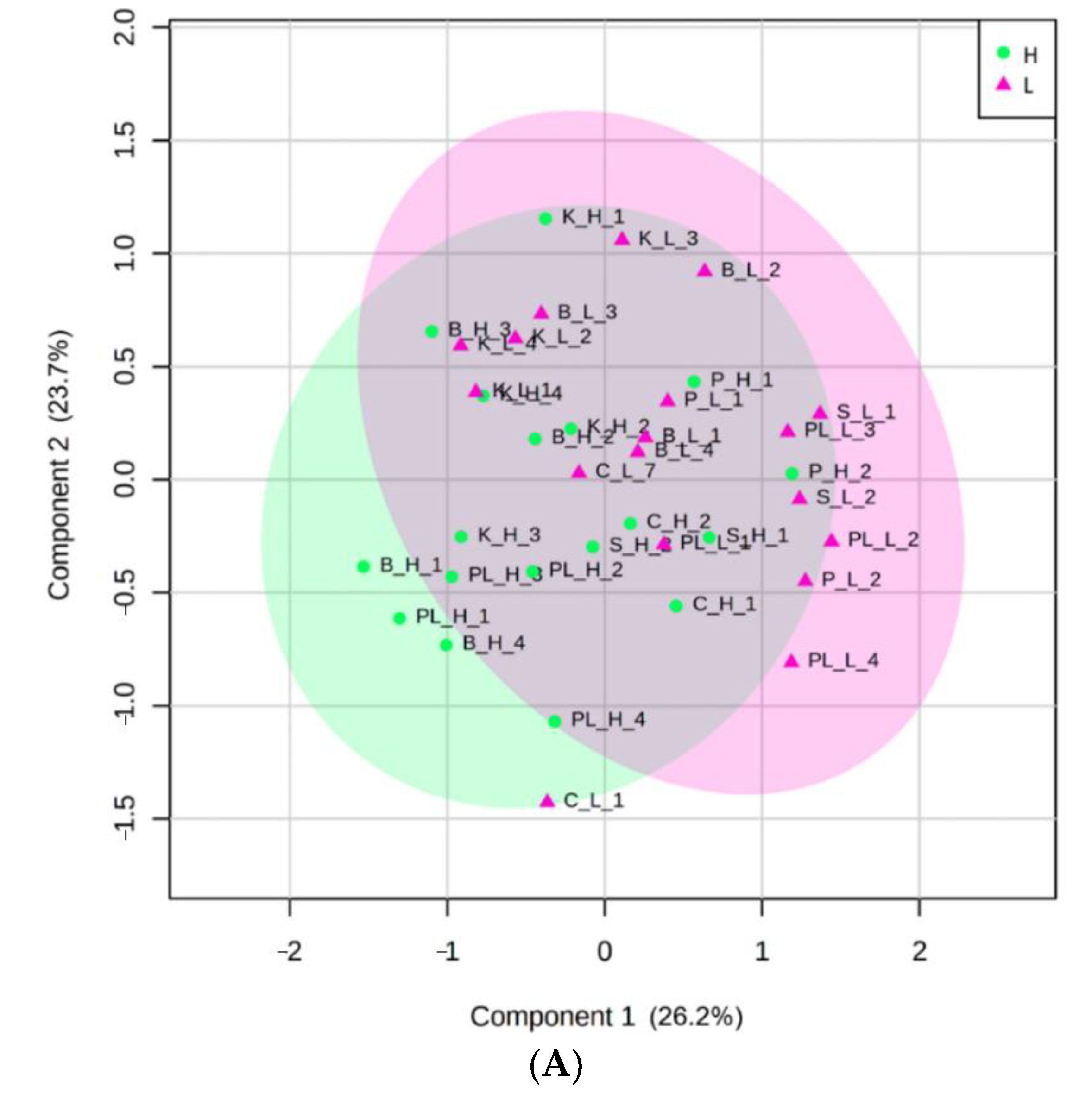
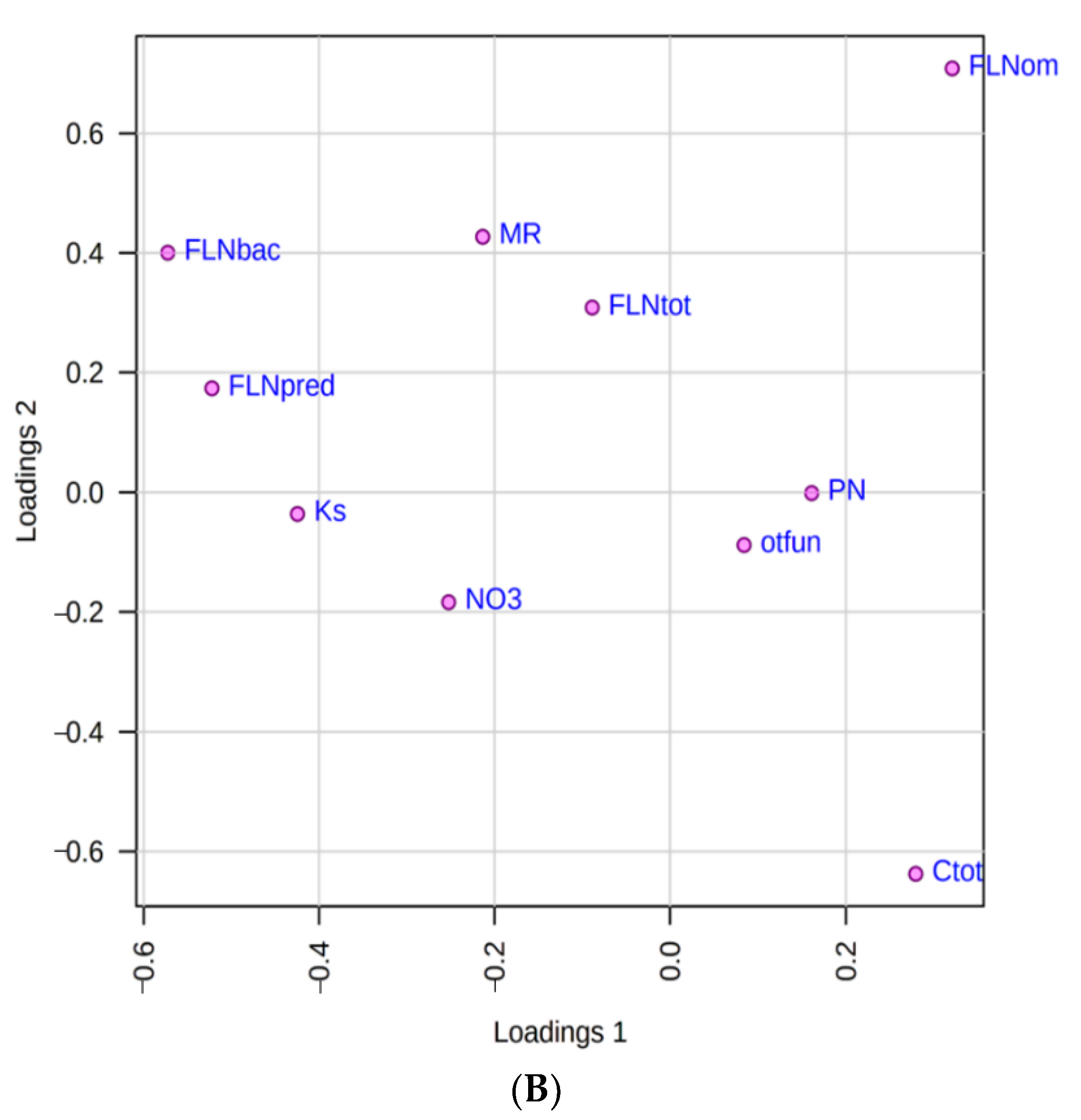
3.2. Identification of the Most Important Variables with PLS-DA
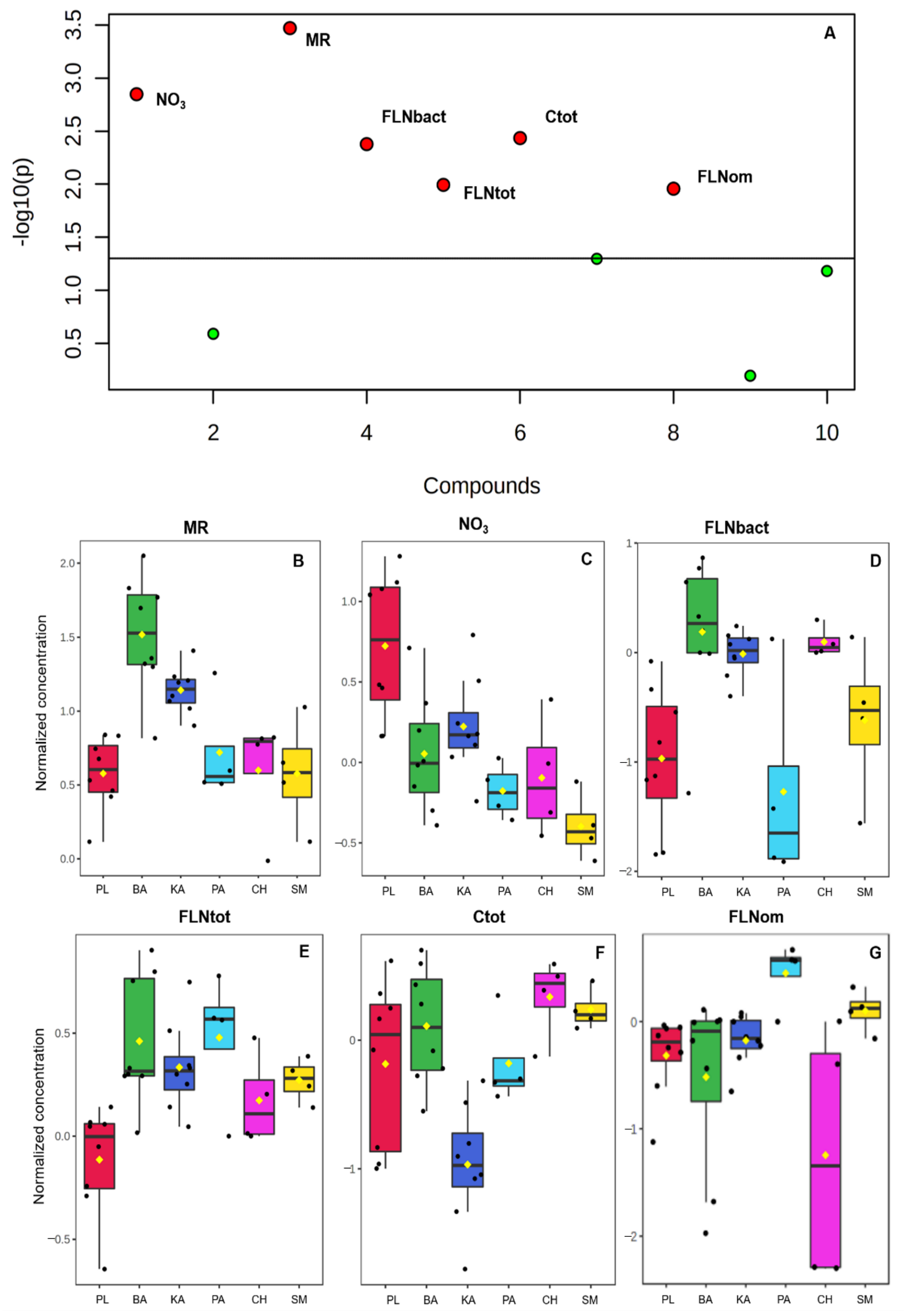
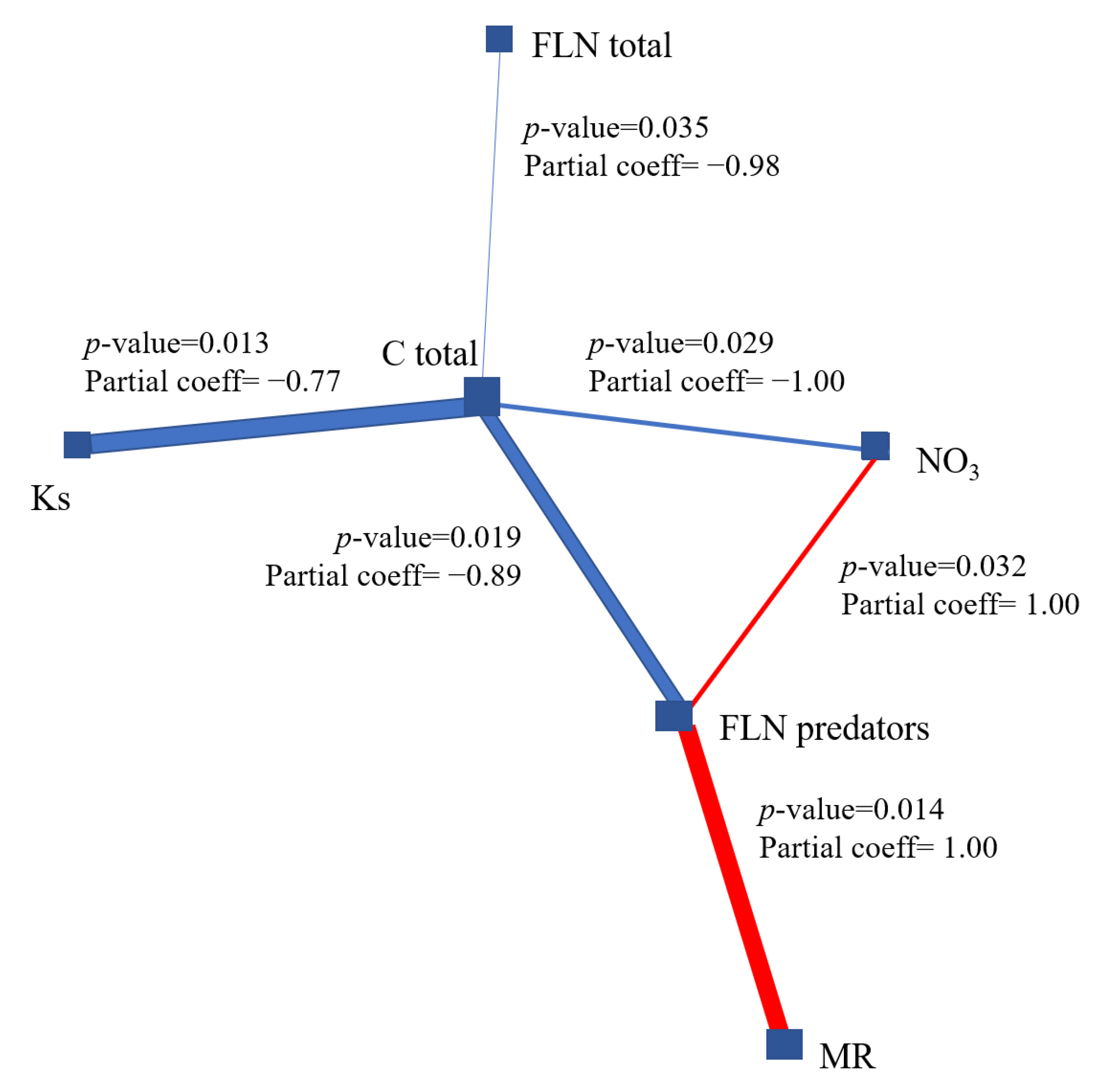
3.3. Debiased Sparse Partial Correlation Algorithm (DSPC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Farm Code | Level | n | Lbact (Log) | Lfung (Log) | Trichoderma CFU·g−1 | Fusarium CFU·g−1 | Other Fungi CFU·g−1 |
|---|---|---|---|---|---|---|---|
| PL | H | 4 | 6.06 ± 0.05 | 4.62 ± 0.05 | 2.50 ± 5.00 | 35.00 ± 31.09 | 30.01 ± 18.26 |
