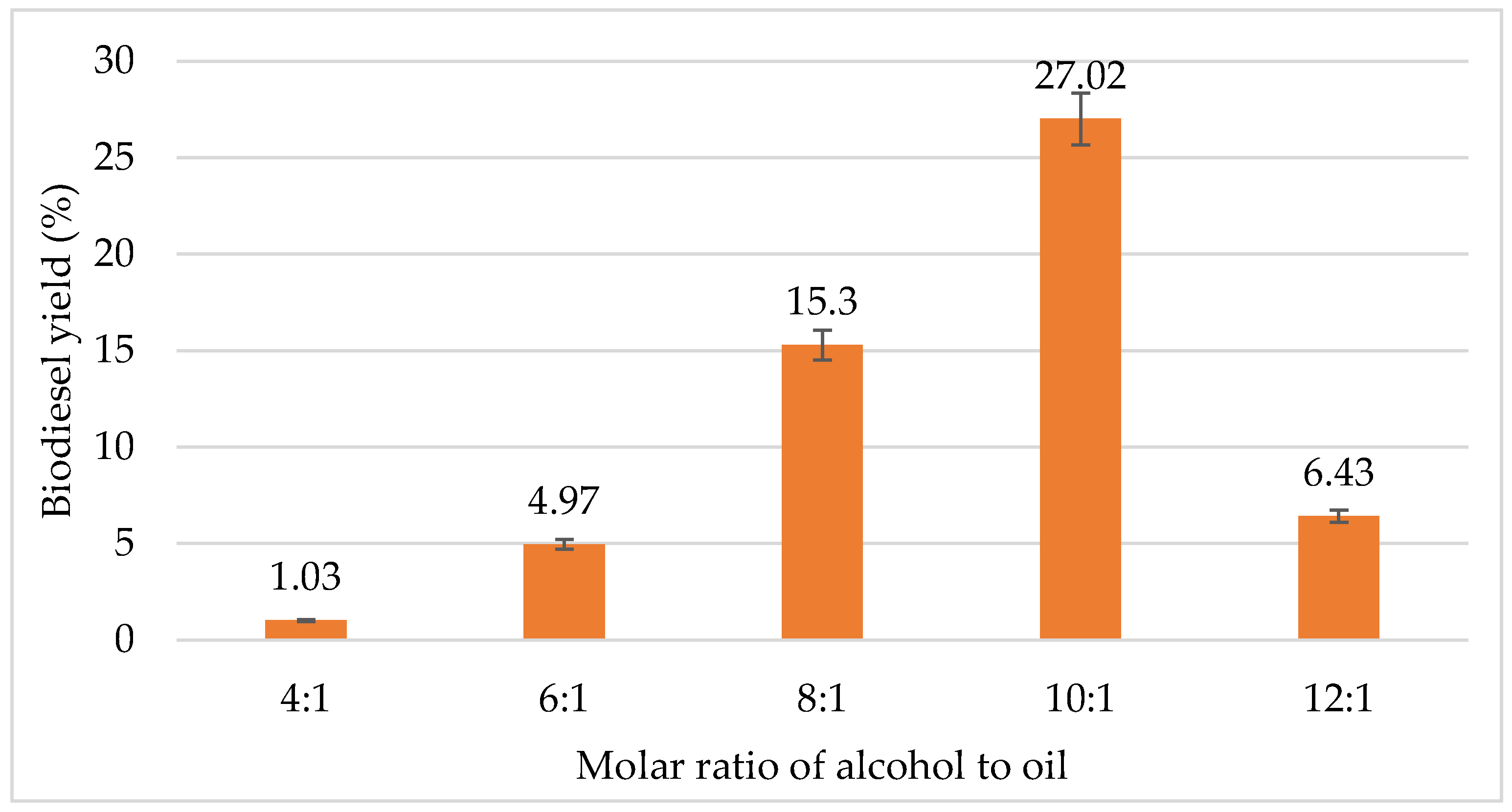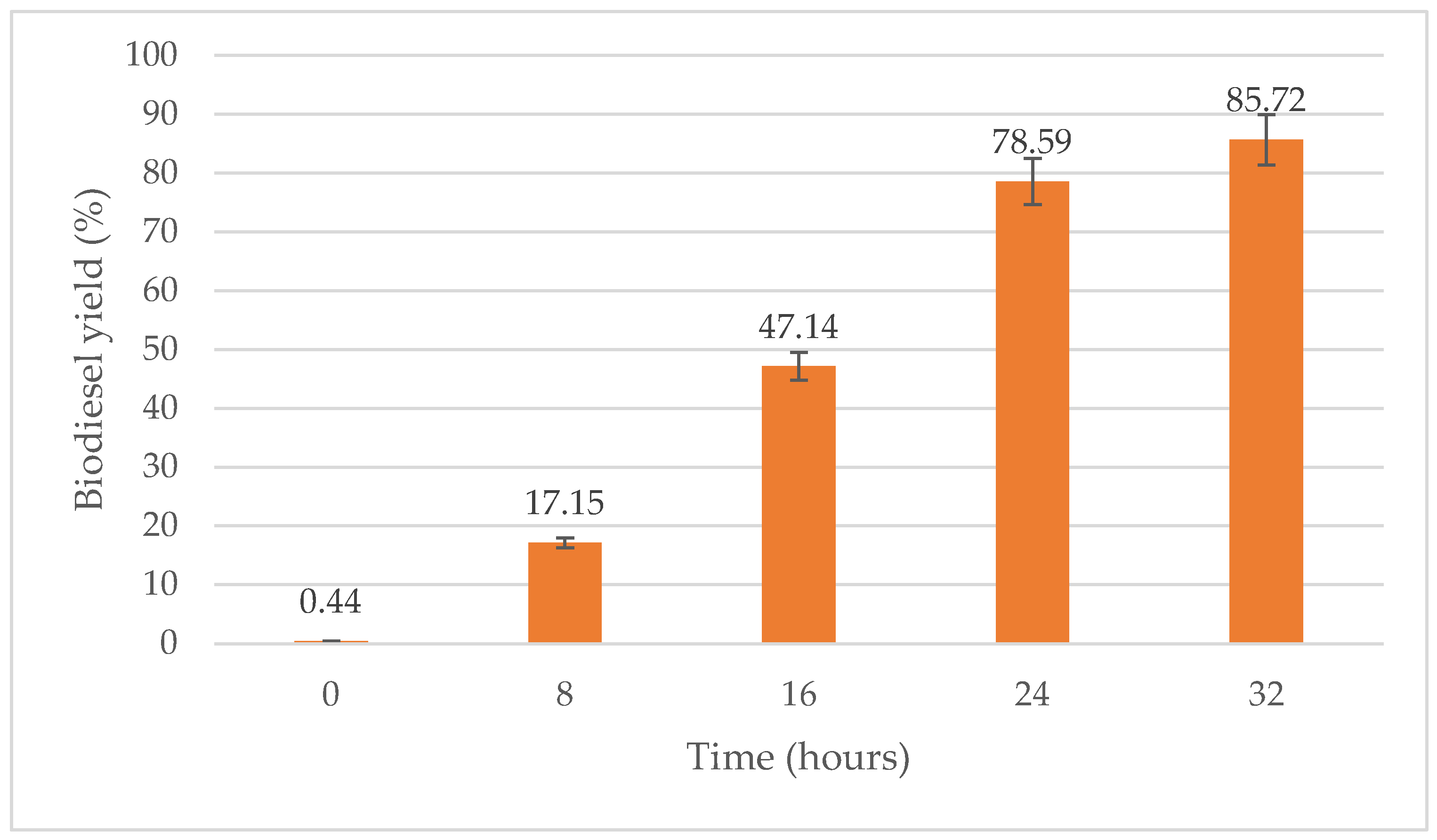Abstract
Biodiesel production from Waste Palm Cooking Oil (WPCO) is of interest to substitute fossil derived diesel fuel, due to its renewable nature, cleaner emissions and non-toxic properties. Thus, in this study, biodiesel production through transesterification process was optimized using immobilized lipase from Candida rugosa and WPCO collected from the faculty’s cafeteria as a feedstock. Interaction between five operating factors: molar ratio of ethanol to oil, water content, lipase loading, reaction temperature and time on the biodiesel yield were investigated. It was observed that, with the optimal conditions of 10:1 molar ratio of ethanol to oil, 1 g water, temperature 40 °C, 0.8 g immobilized lipase and 32 h reaction time, a yield of 85.72% of biodiesel could be achieved. Thus, this study shows that WPCO, an environmental waste, can be utilized as a promising feedstock for biodiesel production using environmentally friendly biocatalysts such as immobilized lipase.
1. Introduction
The need to reduce the reliance on fossil fuels has paved the way to create a renewable and sustainable biofuel for the future. Biodiesel, one of the most favourable biofuels, possesses an important role in substituting the existing fossil derived petrol diesel fuel as they are environmentally friendly, energy efficient, non-toxic and renewable in nature [1]. Cost related with the process of biodiesel production is the main restricting factor of its production on a large-scale [2]. According to Etim et al. [3], almost 80% of the total cost for the biodiesel is from the feedstock utilized during the production. Therefore, the high cost of biodiesel production can be reduced by using low-cost, readily available feedstock or waste by-products and biomass [4].
Waste generated from agriculture or food industries can be efficiently utilized as substrates for production and extraction of value added products [5]. Since these wastes might contain lignocelluloses, carbohydrates, lipids, and protein, it can be reused and recycled into new fuels such as biodiesel and bioethanol. Due to the increasing industrialization in the world, the generated wastes lead to serious environmental concern. Therefore, converting wastes into biofuels is a three win alternative, which can increase energy security, improve food security and reduce environmental pollution [6]. Thereby, the use of waste cooking oil (WCO) as a feedstock might be a good choice for biodiesel production.
WCO is gaining immense attention as a promising sustainable feedstock for biodiesel as it has a rather low commercial price [2]. WCO refers to the processed or produced grease from non-food raw materials such as unqualified poultry or livestock and kitchen wastes [7]. It is also normally found in food processing industries such as from fast foods chains, restaurants, or within households [8]. WCO as energy sources for biodiesel production can promote development of new energy industry; reduce food disposal, safety and pollution problems, as it is simply disposed everywhere into the environment [7]. In particular, waste palm cooking oil (WPCO) utilized in lipase catalyzed process will prevent the direct use of edible oils to produce biodiesel, thus lowering the cost of biodiesel production [9]. Palm oil biodiesel has also been implemented widely as an alternative fuel as it resembles the properties of regular diesel [10,11]. In addition, palm oil has more efficiency to produce biofuel as feedstock as compared to other vegetable oils as it has a similar chain length similar to fossil oil [12].
WPCO, vegetable oils and animals fats can be used to produce biodiesel via the transesterification process in the presence of a catalyst [8]. The transesterification process is the method that is commonly employed for biodiesel production from WCO [13]. Samanta and Sahoo [12] concluded that triglycerides present in WCO were esterified through the transesterification process to produce fatty acid methyl esters (FAME) or biodiesel using a base catalyst. Meanwhile, immobilized lipase from Candida rugosa was used in this study to serve as an environmentally friendly catalyst approach.
Microbial lipase which is obtained from Candida rugosa is shown to have a good transesterification and hydrolysis activity [14]. According to Binhayeeding et al. [15], immobilized lipase was used to overcome the free enzyme limitations which can further facilitate the biodiesel production process. Lipase is able to convert free fatty acids (FFA) into fatty acid ethyl esters (FAEE) or FAME depending on the addition of methanol or ethanol in the transesterification reactions of WPCO [16]. The study by Abdulla and Ravindra [17] used ethanol for the source of alcohol as it can be produced from biomass and is less toxic as compared to methanol. Replacement of methanol using ethanol is also considered interesting as it is safe, easy to handle and can be obtained from renewable sources [18]. Enzyme catalyzed biodiesel production has also received more attention due to its benefits, such as low energy consumption, mild operating conditions, non-toxicity, and environmental friendly processes as compared with the chemical catalyzed methods [19]. Immobilized lipase also could be recovered and reused repeatedly. The study by Marín-Suárez et al. [20] found out that immobilized lipase, Novozyme 435, which was used in the transesterification process of waste fish oil can be used for 10 consecutive cycles. Uniformity of conversions and ease of control can also be derived by immobilizing the enzymes [21].
