Prospective Life Cycle Assessment and Cost Analysis of Novel Electrochemical Struvite Recovery in a U.S. Wastewater Treatment Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goal, Scope and Functional Unit
2.2. Description of Case Study and Scenarios
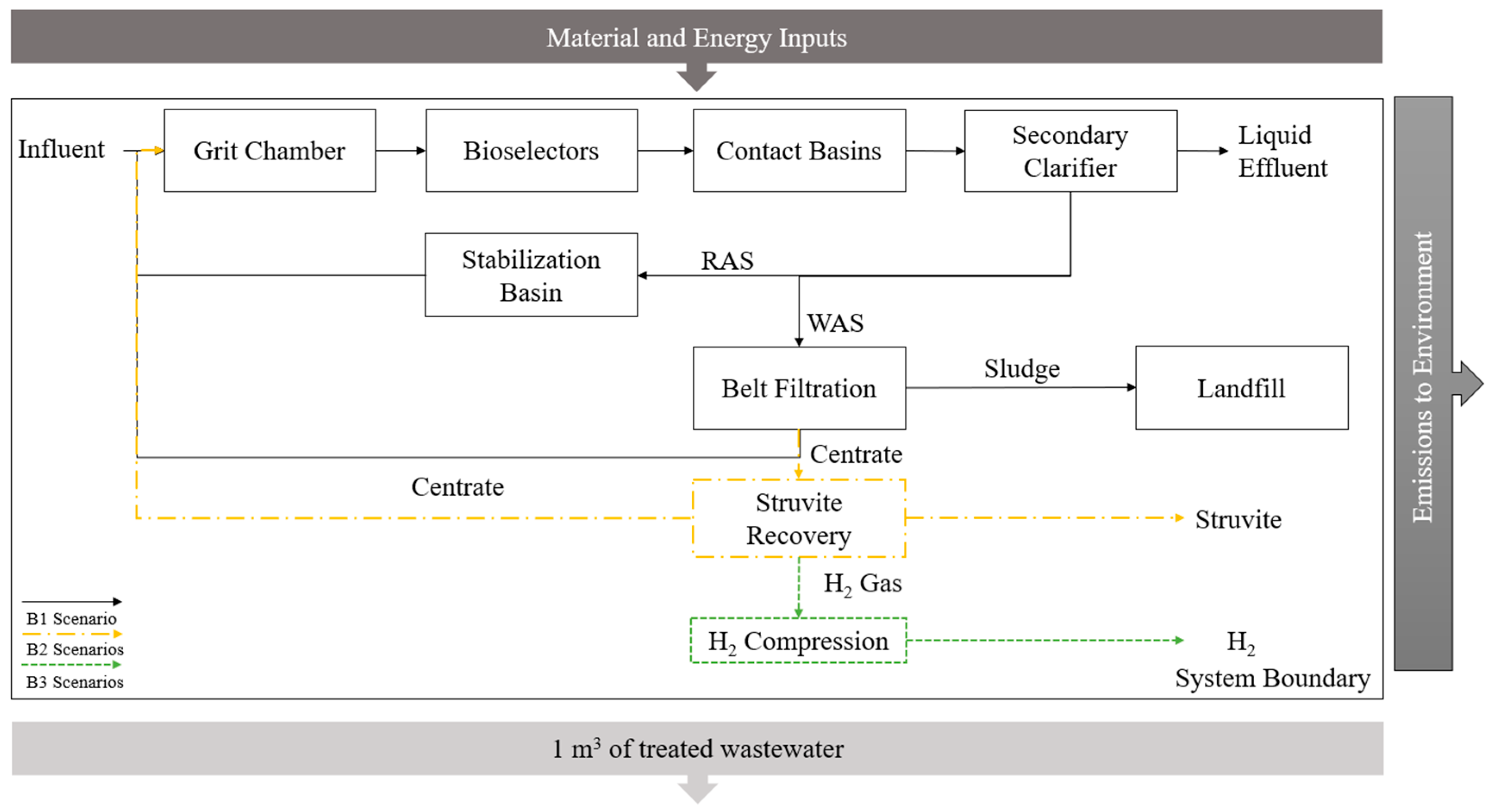
2.3. System Boundary
2.4. Life Cycle Inventory
2.4.1. Plant Infrastructure, Chemicals, and Sludge Disposal
2.4.2. Process Simulation Outputs
2.4.3. Electrochemical Struvite Precipitation Reactor
2.4.4. H2 Collection and Compression
2.4.5. Biogas Combustion and Emissions
2.5. Economic Analysis Methods
2.5.1. Modeling Unit Processes in CapdetWorks
2.5.2. Costs for Struvite and Hydrogen Recovery
2.5.3. Annualization and Functional Unit Conversion
2.5.4. Estimating Potential Revenues and Cost Offsets
3. Results
3.1. GPS-X Model Results
3.2. Life Cycle Impact Assessment Results
3.2.1. Climate Change, Short Term
3.2.2. Freshwater Eutrophication
3.2.3. Terrestrial Acidification
3.2.4. Freshwater Acidification
3.2.5. Mineral Resources Use
3.2.6. Fossil Energy Use
3.2.7. Other Impact Categories
3.3. Economic Analysis and Results
3.3.1. Cost and Revenue Analysis
3.3.2. Break-Even Analysis of Struvite and Hydrogen Production
4. Discussion
4.1. Comparison of Results to Previous Studies
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Jonsson, H.; Stinzing, A.R.; Vinneras, B.; Salomon, E. Guidelines on the Use of Urine and Faeces in Crop Production; EcoSanRes, Stockholm Environment Institute: Stockholm, Sweden, 2004; ISBN 978-91-88714-94-7. [Google Scholar]
- Hallas, J.; Mackowiak, C.; Wilkie, A.; Harris, W. Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater. Sustainability 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- al Rawashdeh, R.; Maxwell, P. The evolution and prospects of the phosphate industry. Miner. Econ. 2011, 24, 15–27. [Google Scholar] [CrossRef]
- Linderholm, K.; Tillman, A.-M.; Mattsson, J.E. Life cycle assessment of phosphorus alternatives for Swedish agriculture. Resour. Conserv. Recycl. 2012, 66, 27–39. [Google Scholar] [CrossRef]
- Ishii, S.K.; Boyer, T.H. Life cycle comparison of centralized wastewater treatment and urine source separation with struvite precipitation: Focus on urine nutrient management. Water Res. 2015, 79, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Tidåker, P.; Sjöberg, C.; Jönsson, H. Local recycling of plant nutrients from small-scale wastewater systems to farmland—A Swedish scenario study. Resour. Conserv. Recycl. 2007, 49, 388–405. [Google Scholar] [CrossRef]