| L | 4 | 6.13 ± 0.09 | 4.65 ± 0.06 | 5.00 ± 5.77 | 52.50 ± 22.17 | 30.00 ± 14.14 | |
| BA | H | 4 | 6.15 ± 0.05 | 4.42 ± 0.15 | 0.00 ± 0.00 | 57.50 ± 30.96 | 0.00 ± 0.00 |
| L | 4 | 5.81 ± 0.09 | 4.42 ± 0.15 | 12.50 ± 15.00 | 57.50 ± 35.94 | 10.00 ± 14.14 | |
| KA | H | 4 | 6.17 ± 0.12 | 4.53 ± 0.15 | 0.00 ± 0.00 | 50.00 ± 14.14 | 20.00 ± 27.08 |
| L | 4 | 5.92 ± 0.16 | 4.57 ± 0.06 | 2.50 ± 5.00 | 15.00 ± 12.91 | 22.50 ± 17.08 | |
| SM | H | 2 | 6.02 ± 0.09 | 5.04 ± 0.62 | 0.00 ± 0.00 | 40.00 ± 56.57 | 20.00 ± 14.14 |
| L | 2 | 6.02 ± 0.09 | 4.84 ± 0.08 | 0.00 ± 0.00 | 40.00 ± 0.00 | 20.00 ± 14.14 | |