Thus, this study provides new insights for further development of biodiesel by producing FAEE instead of FAME from WPCO using immobilized lipase. However, biodiesel production from WPCO still requires in-depth investigation and an optimization process to develop sustainable fuels [22]. Uncertainty of the operating parameters should be addressed by carrying out an optimization process to achieve maximum yield of FAEE. Therefore, this study aims to optimize the biodiesel production from WPCO using immobilized enzyme lipase. The effect of six significant parameters; ethanol to oil molar ratio, temperature, enzyme loading, stirrer speed, time and addition of water into WPCO were investigated to achieve maximum biodiesel yield. The potential of WPCOs in biodiesel production was investigated in order to provide a potentially valuable development of biodiesel using cooking oil waste.
2. Materials and Methods
2.1. WPCO
WPCO was obtained from the cafeteria of Faculty of Science and Natural Resources (FSSA) in the University of Malaysia Sabah (UMS). The collected WPCO was filtered to remove inorganic and food residues [23]. The oil was heated at 105 °C to 110 °C to remove water out of the oil [24]. The WPCO was further blended together using a homogenizer to form a homogeneous batch [25].
2.2. Optimization of Biodiesel
The experiments were done for a total of 25 times depending on its parameters, respectively. Each of the experiments were carried out in duplicate. The error bars presented on the graphs were based on different experimental replicas which represent standard deviation.
The parameters and their levels selected for the study of biodiesel synthesis were 4:1 to 12:1 molar ratio of ethanol to oil, 0 g to 2 g water, 20 °C to 60 °C, 0.2 g to 1.0 g immobilized lipase and 0 h to 32 h reaction time. A standard set of conditions were used as the baseline parameters in the optimization studies. The baseline was set at 40 °C [26], 4:1 ethanol to oil molar ratio, 0.5 g immobilized lipase and 24 h reaction time [27]. The water added was set at 0.5 g with a 200 rpm agitation rate. The amount of WPCO used in each test was 5.0 g. The mixtures of WPCO, ethanol and immobilized lipase were agitated and incubated in an orbital shaking water bath at different speed, reaction temperatures and times. The quantity of biodiesel produced was analyzed using Gas Chromatography-Mass Spectrometry (GC-MS).
2.2.1. Effect of Ethanol to Oil Molar Ratio
Molar ratio of ethanol to oil in the concentration range of 4:1, 6:1, 8:1, 10:1 and 12:1 was evaluated for biodiesel production using slightly modified methods as described in Phan et al. [28]. The ratio was changed accordingly with 24 h reaction time and 0.5 g immobilized lipase. The lipase was first diluted in 0.5 g water, and then was added into the oil to ethanol mixture. Next, it was placed in an orbital shaker at 200 rpm agitation rate at 40 °C. The WPCO molar mass is considered to be 874.8 g/mol according to Felizardo et al. [29]. After 24 h, the mixture was centrifuged and analyzed using GC-MS.
2.2.2. Effect of Water Content
In order to study the effect of water present in the reaction mixture on ethanolysis, the water content was varied from 0 g to 2 g, at constant temperature, enzyme loading and ethanol to oil molar ratio. These parameters were chosen based on the optimum parameters. The orbital shaker rotation was set at 200 rpm. Amount of water added were 0 g (control) followed by 0.5 g, 1.0 g, 1.5 g and 2.0 g of water, respectively. Later, the mixture was collected in a centrifuge tube and analyzed using GC-MS.
2.2.3. Effect of Temperature
Effect of temperature was determined as described by Leung et al. [30] with a slight modification. The transesterification process was conducted at a temperature range of 20 °C to 60 °C. The optimum amount of ethanol determined from the above optimization parameter for water content and oil to ethanol molar ratio, 5.0 g of WPCO and 0.5 g immobilized lipase were added into the mixture. Then, the reaction mixture was placed in an orbital shaker at 20 °C, 200 rpm agitation rate for 24 h. The investigation was done at different temperatures of 30 °C, 40 °C, 50 °C and 60 °C. Finally, the mixture was analyzed using GC-MS. The temperature which gave the highest product yield was set as the optimum temperature, and is used to test other parameters.
2.2.4. Effect of Immobilized Lipase Loading
In this study, immobilized lipase from AYS AMANO was used. The method of Chen et al. [31] was used to evaluate the effect of immobilized lipase loading in this study. The amount of enzyme was varied from 0.2 g to 1.0 g. From the previous optimum temperature, water content and oil to ethanol molar ratio obtained, 0.2 g of lipase was added and the mixture was incubated for 24 h at 200 rpm. The steps were repeated using immobilized lipase of 0.4 g, 0.6 g, 0.8 g and 1.0 g. The lipase which gave the most conversion of biodiesel was set as the optimum immobilized lipase loading.
2.2.5. Effect of Reaction Time
Reaction time was varied to produce a higher yield of biodiesel production using the previous obtained optimum parameters at a rotation speed of 200 rpm. The first reaction was centrifuged and stored before being analyzed because the reaction time was set at 0 h. The method was repeated by replacing the reaction time to 8 h, 16 h, 24 h and 32 h, respectively. The reaction mixture was then centrifuged and run through the GC-MS. The reaction time which gave the highest yield of biodiesel was set as the optimum reaction time.
2.3. Biodiesel Analysis by Gas Chromatography Mass Spectrometry
Three external standards, ethyl palmitate, ethyl oleate and ethyl linoleate, were prepared by diluting 150 µL of the standard in a 500 mL hexane. The standards were analyzed using the GC-MS to obtain the retention time for the peak of each standard. Internal standard (IS), ethyl heptadecanoate, was prepared in a concentration of 2.0 g/L dissolved in hexane. Aliquots of the reaction mixture were withdrawn, and the product concentrations were determined using GC-MS. The samples were analyzed by modifying the method of Pazouki et al. [32]. In addition, 500 µL of FAEE sample from the transesterification process was taken and dissolved in a solution of 9 mL HPLC grade hexane, and an internal standard prepared earlier was injected into the GC-MS for triglycerides analysis [33]. Furthermore, 1 µL of the sample was taken up by automatic syringe in GC-MS and injected into the GC-MS column. The ethyl ester samples were inserted into GC-MS equipped with 30 mm—HP-5 MS Capillary column dimension 0.25 mm × 30 mm × 0.25 mm. Injector and detector temperatures were set at 280 °C and 230 °C, respectively. The column temperature was maintained at 160 °C for 2 min, and was then heated up to 300 °C, at 8 °C/min and kept constant for 5 min. Helium gas was used as the carrier gas. The column pressure was set at 12 psi. The total run time required for analyzing one sample of ethyl ester was 24.5 min. The fatty acid and ethyl ester were determined through peaks with retention time. The areas of the generated peaks were determined and compared with those obtained from the standard for FAEEs, which was ethyl palmitate, ethyl oleate and ethyl linoleate [34]. The percentage of biodiesel yield is calculated as below:
3. Results
3.1. WPCO Properties
WPCO have properties different from the fresh vegetable oil. This is due to the physical and chemical changes involving polymerization, oxidative, thermolytic and hydrolytic reactions which happened during frying between the oil and food [35]. Various chemical and physical changes happened to the cooking oil depending on the degree of heating [36]. WPCO also contains impurities such as high free FFA and water content, which limit its application during the transesterification process [37]. Thus, in order to be used for biodiesel, the oil was filtered to separate out the suspended particles and impurities in the oil [12]. According to Linganiso et al. [38], the collected oil contains a large amount of FFA together with excess moisture that needs to be removed by heating. Hence, properties of WPCO samples such as the pH value, water and moisture content were studied.
The average pH value of WPCO was 5.80, which was slightly acidic. This is because of the presence of FFA in the oil. WPCO is usually comprised of FFA, water, sterols, phospholipids, odorants and other impurities. According to the study by Simasatitkul et al. [39], the highest composition of FFA in WPCO is linoleic acid (53.7%), followed by oleic acid and palmitic acid with 22.8% and 10.2%, respectively. These fatty acids were the reasons that the WPCO is slightly acidic. Acid value is influenced by the FFA content in the oil [36]. Based on research by Samanta and Sahoo [12], high acid value was obtained in the WCO sample because of the appearance of FFA contents during the frying process.
The study by Yahya et al. [36] revealed that repeated food frying caused high moisture content in cooking oil used. This is because, during frying, steam was produced so the more frying completed, the more steam produced, making the moisture content in cooking oil be higher. In this study, the water content in the WPCO was 2.10%. Fuels that are contaminated with water have certain disadvantages as they can cause engine corrosion or react with the glycerides to produce soaps and glycerine. Heating the WPCO to a high temperature was able to remove the water present in it. As a solution, in industrial scales, the dewatering process is carried out by distillation under vacuum at a temperature range of 30 °C to 40 °C. According to Felizardo et al. [29], the dewatering process is able to reduce the water content in biodiesel from 0.21% to 0.04%.
3.2. FFA Composition of WPCO
Fatty acid (FA) can be divided into saturated or unsaturated depending on the carbon-to-carbon bonds [40]. The FFA composition of WPCO is depicted in Table 1. The FA composition of WPCO from each research cited was analyzed and determined using GC-MS. The FFA content of WCO will differ and vary depending on the quality of the feedstock [41]. Research by Ahmad and Zainal [42] and Nayak et al. [43] shows that the highest amount of acid content in WPCO is oleic acid (55.3% and 61.2%), followed by linoleic acid (19.7% and 23.82%). However, the highest acid content in a study by Awogbemi et al. [40] is linoleic acid (45.5%), while, for Kadapure et al. [23], it is lauric acid (46.2%). From the table, it can be seen that stearic and linoleic acid are both frequently occurring acids as both acids appeared in all the WPCO samples studied. According to Krishna and Shivaraj [41], different fatty acids will have different chemical and physical properties.

Table 1.
Chemical composition of waste palm cooking oil.
3.3. Effect of Process Parameters on Biodiesel Yield
Characteristics and parameters of waste cooking oil used are very important as they influence the suitability and quality of biodiesel produced [44]. The effect of each process parameter on the biodiesel yield is discussed in detail in this section to understand how these parameters affect the transesterification process. It is critical to determine the amount of each operating condition to maximize the biodiesel yield.
3.3.1. Effect of Ethanol to Oil Molar Ratio
The process conditions of molar ratio of alcohol to oil are some of the most important parameters for the transesterification process for biodiesel production. There are various types of alcohol used to produce biodiesel such as ethanol, methanol, butanol, pentanol, isopropyl alcohol and propanol [45]. In this study, ethanol is used instead of methanol, as it has lower toxicity as compared to methanol. Ethanol is less polar than methanol, so it is more miscible with triacylglycerols. Moreover, ethanol can be produced from different types of biomass through fermentation, which makes the process more eco-friendly [46]. Based on Figure 1, the biodiesel yield increases with the alcohol to oil molar ratio, up to a certain value and then it decreases. The highest biodiesel yield was achieved at a 10:1 molar ratio of ethanol to oil with about a 30% yield. At an ethanol to oil molar ratio of 4:1 and 6:1, biodiesels produced were 1.03% and 4.97%, respectively. However, the yield increased by 10% at 8:1 molar ratio and reduced to 6.43% ratio at 12:1 ratio. From the results obtained, the optimum molar ratio of ethanol to oil for biodiesel yield was 10:1.
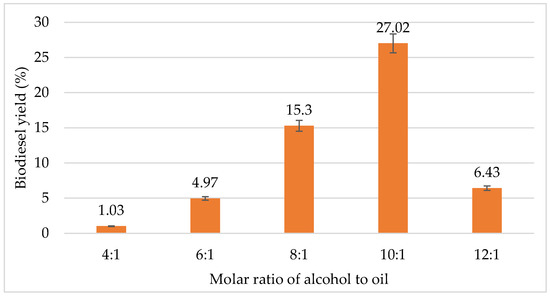
Figure 1.
Effect of molar ratio of alcohol to oil on biodiesel yield (%).
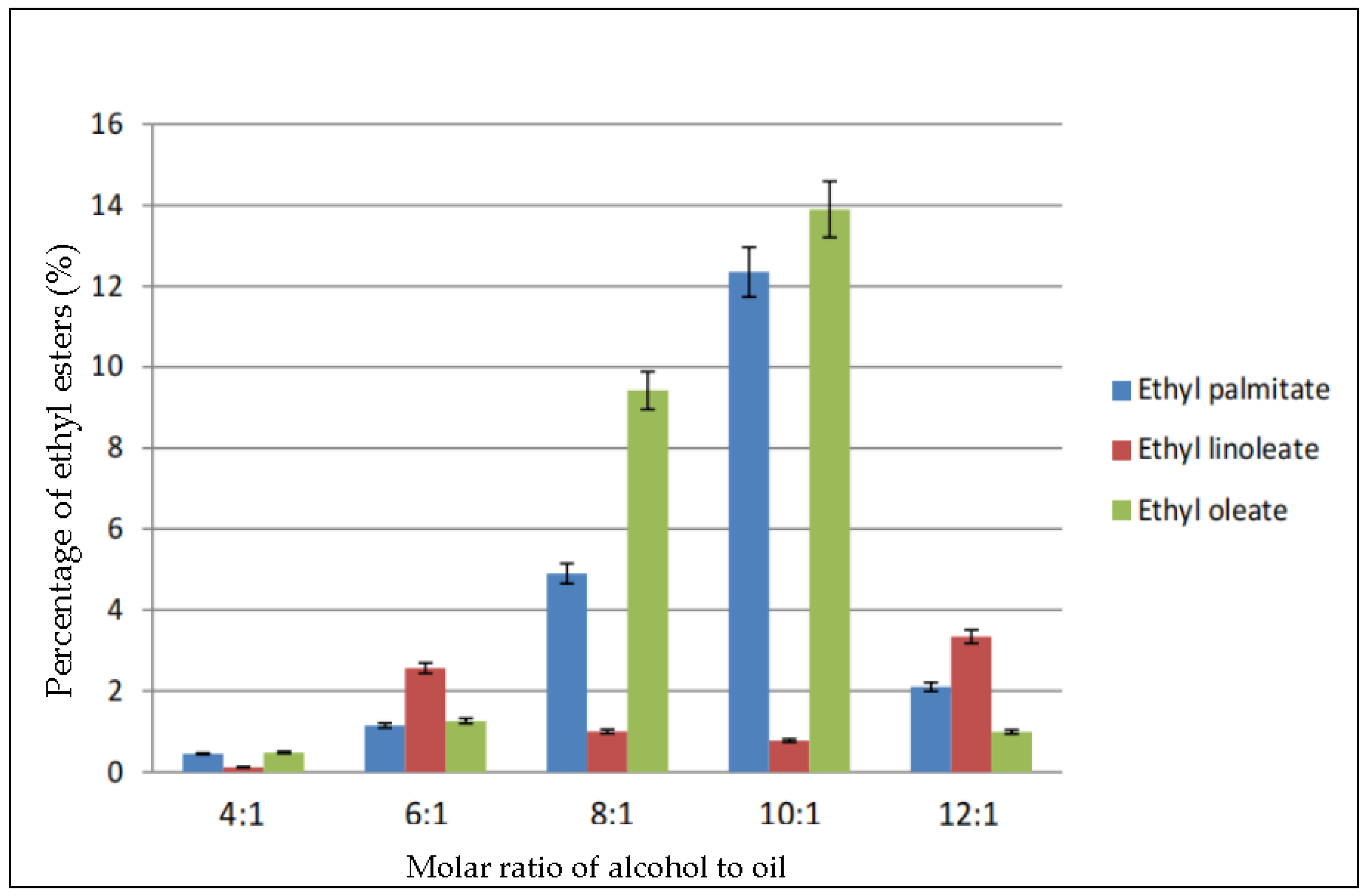
Figure 2 shows the percentage of the three major ethyl esters: ethyl palmitate, ethyl linoleate, and ethyl oleate in the biodiesel produced using the same molar ratio of ethanol to oil as in Figure 1. The molar ratio 4:1 produced very less ethyl esters. The maximum conversion occurred in ratio 10:1, in which 14% of ethyl oleate was produced, followed by 12.35% of ethyl palmitate and 1% ethyl linoleate. Meanwhile, the percentages of the ethyl esters dropped greatly when the ratio was increased to 12:1. According to the figure, ethyl oleate was the most abundant, followed by ethyl palmitate and ethyl linoleate.
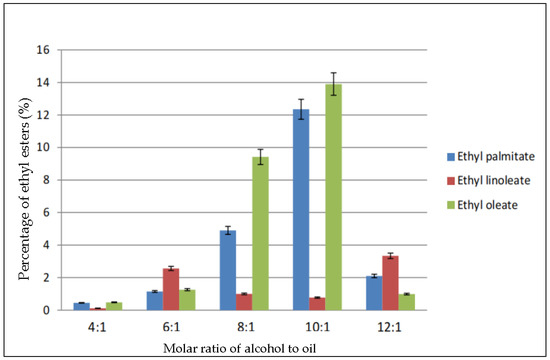
Figure 2.
Percentage of ethyl esters (%) for the effect of molar ratio of alcohol to oil.
The molar ratio of ethanol to oil is crucial to reduce biodiesel production cost and optimize the biodiesel yield [8]. One of the important parameters which affects the FAEE yield is also the molar ratio of alcohol to oil. This is mainly because the yield and viscosity of produced ester depends on the molar ratio of alcohol to oil. Figure 1 shows the graph of the effect of ratio of alcohol to oil on biodiesel yield. The percentage yield of biodiesel initially increased up to a 10:1 molar ratio. However, when the ratio was increased to 12:1, the biodiesel yield decreased by about 20%. Hanif et al. [47] reported that, to achieve a faster reaction rate for an efficient conversion of biodiesel from WPCO, an excess amount of ethanol should be used. This is because it can increase the rate of ethanolysis. Based on the stoichiometric ratio, the transesterification process requires 3 moles of alcohol and 1 mol of triglyceride in order to produce 3 moles of fatty acid alkyl esters and 1 mole of glycerol. Excess ethanol is used to minimize the diffusion limitation. However, the transesterification reaction is an equilibrium reaction. Hence, alcohol is used in excess in order to shift the equilibrium of the reaction towards the desired ethyl esters.
In this study, a molar ratio of 10:1 was found to be effective with maximum biodiesel yield of 27.02%. A study done by Hundie and Akuma [48] also reported that the ratio 10:1 of ethanol to oil was an optimum ratio for biodiesel production. When lower ratios such as 4:1, 6:1 and 8:1 were used, the yield of biodiesel was also lower. This is because, at low ethanol ratios, the ethanol concentration was not sufficient enough to advance the equilibrium reaction towards the completion of transesterification process. Therefore, the transesterification reaction for biodiesel production was found to be highly affected by the ethanol-to-oil ratio.
When the ethanol amount is increased, the conversion of FA to FAEE was higher, with other parameters kept constant. However, when the ratio exceeded 10:1, the increase of conversion was relatively slow. According to Tomasevic and Siler-Marinkovic [49], separations are proved to be difficult, with undesirable suspension when higher ethanol ratios were used. In this study, the yield dropped drastically when the ratio was increased from 10:1 to 12:1. Too much alcohol also inhibits the lipase activity, hence diminishing catalytic activity during transesterification. In addition, it takes a longer amount of time, due to the unreacted ethanol and oil mixture suspension. A high ethanol to oil ratio means greater medium polarity, produced by ethanol and water. This deactivates the enzyme and hence decreases the amount of ethyl ester produced. The molar ratio has no effect on acid, peroxide, saponification and iodine value of methyl esters. These properties of the ester depend on the kind of oil (refined, crude, used frying oil) used for the transesterification reaction. In addition, the reaction of ethanol to triglyceride does not influence changes in the FA composition of obtained esters [49].
3.3.2. Effect of Water Content
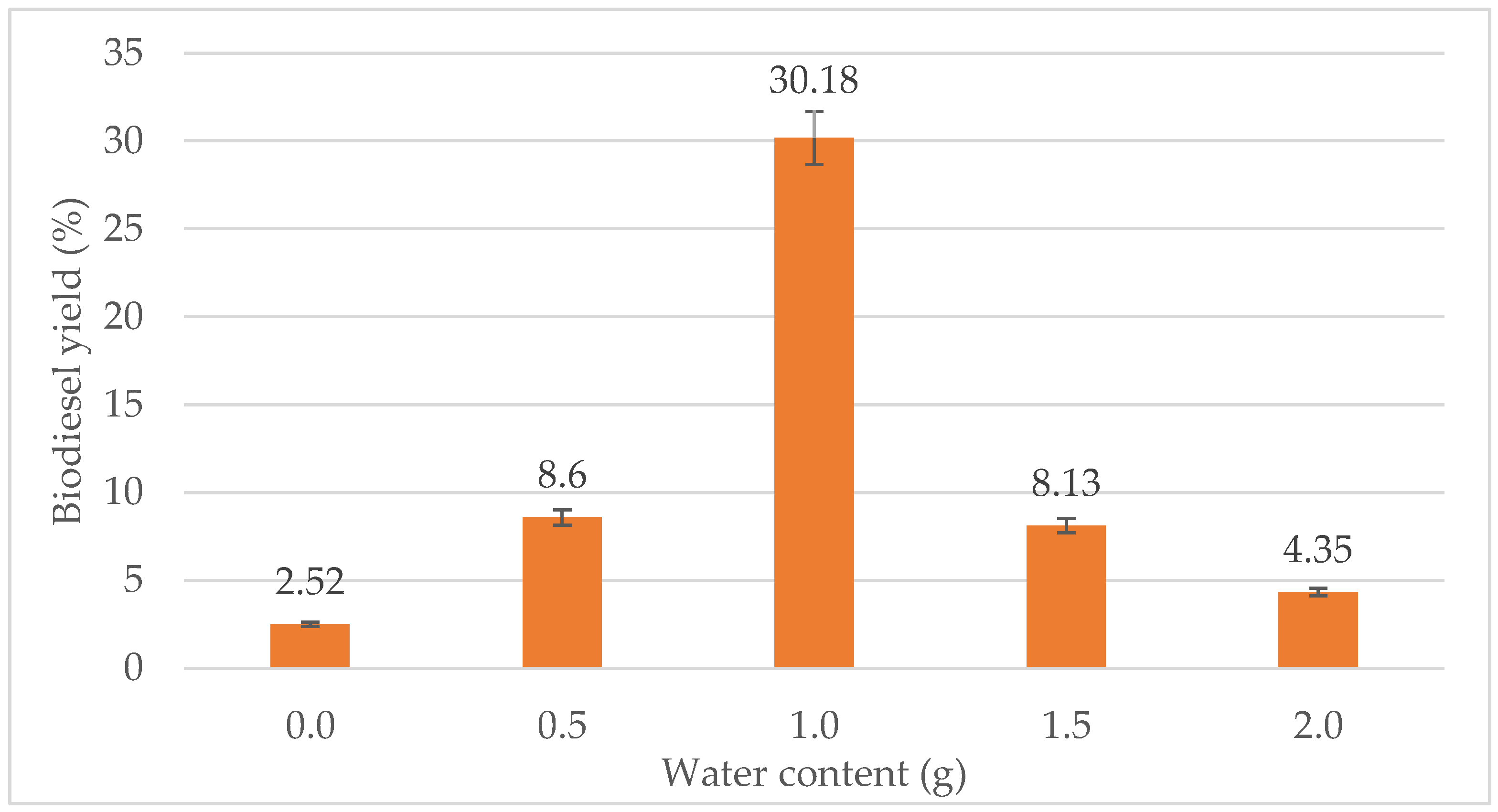
The effect of water content on biodiesel yield can be seen in Figure 3. The biodiesel yield was the lowest when no water was added. However, 0.5 g of water did not show much improvement either for the biodiesel yield as it only increases the yield to 6%. From the figure, the highest yield was achieved when 1.0 g of water was added with a 30.18% biodiesel yield. When the water content was increased to 1.5 g and 2.0 g, the percentage of biodiesel dropped to 8.13% and 4.35%, respectively. Thus, the optimum water content for the transesterification process was 1.0 g.
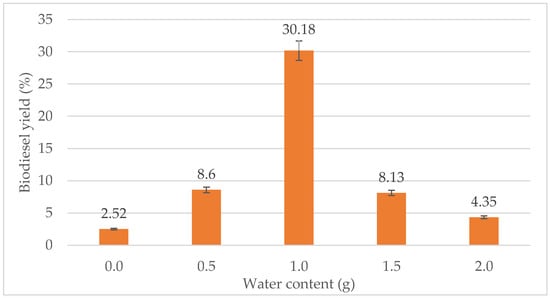
Figure 3.