| PZ | H | 2 | 5.78 ± 0.11 | 5.04 ± 0.62 | 10.00 ± 14.14 | 90.00 ± 14.14 | 30.00 ± 14.14 |
| L | 2 | 5.88 ± 0.04 | 4.60 ± 0.00 | 0.00 ± 0.00 | 70.00 ± 42.43 | 80.00 ± 0.10 | |
| CH | H | 2 | 5.74 ± 0.06 | 5.04 ± 0.62 | 45.00 ± 21.21 | 45.00 ± 49.50 | 40.00 ± 0.00 |
| L | 2 | 5.74 ± 0.06 | 4.50 ± 0.28 | 5.00 ± 7.07 | 80.00 ± 28.28 | 20.00 ± 0.00 |
| Farm Code | Level | n | log10FLN | log10PN |
|---|---|---|---|---|
| PL | H | 4 | 1.41 ± 0.73 | 2.70 ± 1.86 |
| PL | L | 4 | 2.16 ± 1.40 | 2.64 ± 1.67 |
| KA | H | 4 | 2.21 ± 1.00 | 3.03 ± 2.30 |
| KA | L | 4 | 2.12 ± 1.30 | 2.52 ± 1.89 |
| BA | H | 4 | 2.19 ± 1.35 | 1.60 ± 1.09 |