Effect of water content (g) on biodiesel yield (%).
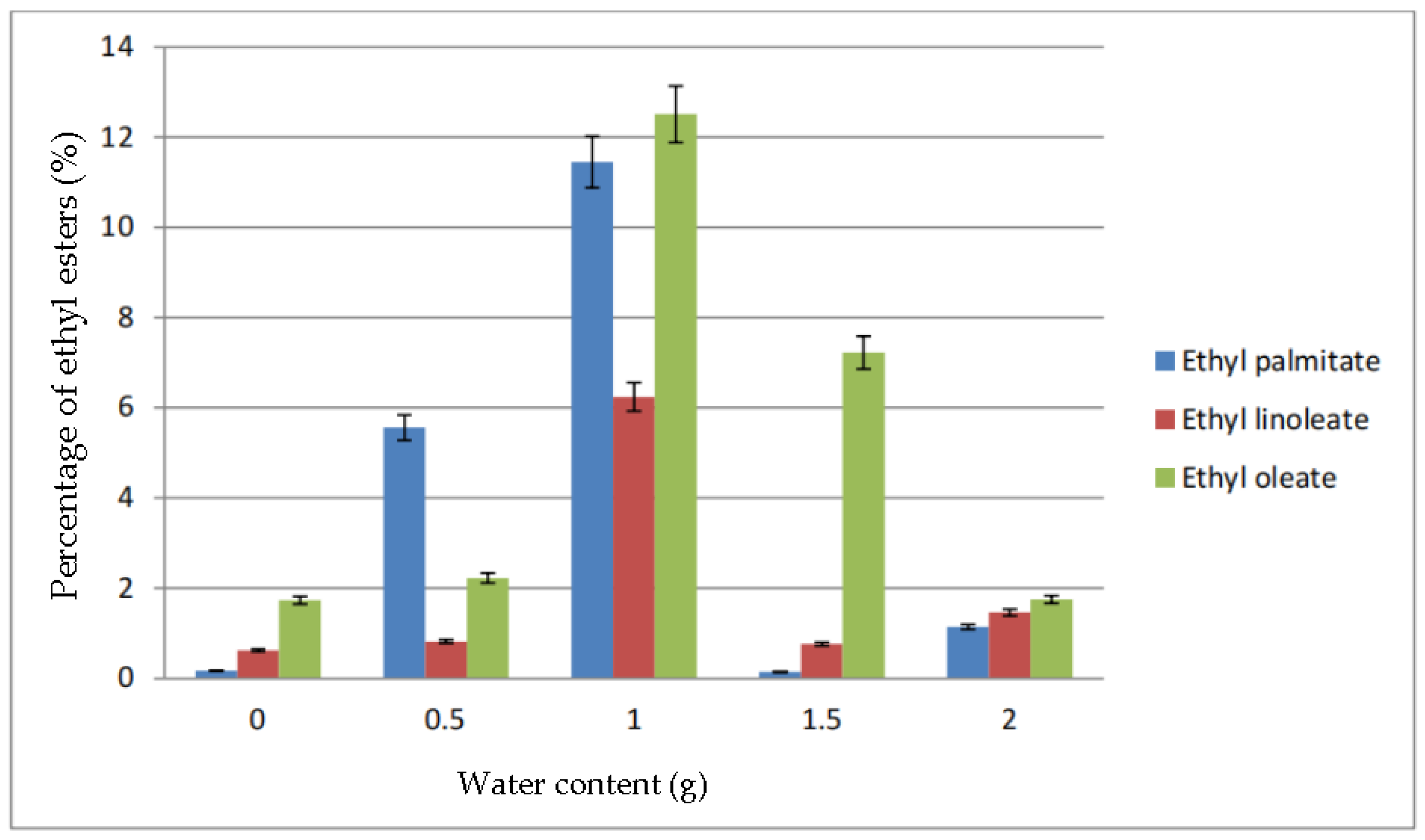
Figure 4 shows the percentage of ethyl palmitate, ethyl linoleate, and ethyl oleate in the biodiesel produced due to the addition of water. All ethyl ester produced was lower in percentage when no water was added. However, at 0.5 g of water, the percentage of ethyl palmitate increased to almost 6%. The other two ethyl esters almost maintained the same as with no addition of water. The highest percentage of ethyl esters was at 1.0 g of water, which produced 12.51% of ethyl palmitate, 11.45% ethyl palmitate and 6.24% ethyl oleate. As the water content increased to 1.5 g and 2.0 g, the percentage of ethyl esters in the biodiesel dropped.
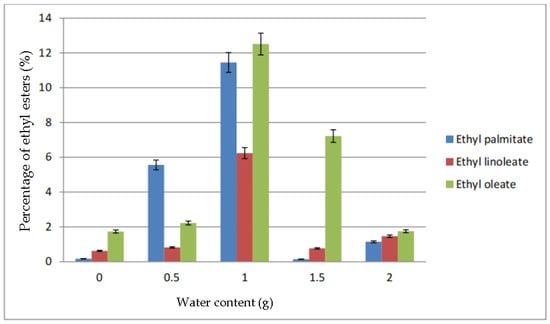
Figure 4.
Percentage of ethyl esters (%) for the effect of water content (g).
Water plays a major role in the transesterification, especially when lipase catalyzed reaction is performed. It plays multiple roles, and it has strong influence on the catalytic activity and stability of the lipase [50]. From Figure 3, the optimum amount of water needed for transesterification is 1.0 g. Any amount below or above 1.0 g reduces the biodiesel yield. All starting materials including the WPCO, ethanol and lipase should be substantially anhydrous. Generally, less than a mono-layer of water is required for an enzyme to show biological activity [27]. A certain volume of water is required to keep the enzyme active in organic solvents. The water might also take part in the transesterification, thus influencing the equilibrium. The activity of lipase depends on interfacial area due to its feature where it acts between an aqueous and an organic phase. Hence, the presence of water helps to increase the available interfacial area, to maintain the lipase activity.
In this study, the lipase used is from Candida rugosa. Tan et al. [51] reported that the optimum water amount required for Candida sp. to maintain the highest transesterification activity was 10% to 20% based on the oil weight. This supports the result obtained, where 1.0 g of water was the optimum water content, as 1.0 g is about 20% of the oil weight. However, too much water could make the lipase more flexible, which results in other side reactions which include hydrolysis, especially in the transesterification process. In addition, water can promote hydrolysis of ester to form FFA, which intensely reduces the biodiesel yield. When the water content exceeded 1.0 g, the yield reduced by more than 20%. The amount of water required is influenced by the type of feedstock and lipase, the immobilized support and the organic solvent used [52]. As the water level increased, the enzyme flexibility and expressed activity also increased. After an optimum level of water, hydrolytic reactions become more significant, and the transesterification yield was expected to decrease.
3.3.3. Effect of Temperature
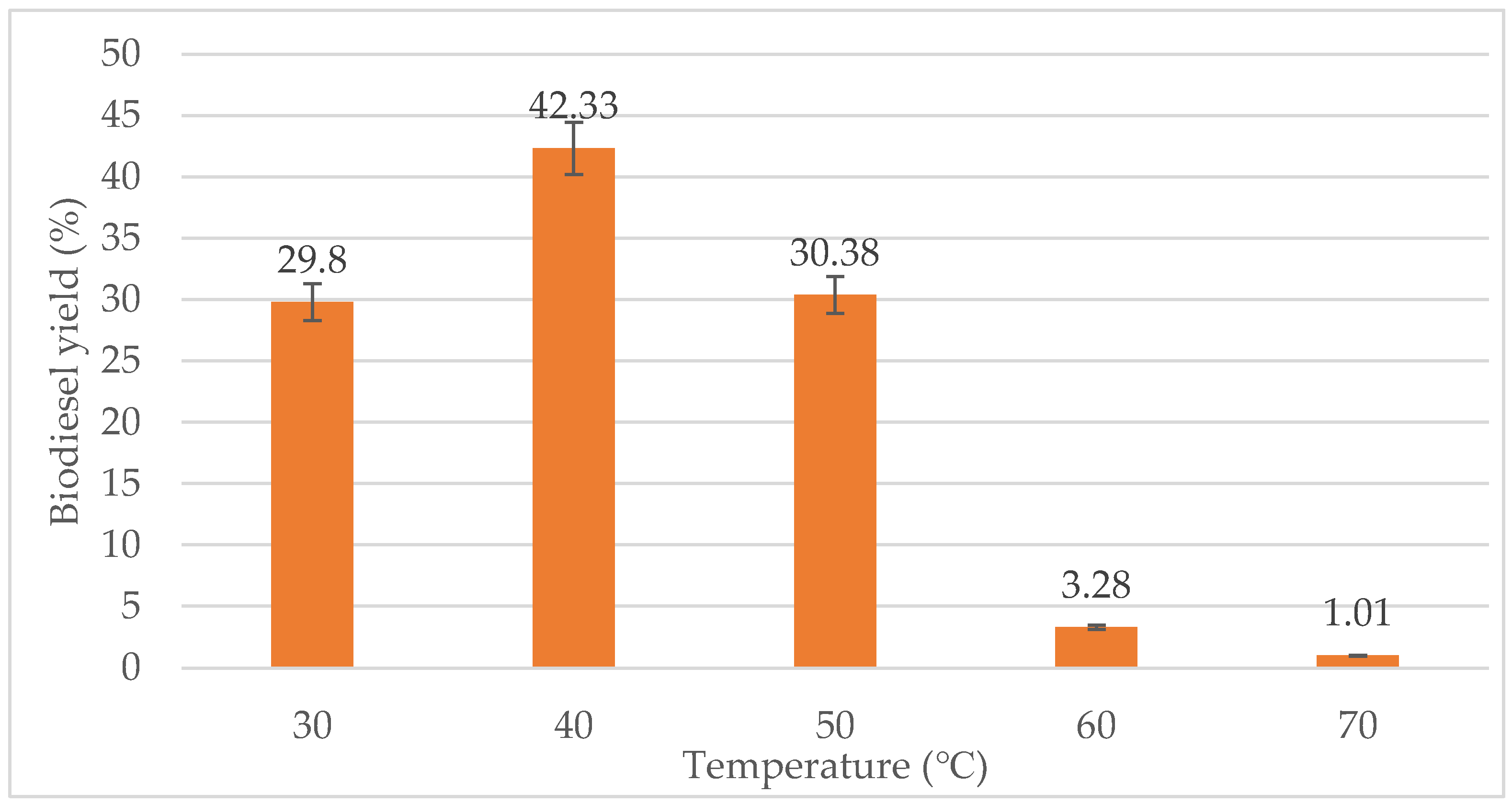
The effect of reaction temperature on the biodiesel yield was studied by varying the temperature in the range from 30 °C to 70 °C. From Figure 5, a reaction temperature of 30 °C gave quite a high yield of biodiesel, which is almost 30%. However, the highest biodiesel yield was produced at 40 °C with 42.33%. When the temperature exceeded 40 °C, the biodiesel yield reduces. At 50 °C, the biodiesel yield has decreased drastically to 3.28%. Almost no conversion occurred at 70 °C with only about 1.01% biodiesel yield. Thus, the optimum temperature for the transesterification process for this study is 40 °C. Based on the study by Chen et al. [53], the optimum temperature for transesterification of WCO was 40 °C.
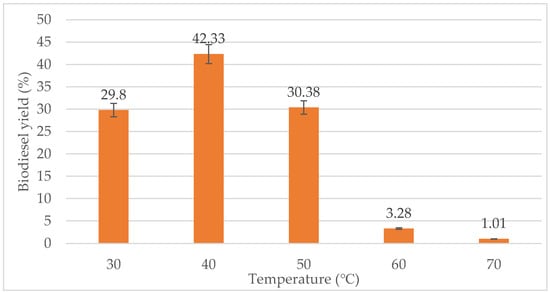
Figure 5.
Effect of temperature (°C) on biodiesel yield (%).
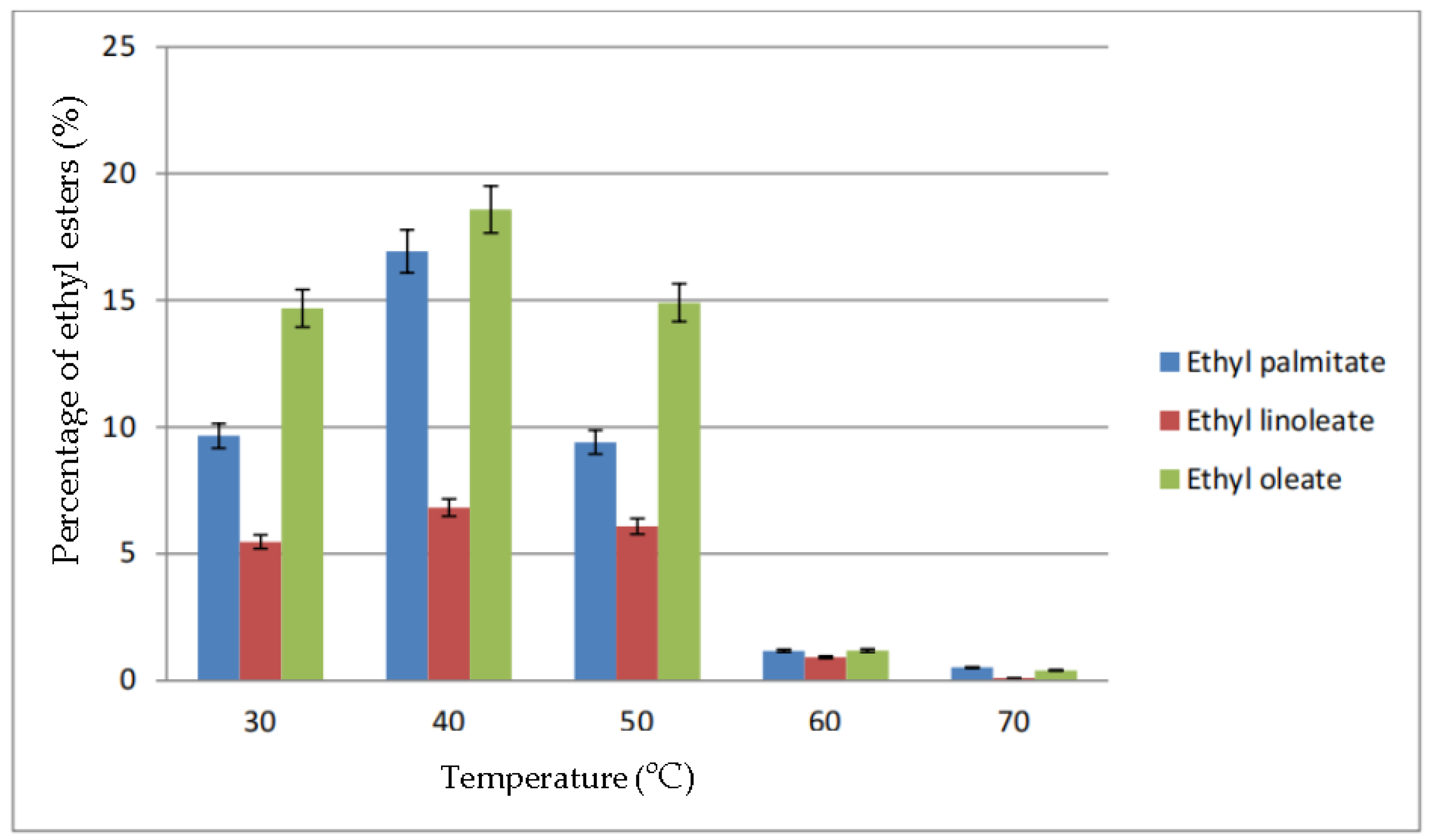
The percentage of ethyl esters in biodiesel based on the parameter of reaction temperature is shown in Figure 6. At a temperature of 30 °C until 70 °C, the highest percentage of ethyl esters obtained was ethyl oleate followed by ethyl palmitate and ethyl linoleate. From the figure, the highest conversion was at 40 °C, in which 18.58% ethyl oleate, 16.93% ethyl palmitate and 6.82% ethyl linoleate were produced. At a temperature above 50 °C, the percentage of each ethyl esters dropped drastically to below 2%
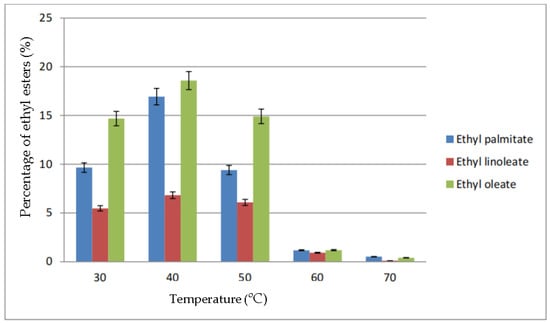
Figure 6.
Percentage of ethyl esters (%) for the effect of temperature (°C).
In this study, the temperature was varied from 30 °C to 70 °C, with a fixed 10:1 molar ratio of ethanol to oil and 1.0 g water content. The reaction increased at the beginning; however, at a temperature above 40 °C, the yield was decreased due to slightly deactivated lipase. At a temperature of 70 °C, almost no biodiesel was produced. This is due to its high temperature, which is close to the boiling point of ethanol, which is 78.37 °C. Hence, the elevation of temperature may result in evaporation of the ethanol. Increase in reaction temperature decreases the biodiesel synthesis by evaporating the alcohol [13]. The evaporation of the alcohol transesterification due to a reaction temperature greater than the alcohol’s boiling point lowered the oil-alcohol contact duration as its availability decreased, thus reducing the total biodiesel output [54].
Temperature is a crucial parameter for the esterification process as extended heating can reverse the process [23]. The transesterification process can occur at different temperatures, influenced by the properties of oils. The effect of temperature on the material balance responses is the same as for the lipase concentration. The FAEE weight and molar yield reduce with temperature because of the increase of triglyceride saponification and the subsequent dissolution of ethyl ester into glycerol [55].
In this study, the lipase activity increased with temperature, until a specific temperature as extreme temperatures would inhibit the activity of lipase. From 30 °C to 40 °C, the temperature was elevated making the possibility of collision between the lipase and substrate molecules increase. This helps the formation of enzyme substrate complexes which caused the reaction to increase. An increase in temperature enhances the reaction rate and thus shortens the time to complete the transesterification reaction [56]. Hence, more than 10% of conversion occurred at 30 °C to 40 °C. However, there was a slight reduction in the conversion at temperature above 40 °C. Certain enzymes are partially deactivated at 60 °C by ethanol within the first 24 h while others were not affected during such a short reaction time.
3.3.4. Effect of Lipase Loading
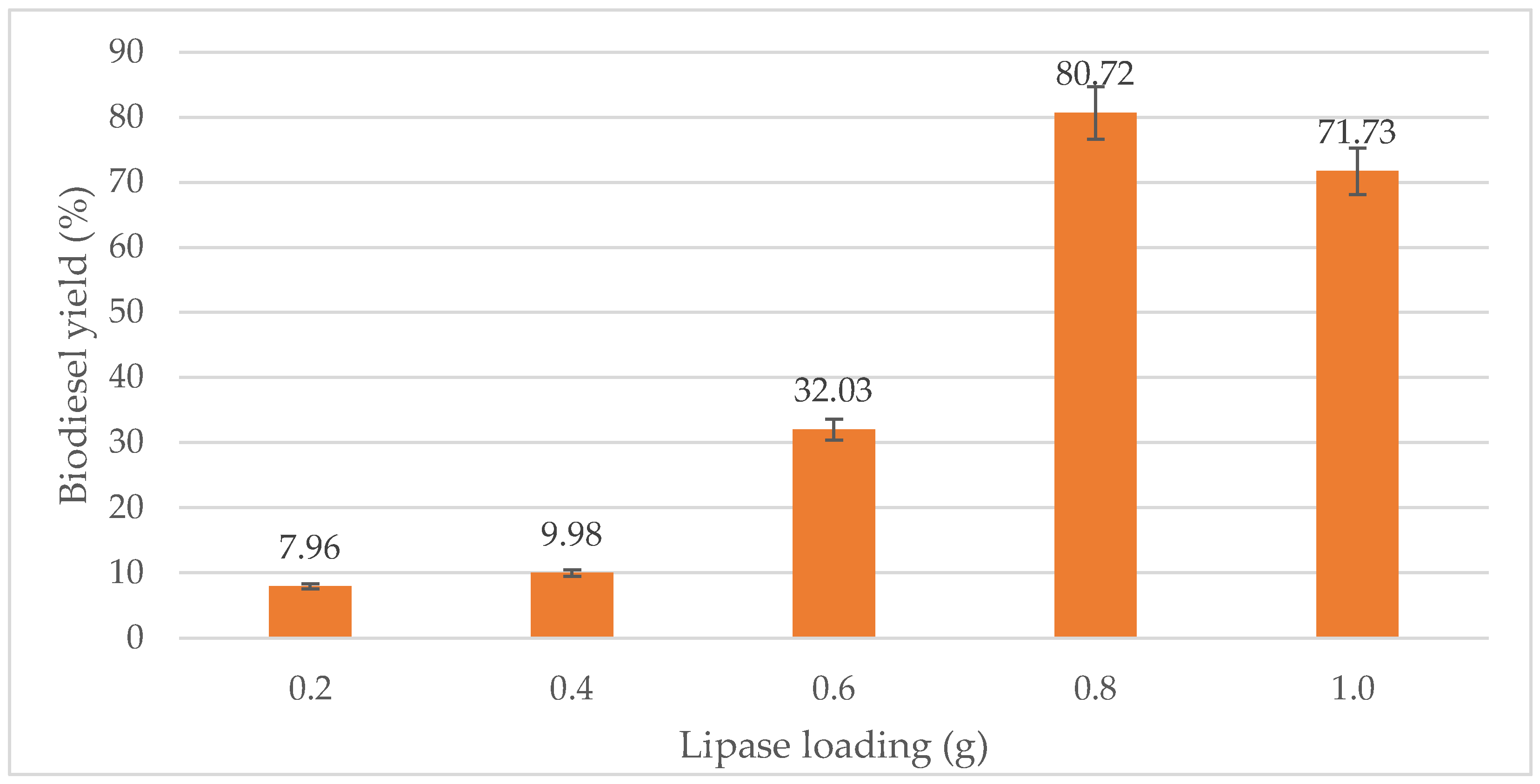
From Figure 7, it is clear that the biodiesel yield increased proportionally with the amount of lipase until optimum lipase loading 0.8 g was reached, giving an optimum yield of 80.72%. When 0.2 g of lipase is added, 7.96% of biodiesel was produced. Meanwhile, 10.00% and 32.03% of biodiesel were produced, respectively, when 0.4 g and 0.6 g of lipase were added. However, when the lipase amount was increased to 1.2 g, the biodiesel percentage slightly reduced to 71.73%. Thus, 0.8 g of lipase was the optimum amount of lipase required for the transesterification process. This result is similar to Ali et al. [9], in which the optimal conditions for biodiesel production achieved up to 86% yield using 0.782 g lipase with a temperature of 44.2 °C.
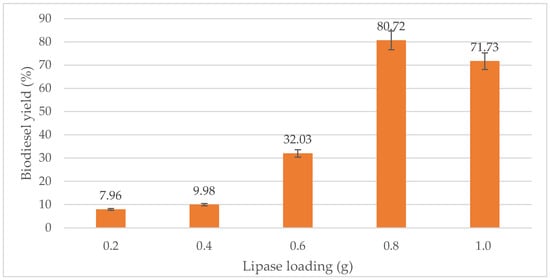
Figure 7.
Effect of lipase loading (g) on biodiesel yield (%).