| BA | L | 4 | 2.25 ± 1.58 | 2.54 ± 1.82 |
| SM | H | 2 | 2.20 ± 1.15 | 2.40 ± 2.03 |
| SM | L | 2 | 2.20 ± 1.93 | 3.10 ± 2.39 |
| PA | H | 2 | 2.48 ± 1.63 | 2.99 ± 1.93 |
| PA | L | 2 | 1.85 ± 0.85 | 2.23 ± 2.03 |
| CH | H | 2 | 1.90 ± 0.00 | 2.41 ± 2.2 |
| CH | L | 2 | 2.00 ± 1.15 | 2.77 ± 1.8 |
References
- Martínez-Solórzano, G.E.; Rey-Brina, J.C. Bananos (Musa AAA): Importance, production, and trade in COVID-19 times. Agron. Mesoam. 2021, 32, 1034–1046. [Google Scholar] [CrossRef]
- Pitti, J.; Olivares, B.O.; Montenegro, E.; Miller, L.; Ñango, Y. The role of agriculture in the Changuinola district: A case of applied economics in Panama. Trop. Subtrop. Agroecosystems 2021, 25, 017. [Google Scholar]
- Montenegro, E.J.; Pitti-Rodríguez, J.E.; Olivares-Campos, B.O. Identification of the main subsistence crops of Teribe: A case study based on multivariate techniques. Idesia 2021, 39, 83–94. [Google Scholar] [CrossRef]
- Olivares, B.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A review of agro-environmental factors in the Venezuelan production system affecting its development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. Available online: https://n9.cl/2axkm (accessed on 7 August 2021).