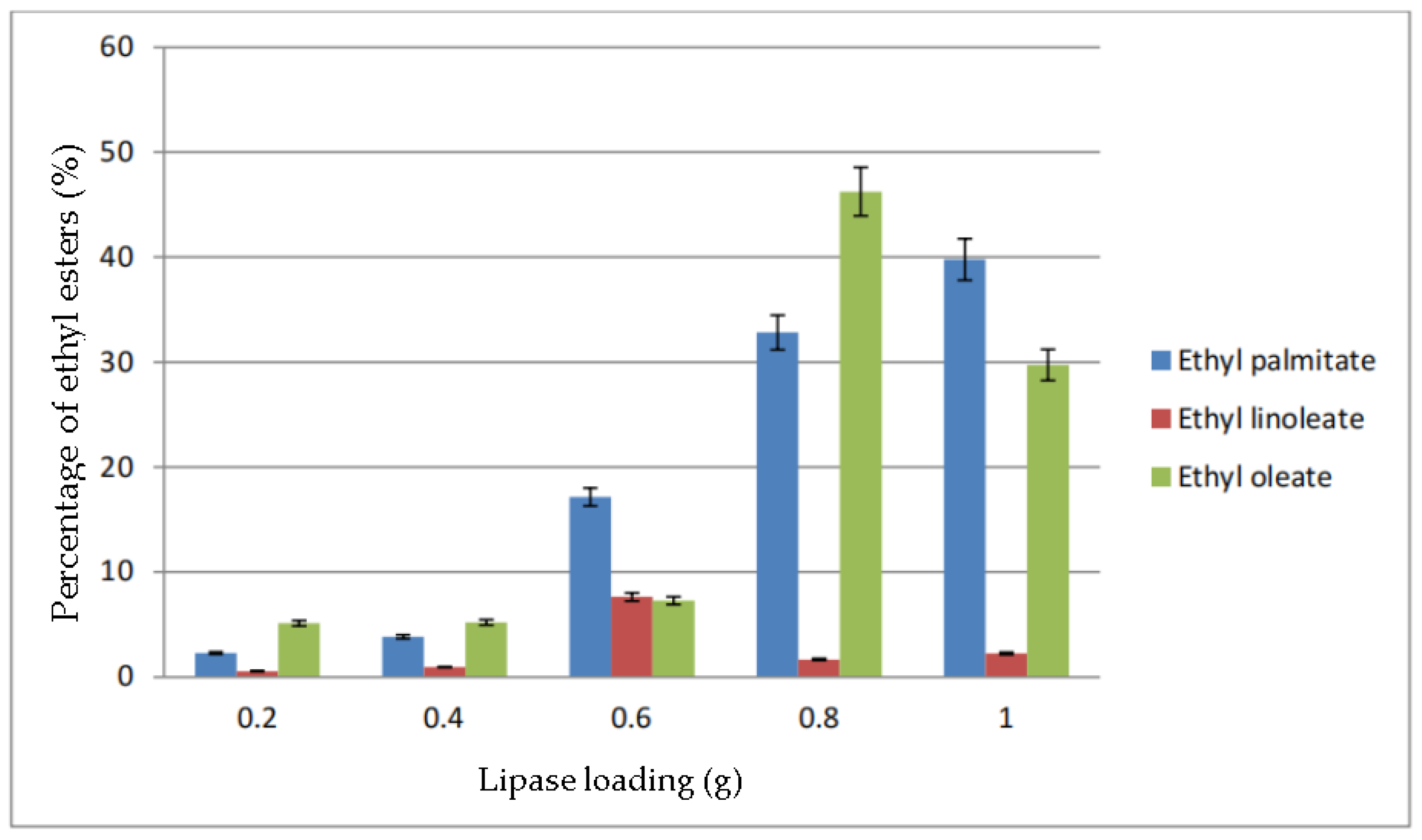
The percentage of ethyl esters in biodiesel based on the parameter of lipase loading is shown in Figure 8. When 0.2 g of lipase was added, the maximum percentage of ethyl ester produced was ethyl oleate, which is about 5%, followed by ethyl palmitate, and almost no ethyl linoleate was produced. The total percentage of ethyl esters when 0.4 g of lipase used was almost the same as 0.2 g. However, the percentage of ethyl palmitate increased during the transesterification with 0.6 g lipase to 17.14% while the other two ethyl esters increased slightly. Ethyl oleate was found to be the highest when 0.8 g lipase was used, where it reached up to almost 50%, while ethyl palmitate reached 32.82%. However, the ethyl linoleate reduced to 1.66%. Both ethyl palmitate and linoleate increased when the lipase amount was increased to 1.0 g. However, the percentage of ethyl oleate dropped about 20%, which caused the biodiesel yield to decrease.
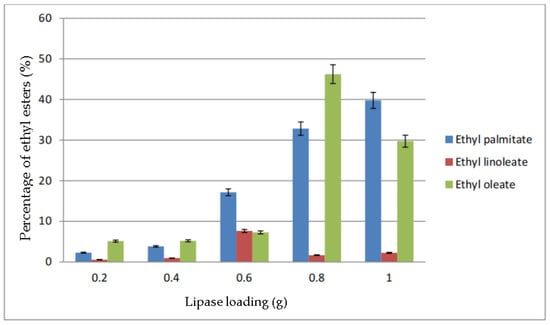
Figure 8.
Percentage of ethyl esters (%) for the effect of lipase loading (g).
Enzymes are an alternative to chemical catalysts for the synthesis of biodiesel. For instance, enzyme lipases are known to catalyze esterification, hydolysis and transesterification reactions [57]. FAEE yield is greatly affected by the amount of enzyme lipase. The greater the concentration of lipase, the higher the reaction yield [25]. In this study, enzyme concentration had a positive effect, producing a higher yield when a greater quantity of enzyme was used. However, when 0.2 g and 0.4 g lipase were used, the yield was significantly lower. The best results were obtained with 0.8 g, followed by 1.0 g of lipase, which gives 80.72% and 71.73% conversions, respectively. When the lipase amount was increased from 0.8 g to 1.0 g, the yield decreased about 10%. This could be due to the increase in the viscosity which causes a reduction in the rate of reaction up to a point where even additional amounts of enzymes did not help. The amount of lipase has an overall positive effect on the purity of the biodiesel. The content of ester in the FAEE increases with the variable. According to Bautista et al. [55], the quadratic effect of this factor has a significant negative influence on the FAEE purity. However, its absolute value is smaller than its corresponding main effect. Hence, the increase in the lipase concentration does not produce a constant rise in the FAEE purity because of the previously mentioned significant curvature effect. The curvature effect is present at high values of this variable. At this stage, the FAEE purity achieves maximum values and the influence of catalyst concentration is no longer significant. Conversely, initial catalyst concentration has a negative influence on the FAEE weight and molar yields. However, it has an obviously positive influence on the yield losses due to triglyceride saponification and ethyl ester dissolution in glycerol. Both yield losses follow a similar negative tendency, although the influence on the yield loss due to ethyl ester dissolution in glycerol is more significant. As a result, the FAEE yield decreases.
3.3.5. Effect of Reaction Time
As shown in Figure 9, the biodiesel yield increases as the reaction time increases from 8 h to 32 h, the latter being the optimum reaction time. Biodiesel yield produced at a reaction time of 8 h, 16 h and 24 h were 17.15%, 47.14% and 78.59%, respectively. In this study, the optimum reaction time for transesterification process of WPCO was 32 h with a 85.72% yield.
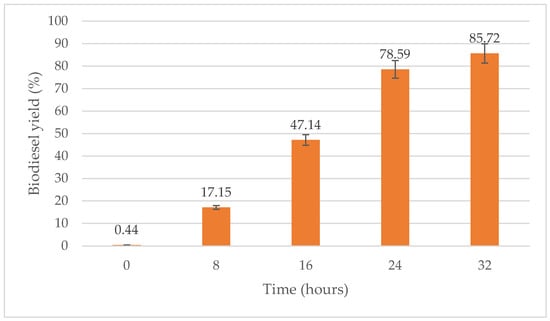
Figure 9.
Effect of time (hours) on biodiesel yield (%).
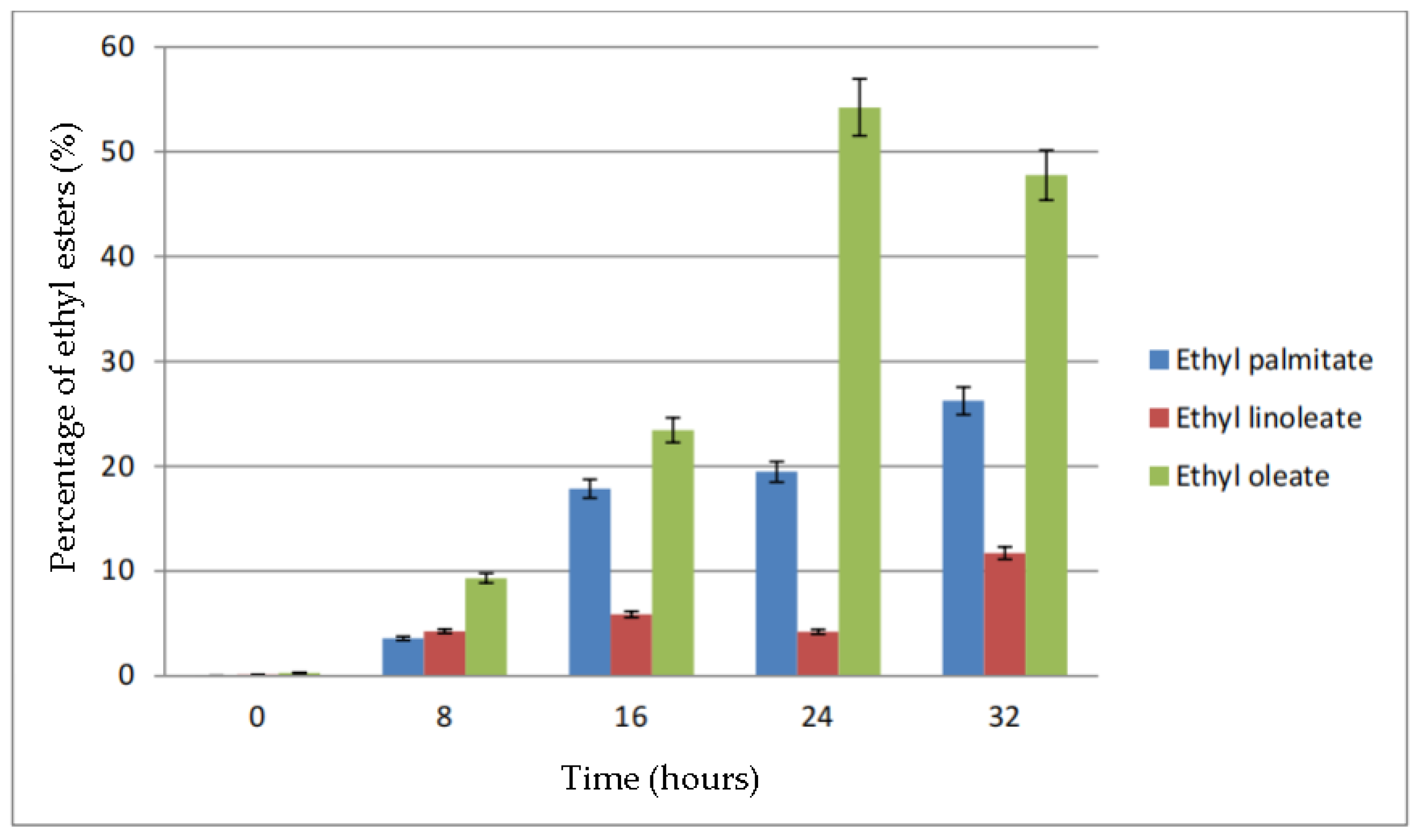
The effect of time on percentage of ethyl esters in biodiesel is shown in Figure 10. From the figure, it can be seen that the percentage of ethyl palmitate increases as the reaction time increases. Meanwhile, the ethyl linoleate percentage was below 15% for all reaction times. The percentage of ethyl oleate kept increasing until 24 h and then it dropped about 5% when the reaction time was increased to 32 h. The decrease in percentage of ethyl oleate was, however, replaced by the increase in percentage of ethyl linoleate and ethyl palmitate.
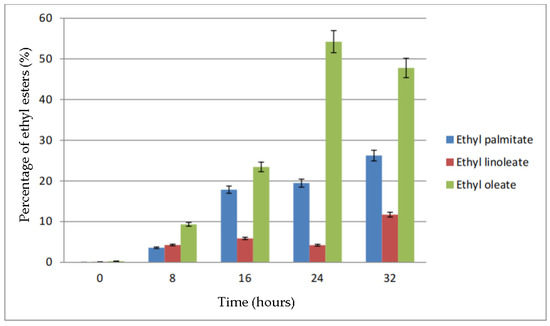
Figure 10.
Percentage of ethyl esters (%) for the effect of time (hours).
The reaction time also plays a role in the rate of production of biodiesel. The biodiesel produced will increase as the reaction time increases [13]. This is supported by the study done by Akhtar et al. [58], in which, at a shorter reaction time, the reactants still did not reach the equilibrium stage, causing the conversion of triglycerides into biodiesel to be incomplete. This is due to the increase in mixing and dispersion of ethanol in oil phase with reaction time. Research by Leung and Guo [30] reported that sufficient time should be provided in the transesterification process of used frying oil. This is because excess reaction time does not increase the conversion but favors the backward reaction by hydrolysis of esters, which results in a reduction of product yield. Bharathiraja et al. [59] also reported that a reaction time above 24 h was required for a maximum biodiesel yield. In a study of transesterification of waste cooking palm oil, Al-Widyan and Al-Shyoukh [60] noticed that product specific gravity changes with reaction time in a roughly exponential trend. Hence, it was observed that higher reaction time enhances the conversion of triglycerides to FAEE during a transesterification reaction at a given temperature.
3.4. Ethyl Ester Produced from Transesterification
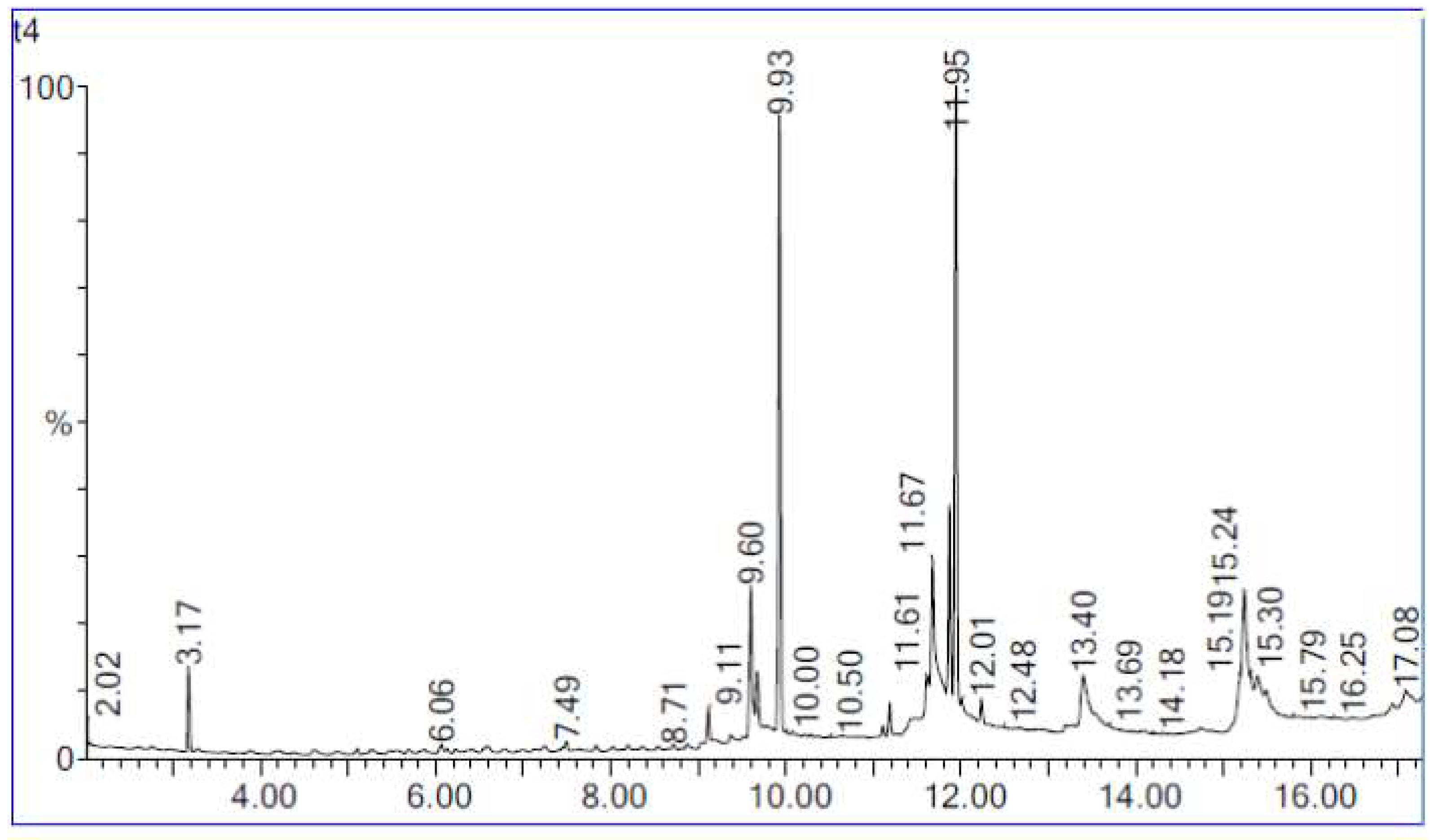
Biodiesel is composed of mono-alkyl esters of long-chain FA made by esterification of FFA with alcohols or through transesterification of oils [41]. Figure 11 shows the chromatogram of the maximum percentage of biodiesel yield in this study. The optimum parameters include 10:1 molar ratio of ethanol to oil, 1.0 g of water addition, 40 °C reaction temperature, 0.8 g lipase loading and 32 h reaction time. The three main peaks formed at retention times 8.6, 10.5 and 10.6 were ethyl palmitate, ethyl linoleate and ethyl oleate, respectively. Both ethyl palmitate and ethyl oleate had high peaks. Meanwhile, the peak for ethyl linoleate was found to be shorter than the two other peaks. The three main ethyl esters produced are ethyl palmitate, ethyl linoleate and ethyl oleate. The FA is converted into ethyl ester by the transesterification. They are converted into ethyl linoleate, ethyl oleate and ethyl palmitate, respectively. In the presence of high temperature and water, transesterification leads to hydrolysis of triglycerides, which increases the FFA content in oil [57]. According to the results obtained, the most abundant ethyl ester produced was ethyl oleate, followed by ethyl palmitate and lastly ethyl linoleate. Ethyl linoleate was produced at a very low percentage. The ethyl esters were produced by transesterification of the triglycerides in WPCO. The products of this reaction were FAEE, or known as biodiesel and glycerol. Biodiesel is made from a reaction between alcohol and triglyceride, in which triglyceride is found primarily in plants and animals [13]. The major component of the WPCO is triacylglycerols or triglycerides, which consist of three long chain FA esterified to a glycerol backbone. The reaction between triacylglycerols with ethanol results in the three FA chains being released from the glycerol skeleton and combining with the alcohol to yield fatty acid alkyl esters and glycerol as the by product. In this study, the fatty acid alkyl ester produced was FAEE.
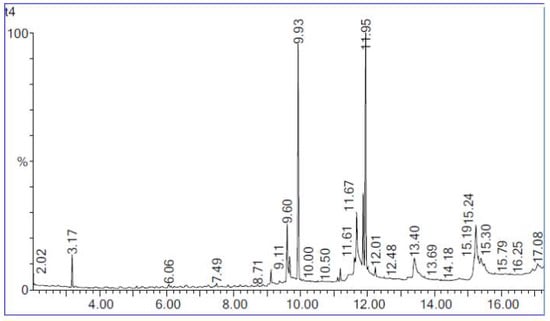
Figure 11.
Biodiesel yield chromatogram from GC-MS analysis.
3.5. Biodiesel Optimization
With the formation of biodiesel from transesterification, the reaction mixture was separated into two immiscible phases: a biodiesel phase and an ethanol phase containing lipase. The biodiesel layer was analysed for FAEE. At optimum operating parameters of 10:1 ethanol to oil molar ratio, 1.0 g of water addition, 40 °C reaction temperature, 0.8 g lipase loading and 32 h reaction time, the maximum biodiesel yield obtained was 85.72%. The biodiesel yield in this study could not reach up to 100%. This could be because the WPCO contains water and is low in FFA. Due to water contamination and low value of FFA, the conversion could not be completed. The sums of ethyl esters of in each biodiesel sample were not 100 wt.% because some unrecognized peaks, found in the biodiesel chromatograms, prevented all the fatty acids from being identified [61]. Table 2 shows the comparison of optimum biodiesel yield obtained in this study with the findings reported by other researchers. The differences in the biodiesel yield could be due to the type of lipase and feedstock used as well as its immobilization techniques employed.

Table 2.
Comparisons of biodiesel yield (FAEE) obtained in this study and with other related works.
4. Conclusions
The world’s energy needs that are supplied from fossil fuels, such as petroleum, coal and natural gas, will eventually be exhausted in the future. Hence, an effort has been taken to overcome this problem of depletion by using sustainable biofuels. This study was carried out to determine the optimum parameters required to produce maximum biodiesel from WPCO. Results obtained revealed that operating parameters of 10:1 molar ratio of ethanol to oil, 1 g water, temperature of 40 °C, 0.8 g immobilized lipase and 32 h reaction time are the optimum conditions for biodiesel production. The improvement in the optimization of the parameters gave a maximum yield of 85.72%. In short, this work concludes that WPCO has the potential to be developed as a feedstock for producing biodiesel commercially using environmentally friendly immobilized lipase. By employing WPCO together with immobilized lipase, the production cost of biodiesel can be reduced, and it also solves the disposal problems associated with used cooking oil. In the near future, WPCO will be more likely to play a larger part in biodiesel manufacturing than other raw materials due to its availability and lower costs. This research is also in line with the “waste to wealth approach” on production of sustainable biofuels like biodiesel.