- Hernández, Y.; Marín, M.; García, J. Response to the plantain (Musa AAB cv. Horn) yield as a function of the mineral nutrients and its phenologycal cycle. Part I. Growth and Production. Rev. Fac. Agron. LUZ 2007, 24, 607–626. [Google Scholar]
- Delgado, E.; Trejos, J.; Villalobos, M.; Martinez, G.; Lobo, D.; Rey, J.C.; Rodriguez, G.; Rosales, F.E.; Pocasangre, L.E. Determination of a soil quality and health index for banana plantations in Venezuela. Interciencia 2010, 35, 927–933. [Google Scholar]
- González-Pedraza, A.F.; Atencio, J.; Cubillán, K.; Almendrales, R.; Ramírez, L.; Barrios, O. Microbial activity in soils cultivated with plantain (Musa AAB plantain subgroup cv. Harton) with different vigor of plants. Rev. Fac. Agron. LUZ 2014, 31, 526–538. [Google Scholar]
- Olivares, B.O.; Araya-Alman, M.; Acevedo-Opazo, C.; Rey, J.C.; Cañete-Salinas, P.; Kurina, F.G.; Balzarini, M.; Lobo, D.; Navas-Cortés, J.A.; Landa, B.B. Relationship Between Soil Properties and Banana Productivity in the Two Main Cultivation Areas in Venezuela. J. Soil Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
- Olivares, B.O.; Vega, A.; Calderón, M.A.R.; Rey, J.C.; Lobo, D.; Gómez, J.A.; Landa, B.B. Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants 2022, 11, 2070. [Google Scholar] [CrossRef]
- Olivares, B.O.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.B.; Gómez, J.A. Correlation of banana productivity levels and soil morphological properties using regularized optimal scaling regression. Catena 2022, 208, 105718. [Google Scholar] [CrossRef]
- González-García, H.; Pedraza, A.F.G.; Atencio, J.; Soto, A. Evaluación de calidad de suelos plataneros a través de la actividad microbiana en el sur del lago de Maracaibo, estado de Zulia, Venezuela. Rev. Fac. Agron. LUZ 2021, 38, 216–240. [Google Scholar] [CrossRef]
- García, H.G.; González, A.F.; Pineda, M.; Escalante, H.; Yzquierdo, G.A.R.; Bracho, A.S. Microbiota edáfica en lotes de plátano con vigor contrastante y su relación con propiedades del suelo. Bioagro 2021, 33, 143–148. [Google Scholar] [CrossRef]
- Ferris, H.; Pocasangre, L.E.; Serrano, E.; Muñoz, J.; Garcia, S.; Perichi, G.; Martinez, G. Diversity and complexity complement apparent competition: Nematode assemblages in banana plantations. Acta Oecologica 2012, 40, 11–18. [Google Scholar] [CrossRef]
- Sial, T.A.; Khan, M.N.; Lan, Z.; Kumbhar, F.; Ying, Z.; Zhang, J.; Sun, D.; Li, X. Contrasting effects of banana peels waste and its biochar on greenhouse gas emissions and soil biochemical properties. Process Saf. Environ. Prot. 2019, 122, 366–377. [Google Scholar] [CrossRef]
- Rondon, T.; Hernandez, R.M.; Guzman, M. Soil organic carbon, physical fractions of the macro-organic matter, and soil stability relationship in lacustrine soils under banana crop. PLoS ONE 2021, 16, e0254121. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67. [Google Scholar]
- Bautista, L.G.; Bolaños, M.M.; Massae Asakawa, N.; Villegas, B. Plantain Musa AAB simmonds phytonematodes response to soil integrated management strategies and nutrition. Luna Azul. 2015, 40, 69–84. [Google Scholar] [CrossRef]
- Sun, J.; Zou, L.; Li, W.; Wang, Y.; Xia, Q.; Peng, M. Soil microbial and chemical properties influenced by continuous cropping of banana. Sci. Agric. 2018, 75, 420–425. [Google Scholar] [CrossRef]
- Olivares, B.O.; Paredes, F.; Rey, J.C.; Lobo, D.; Galvis-Causil, S. The relationship between the normalized difference vegetation index, rainfall, and potential evapotranspiration in a banana plantation of Venezuela. ST-JSSA 2021, 18, 58–64. [Google Scholar] [CrossRef]
- Olivares, B.O. Tropical rainfall conditions in rainfed agriculture in Carabobo, Venezuela. GRANJA Rev. Cienc. Vida 2018, 27, 86–102. [Google Scholar] [CrossRef]