Author Contributions
R.A.: supervision, conceptualization, overall guidance, writing—review and editing, validation, revision of the paper. E.D.: conceptualization, investigation, formal analysis, writing. T.K.: conceptualization, investigation, formal analysis, data curation, methodology, writing—original draft. A.Z.Y.: writing—review and editing. M.A.A.S.: writing—review and editing. J.A.G.: writing—review and editing. S.U.F.S.N.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study did not require any ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank and acknowledge the Faculty of Science and Natural Resources, and the Faculty of Engineering at Universiti Malaysia Sabah and Kulliyyah of Science at International Islamic University Malaysia, Kuantan Campus.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Nomenclature
| WPCO | Waste palm cooking oil |
| WCO | Waste cooking oil |
| FAME | Fatty acid methyl esters |
| FAEE | Fatty acid ethyl esters |
| FFA | Free fatty acids |
| FA | Fatty acid |
| IS | Internal standard |
| GCMS | Gas chromatography mass spectrometry |
| HPLC | High performance liquid chromatography |
References
- Peng, Y.; Amesho, K.T.T.; Chen, C.; Jhang, S.; Chou, F.; Lin, Y. Optimization of Biodiesel Production from Waste Cooking Oil Using Waste Eggshell as a Base Catalyst under a Microwave Heating System. Catalysts 2018, 8, 81. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Adamu, A.; Zhu, Z. Current Research in Green and Sustainable Chemistry Economic Evaluation and Production Process Simulation of Biodiesel Production from Waste Cooking Oil. Curr. Res. Green Sustain. Chem. 2021, 4, 100091. [Google Scholar] [CrossRef]
- Etim, A.O.; Musonge, P.; Eloka-Eboka, A.C. Effectiveness of biogenic waste-derived heterogeneous catalysts and feedstock hybridization techniques in biodiesel production. Biofuels Bioprod. Biorefining 2020, 14, 620–649. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Obahiagbon, K.; Isesele, V.; Usman, F. Optimized Biodiesel Production from Waste Cooking Oil Using a Functionalized Bio-Based Heterogeneous Catalyst. Clean. Eng. Technol. 2022, 8, 100501. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.; Pandey, A. Assessing the Impact of Industrial Waste on Environment and Mitigation Strategies: A Comprehensive Review. J. Hazard. Mater. 2020, 398, 123019. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Kazmi, M.; Fayaz, H.; Mujtaba, M.A.; Ali, C.H.; Kalam, M.A.; Soudagar, M.E.M.; et al. Production and Utilization Aspects of Waste Cooking Oil Based Biodiesel in Pakistan. Alex. Eng. J. 2021, 60, 5831–5849. [Google Scholar] [CrossRef]
- Yang, J.; Shan, H. The Willingness of Submitting Waste Cooking Oil (WCO) to Biofuel Companies in China: An Evolutionary Analysis in Catering Networks. J. Clean. Prod. 2021, 282, 125331. [Google Scholar] [CrossRef]
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Sukiman, N.L.; Kalam, M.A.; Masjuki, H.H.; Syahir, A.Z.; Awang, M.S.N.; Mujtaba, M.A.; Milano, J.; Shamsuddin, A.H. Microwave Irradiation-Assisted Transesterification of Ternary Oil Mixture of Waste Cooking Oil–Jatropha Curcas—Palm Oil: Optimization and Characterization. Alex. Eng. J. 2022, 61, 9569–9582. [Google Scholar] [CrossRef]
- Ali, C.H.; Qureshi, A.S.; Mbadinga, S.M.; Liu, J.; Yang, S.; Mu, B. Biodiesel Production from Waste Cooking Oil Using Onsite Produced Purified Lipase from Pseudomonas Aeruginosa FW_SH-1: Central Composite Design Approach. Renew. Energy 2017, 109, 93–100. [Google Scholar] [CrossRef]
- Vergel-ortega, M.; Valencia-ochoa, G.; Duarte-forero, J. Experimental Study of Emissions in Single-Cylinder Diesel Engine Operating with Diesel-Biodiesel Blends of Palm Oil-Sunflower Oil and Ethanol. Case Stud. Therm. Eng. 2021, 26, 101190. [Google Scholar] [CrossRef]
- Zahan, K.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Samanta, S.; Sahoo, R.R. Waste Cooking (Palm) Oil as an Economical Source of Biodiesel Production for Alternative Green Fuel and Efficient Lubricant. BioEnergy Res. 2021, 14, 163–174. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Kalthum, U.; Abdullah, S.; Haron, N. Materials Today: Proceedings Biodiesel Production from Waste Cooking Oil: A Brief Review. Mater. Today Proc. 2022, 63, S484–S489. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of Biodiesel Production Using Waste Cooking Oil and Applying Single and Mixed Immobilised Lipases on Polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Jung, S.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Park, Y.; Aminabhavi, T.M.; Kwon, E.E. Synthesis of Different Biofuels from Livestock Waste Materials and Their Potential as Sustainable Feedstocks—A Review. Energy Convers. Manag. 2021, 236, 114038. [Google Scholar] [CrossRef]
- Abdulla, R.; Ravindra, P. Immobilized Burkholderia Cepacia Lipase for Biodiesel Production from Crude Jatropha Curcas L. Oil. Biomass Bioenergy 2013, 56, 8–13. [Google Scholar] [CrossRef]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.I.; Lv, Y.; Xiong, W.; Xu, J. Modern developmental aspects in the field of economical harvesting and biodiesel production from microalgae biomass. Renew. Sustain. Energy Rev. 2022, 135, 110209. [Google Scholar] [CrossRef]
- Dwiarti, L.; Ali, E.; Park, E.Y. Enhancement of lipase catalyzed-fatty acid methyl esters production from waste activated bleaching earth by nullification of lipase inhibitors. Bioresour. Technol. 2010, 101, 14–20. [Google Scholar] [CrossRef][Green Version]
- Marín-Suárez, M.; Mendez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of Immobilized Lipases in the Transesterification of Waste Fish Oil for the Production of Biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Abdulla, R.; Pogaku, R. Advances in Biofuels; Pogaku, R., Sarbatly, R.H., Eds.; Springer Science and Business Media: New York, NY, USA, 2013; pp. 191–216. ISBN 9781461462484. [Google Scholar]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. Biotechnology for Biofuels An Overview to Process Design, Simulation and Sustainability Evaluation of Biodiesel Production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef]
- Kadapure, S.A.; Kirti, P.; Singh, S.; Kokatnur, S.; Hiremath, N.; Variar, A.; Shaikh, S.; Chittaragi, R. Studies on Process Optimization of Biodiesel Production from Waste Cooking and Palm Oil. Int. J. Sustain. Eng. 2018, 11, 167–172. [Google Scholar] [CrossRef]
- Thoai, D.N.; Le Hang, P.T.; Lan, D.T. Pre-Treatment of Waste Cooking Oil with High Free Fatty Acids Content for Biodiesel Production: An Optimization Study via Response Surface Methodology. Vietnam J. Chem. 2019, 57, 568–573. [Google Scholar] [CrossRef]
- Akoh, C.C.; Shaw, J.F.; Chang, S.W.; Lin, S.C.; Wu, T.T.; Ju, H.Y.; Shieh, C.J. Continuous enzymatic synthesis of biodiesel with Novozym 435. Energy Fuels 2008, 22, 840–844. [Google Scholar]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, S.; Gupta, M.N. Biodiesel preparation by lipase-catalyzed transesterification of Jatropha oil. Energy Fuels 2004, 18, 154–159. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Felizardo, P.; Neiva Correia, M.J.; Raposo, I.; Mendes, J.F.; Berkemeier, R.; Bordado, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, B.; Chang, J.; Fu, Y.; Lv, P.; Wang, X. Synthesis of biodiesel from waste cooking oil using immobilized lipase in fixed bed reactor. Energy Convers. Manag. 2009, 50, 668–673. [Google Scholar] [CrossRef]
- Pazouki, M.; Zamani, F.; Zamzamian, A.H.; Fahar, M.; Najafpour, G.H. Esterification of free fatty acids by Rhizopus oryzae as cell-catalyzed from used cooking oil for biodiesel production. World Appl. Sci. 2010, 8, 719–724. [Google Scholar]
- Ghazali, M.; Hidayah, N.; Gimbun, J.; Nurdin, S. Transesterification of Waste Cooking Oil using Chemically Treated Catalyst. J. Appl. Sci. 2014, 14, 1425–1429. [Google Scholar]
- Al-Zuhair, S.; Dowaidar, A.; Kamal, H. Dynamic modeling of biodiesel production from simulated waste cooking oil using immobilized lipase. Biochem. Eng. J. 2009, 44, 256–262. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) Using Calcium Oxide (CaO) Nano-Catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef]
- Yahya, S.; Razali, F.H.; Harun, F.W. Physicochemical Properties of Refined Palm Cooking Oil and Used Palm Cooking Oil. Mater. Today Proc. 2019, 19, 1166–1172. [Google Scholar] [CrossRef]
- Anantapinitwatna, A.; Ngaosuwan, K.; Kiatkittipong, W. Effect of Water Content in Waste Cooking Oil on Biodiesel Production via Ester-Transesterification in a Single Reactive Distillation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 559, 012014. [Google Scholar] [CrossRef]
- Linganiso, E.C.; Tlhaole, B.; Magagula, L.P.; Dziike, S.; Linganiso, L.Z.; Motaung, T.E.; Moloto, N.; Tetana, Z.N. Biodiesel Production from Waste Oils: A South African Outlook. Sustainability 2022, 14, 1983. [Google Scholar] [CrossRef]
- Simasatitkul, L.; Gani, R.; Arpornwichanop, A. Optimal Design of Biodiesel Production Process from Waste Cooking Palm Oil. Procedia Eng. 2012, 42, 1411–1420. [Google Scholar] [CrossRef]
- Awogbemi, O.; Onuh, E.I.; Inambao, F.L. Comparative Study of Properties and Fatty Acid Composition of Some Neat Vegetable Oils and Waste Cooking Oils. Int. J. Low-Carbon Technol. 2019, 14, 417–425. [Google Scholar] [CrossRef]
- Krishna, B.S.V.S.R.; Shivaraj, B.K. Estimation of Properties of Mixed Waste Cooking Oil for Production of Biodiesel. Int. J. Eng. Technol. 2018, 7, 552–555. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.A.; Zainal, Z.A. Performance and Chemical Composition of Waste Palm Cooking Oil as Scrubbing Medium for Tar Removal from Biomass Producer Gas. J. Nat. Gas Sci. Eng. 2016, 32, 256–261. [Google Scholar] [CrossRef]
- Nayak, S.K.; Mishra, P.C.; Behera, G.R. Experimental Investigation on Dual-Fuel Engine Utilizing Waste Cooking Oil and Producer Gas. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 369–376. [Google Scholar] [CrossRef]
- Alias, N.I.; Jayakumar, J.K.; Md Zain, S. Characterization of Waste Cooking Oil for Biodiesel Production. J. Kejuruter. SI 2018, 1, 79–83. [Google Scholar]
- Mansir, N.; Teo, S.H.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, G.A.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized Production and Advanced Assessment of Biodiesel: A Review. Int. J. Energy Resour. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Hundie, K.B.; Akuma, D.A. Heliyon Optimization of Biodiesel Production Parameters from Prosopis Julifera Seed Using definitive Screening Design. Heliyon 2022, 8, e08965. [Google Scholar] [CrossRef]
- Tomasevic, A.V.; Siler-Marinkovic, S.S. Methanolysis of used frying oil. Fuel Process. Technol. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Wang, F.; Tan, T. Effect of water on methanolysis of glycerol trioleate catalyzed by immobilized lipase Candida sp. in organic solvent system. J. Mol. Catal. B Enzym. 2009, 56, 122–125. [Google Scholar] [CrossRef]
- Tan, T.; Nie, K.; Wang, F. Production of biodiesel by immobilized Candida sp. lipase at high water content. Appl. Biochem. Biotechnol. 2006, 128, 109–116. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Abang, S.; Poncelet, D.; Chan, E.S.; Ravindra, P. Production of biodiesel using immobilized lipase—A critical review. Crit. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ying, M.; Li, W. Enzymatic conversion of waste cooking oils into alternative fuel—Biodiesel. Appl. Biochem. Biotechnol. 2006, 132, 911–921. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Bora, A.P.; Kumar, T.; Halder, G. Parametric optimization of biodiesel synthesis from rubber seed oil using iron doped carbon catalyst by Taguchi approach. Renew. Energy 2017, 105, 616–624. [Google Scholar] [CrossRef]
- Bautista, L.F.; Vicente, G.; Rodriguez, R.; Pacheco, M. Optimisation of FAME production from waste cooking oil for biodiesel use. Biomass Bioenergy 2009, 33, 862–872. [Google Scholar] [CrossRef]
- Zulqarnain; Ayoub, M.; Yusoff, M.H.M.; Nazir, M.H.; Zahid, I.; Ameen, M.; Sher, F.; Floresyona, D.; Budi Nursanto, E. A Comprehensive Review on Oil Extraction and Biodiesel Production Technologies. Sustainability 2021, 13, 788. [Google Scholar] [CrossRef]
- Karmee, S.K. Liquid Biofuels from Food Waste: Current Trends, Prospect and Limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Ahmad, M.; Asma, M.; Munir, M.; Zafar, M.; Sultana, S.; Mujtaba, M.A.; Mohamed, A.; Kalam, M.A. Efficient Production of Wild and Non-Edible Brassica juncea (L.) Czern. Seed Oil into High-Quality Biodiesel via Novel, Green and Recyclable NiSO 4 Nano-Catalyst. Sustainability 2022, 14, 10188. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Raj, A.S.; Jayamuthunagai, J.; Jayakumar, M.; Kirubakaran, M.A.; Vivek, P.; Palani, S. Optimization of biodiesel production from waste cooking oil using pure lipase and rhizopus oryzae through response surface methodology. Asian J. Microbiol. Biotechnol. Environ. Sci. 2013, 16, 63–70. [Google Scholar]
- Al-Widyan, M.I.; Al-Shyoukh, A.O. Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour. Technol. 2002, 85, 253–256. [Google Scholar] [CrossRef]
- Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Danane, F.; Bessah, R.; Alloune, R.; Tebouche, L.; Madjene, F.; Kheirani, A.Y.; Bouabibsa, R. Experimental Optimization of Waste Cooking Oil Ethanolysis for Biodiesel Production Using Response Surface Methodology (RSM). Sci. Technol. Energy Transit. 2022, 77, 14. [Google Scholar] [CrossRef]
- Moreira, K.S.; Junior, L.S.M.; Monteiro, R.R.C.; De Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; De Souza, M.C.M.; Fernandez-lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya Sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef]
- Guo, M.; Jiang, W.; Chen, C.; Qu, S.; Lu, J.; Yi, W.; Ding, J. Process Optimization of Biodiesel Production from Waste Cooking Oil by Esterification of Free Fatty Acids Using La3+/ZnO-TiO2 Photocatalyst. Energy Convers. Manag. 2021, 229, 113745. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).