- Olivares, B.O.; Cortez, A.; Lobo, D.; Parra, R.; Rey, J.; Rodriguez, M. Evaluation of agricultural vulnerability to drought weather in different locations of Venezuela. Rev. Fac. Agron. LUZ 2017, 34, 103–129. [Google Scholar]
- Rosales, F.E.; Pocasangre, L.E.; Trejos, J.; Serrano, E.; Peña, W. Guía de Diagnóstico de la Calidad y Salud de Suelos Bananeros; Bioversity International: Roma, Italy, 2008; pp. 48–80. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [PubMed]
- Mevik, B.; Wehrens, R. R Package: ‘pls’: Partial Least Squares and Principal Component regression. J. Stat. Softw. 2015, 18, 1–23. [Google Scholar]
- Li, R.; Zeng, L.; Xie, S.; Chen, J.; Yu, Y.; Zhong, L. Targeted metabolomics study of serum bile acid profile in patients with end-stage renal disease undergoing hemodialysis. PeerJ 2019, 7, e7145. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Jankova, J. Confidence intervals for high-dimensional inverse covariance estimation. Electron. J. Stat. 2015, 9, 1205–1229. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. A review of soil nematodes as biological indicators for the assessment of soil health. Front. Agric. Sci. Eng. 2020, 7, 275–281. [Google Scholar] [CrossRef]
- Talavera, M.; Thoden, T.C.; Vela-Delgado, M.D.; Verdejo-Lucas, S.; Sánchez-Moreno, S. The impact of fluazaindolizine on free-living nematodes and the nematode community structure in a root-knot nematode infested vegetable production system. Pest Manag. Sci. 2021, 77, 5220–5227. [Google Scholar] [CrossRef]
- Castilla-Díaz, E.; Millán-Romero, E.; Mercado-Ordoñez, J.; Millán-Páramo, C. Relation of soil parameters on the diversity and spatial distribution of free-living nematodes. Tecnol. Marcha 2017, 30, 24–34. [Google Scholar] [CrossRef]
- Landi, S.; D’errico, G.I.A.D.A.; Papini, R.; Gargani, E.; Simoncini, S.; Amoriello, T.; Ciccoritti, R.; Carbone, K. Communities of plant parasitic and free-living nematodes in Italian hop crops. Redia 2019, 102, 141–148. [Google Scholar] [CrossRef]
- Arie-Vonk, J.; Breure, A.M.; Mulder, C. Environmentally-driven dissimilarity of trait-based indices of nematodes under different agricultural management and soil types. Agric. Ecosyst. Environ. 2013, 179, 133–138. [Google Scholar] [CrossRef]
- Holterman, M.H.; Korthals, G.W.; Doroszuk, A.; van Megen, H.H.; Bakker, J.; Bongers, T.; Helder, J.; van der Wurff, A. A strategy in searching for stress tolerance-correlated characteristics in nematodes while accounting for phylogenetic interdependence. Nematology 2011, 13, 261–275. [Google Scholar] [CrossRef]
- Crozzoli, R. Nematodes of tropical fruit crops in Venezuela. In Integrated Management of Fruit Crops and Forest Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 63–83. [Google Scholar] [CrossRef]
- Crozzoli, R. La Nematología Agrícola en Venezuela; Ediciones Facultad de Agronomía; UCV: Maracay, Venezuela, 2014; p. 500. [Google Scholar]
- Davide, R.G. Influence of cultivar, age, soil texture, and pH on Meloidogyne incognita and Radopholus similis on banana. Plant Dis. 1980, 64, 571–573. [Google Scholar] [CrossRef][Green Version]
- Bwamiki, D.P.; Duxbury, J.M.; Esnard, J. Effect of banana nutritional status on host tolerant to the burrowing nematode Radopholus similis. In 42nd Annual Meeting of Society of Nematologists; Cornell University, Ithaca: New York, NY, USA, 2003; pp. 328–329. [Google Scholar]
- Cássia Ferreira, R.R.; Aparecida, A.; Mizobutsi, E.H.; Pereira, F.R.; Ribeiro, H.B.; Alexandre, P.A.A.; Ferraz, S. Influencia de fatores edáficos sobre a população de Meloidogyne javanica, Helicotylenchus multicinctus e Radopholus similis em bananeira. Proceedings of XVII ACORBAT Meeting, Joinville, Santa Catarina, Brazil; ACORBAT. Soprano, E., Tcacenco, F.A., Lichtemberg, L.A., Silva, M.C., Eds.; 2006; pp. 813–817. [Google Scholar]
- Yang, B.; Zhang, C.; Cheng, S.; Li, G.; Griebel, J.; Neuhaus, J. Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine. Diagnostics 2021, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.S.; Di Bernardo, D.; Lorenz, D.; Collins, J.J. Inferring genetic networks and identifying compound mode of action via expression profiling. Science 2003, 301, 102–105. [Google Scholar] [CrossRef]
- Jeong, H.; Mason, S.P.; Barabási, A.L.; Oltvai, Z.N. Lethality and centrality in protein networks. Nature 2001, 411, 41–42. [Google Scholar] [CrossRef]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef]
- Culman, S.W.; Young-Mathews, A.; Hollander, A.D.; Ferris, H.; Sánchez-Moreno, S.; O’Geen, A.T.; Jackson, L.E. Biodiversity is associated with indicators of soil ecosystem functions over a landscape gradient of agricultural intensification. Landsc. Ecol. 2010, 25, 1333–1348. [Google Scholar] [CrossRef]
- Chávez-Velazco, C.; Araya-Vargas, M. Correlation between soil characteristics and banana root nematodes (Musa AAA) in Ecuador. Agron. Mesoam. 2009, 20, 361–369. [Google Scholar] [CrossRef][Green Version]
- Millán, E.; Castilla, D.; Millán, C. Comunidades de nematodos de vida libre del suelo y su correspondencia con la calidad. Ing. Reg. 2016, 16, 25–34. [Google Scholar] [CrossRef]
- Jaurixje, M.; Torres, D.; Mendoza, B.; Henríquez, M.; Contreras, J. Physical, and chemical soil properties and their relationship with biological activity under different soil managements in Quibor, Lara State, Venezuela. Bioagro 2013, 25, 47–56. [Google Scholar]

| Farm Code | Latitude | Longitude | Height (masl) | State | Planted Area (ha) | Average Yield (t ha−1 year−1) |
|---|---|---|---|---|---|---|
| PL | 10°12′20″ N | 67°30′10″ W | 435 | Aragua | 135 | 74.9 |
| BA | 09°29′14″ N | 70°57′05″ W | 16 | Trujillo | 300 | 69.6 |
| KA | 09°28′31″ N | 70°55′46″ W | 17 | Trujillo | 270 | 64.9 |
| SM | 10°12′55″ N | 67°23′42″ W | 502 | Aragua | 11 | 11.1 |
| PZ | 10°11′30″ N | 67°31′04″ W | 514 | Aragua | 20 | 11.4 |
| CH | 10°11′34″ N | 67°31′34″ W | 498 | Aragua | 9 | 12.3 |
| Variable | VIP 1 | VIP 2 | VIP Average | Coefficient Score |
|---|---|---|---|---|
| FLN predators | 1.72 | 1.55 | 1.64 | 100.00 |
| FLN bacterivore | 1.48 | 1.40 | 1.44 | 77.58 |
| Ks | 1.41 | 1.27 | 1.34 | 76.43 |
| FLN omnivores | 1.29 | 1.22 | 1.26 | 75.68 |
| NO3 | 0.94 | 0.86 | 0.90 | 47.20 |
| Phytonematodes | 0.31 | 0.37 | 0.34 | 22.31 |
| FLN total | 0.27 | 0.25 | 0.26 | 15.16 |
| Other fungi | 0.20 | 0.20 | 0.20 | 14.95 |
| Total organic carbon (Ctot) | 0.16 | 0.94 | 0.55 | 11.91 |
| Microbial respiration (MR) | 0.08 | 0.76 | 0.42 | 0.00 |
| Variable | Chi-Squared | p-Value | −log10(p) | FDR |
|---|---|---|---|---|
| Microbial respiration (MR) | 22.995 | 0.001 | 34.708 | 0.003 |
| NO3 | 19.706 | 0.001 | 28.481 | 0.007 |
| FLN bacteriophage | 17.479 | 0.003 | 24.347 | 0.010 |
| Total organic carbon (Ctot) | 17.169 | 0.004 | 23.778 | 0.010 |
| FLN total | 15.051 | 0.010 | 19.938 | 0.018 |
| FLN omnivores | 14.85 | 0.011 | 19.577 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares, B.O.; Rey, J.C.; Perichi, G.; Lobo, D. Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela. Sustainability 2022, 14, 13531. https://doi.org/10.3390/su142013531
Olivares BO, Rey JC, Perichi G, Lobo D. Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela. Sustainability. 2022; 14(20):13531. https://doi.org/10.3390/su142013531
Chicago/Turabian StyleOlivares, Barlin O., Juan C. Rey, Guillermo Perichi, and Deyanira Lobo. 2022. "Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela" Sustainability 14, no. 20: 13531. https://doi.org/10.3390/su142013531
APA StyleOlivares, B. O., Rey, J. C., Perichi, G., & Lobo, D. (2022). Relationship of Microbial Activity with Soil Properties in Banana Plantations in Venezuela. Sustainability, 14(20), 13531. https://doi.org/10.3390/su142013531