- Amann, A.; Zoboli, O.; Krampe, J.; Rechberger, H.; Zessner, M.; Egle, L. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 2018, 130, 127–139. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, G.; Frison, N.; Vázquez-Padín, J.; Hospido, A.; Garrido, J.; Fatone, F.; Bolzonella, D.; Moreira, M.; Feijoo, G. Life cycle assessment of nutrient removal technologies for the treatment of anaerobic digestion supernatant and its integration in a wastewater treatment plant. Sci. Total Environ. 2014, 490, 871–879. [Google Scholar] [CrossRef]
- Jossa, P.; Remy, C. Life Cycle Assessment of Selected Processes for P Recovery from Sewage Sludge, Sludge Liquor, or Ash. In Sustainable Sewage Sludge Management Fostering Phosphorus Recovery and Energy Efficiency; Kompetenzzentrum Wasser Berlin gGmbH: Berlin, Germany, 2015. [Google Scholar]
- Johansson, K.; Perzon, M.; Fröling, M.; Mossakowska, A.; Svanström, M. Sewage sludge handling with phosphorus utilization–Life cycle assessment of four alternatives. J. Clean. Prod. 2008, 16, 135–151. [Google Scholar] [CrossRef]
- Lundin, M.; Olofsson, M.; Pettersson, G.; Zetterlund, H. Environmental and economic assessment of sewage sludge handling options. Resour. Conserv. Recycl. 2004, 41, 255–278. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [Green Version]
- Sena, M.; Seib, M.; Noguera, D.R.; Hicks, A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 2021, 280, 124222. [Google Scholar] [CrossRef]
- Shaddel, S.; Grini, T.; Ucar, S.; Azrague, K.; Andreassen, J.-P.; Østerhus, S.W. Struvite crystallization by using raw seawater: Improving economics and environmental footprint while maintaining phosphorus recovery and product quality. Water Res. 2020, 173, 115572. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhang, H.; Krampe, J.; Muster, T.; Gao, B.; Zhu, N.; Jin, B. Applying a chemical equilibrium model for optimizing struvite precipitation for ammonium recovery from anaerobic digester effluent. J. Clean. Prod. 2017, 147, 297–305. [Google Scholar] [CrossRef]
- Marchi, A.; Geerts, S.; Saerens, B.; Weemaes, M.; De Clercq, L.; Meers, E. Struvite Recovery from Domestic Wastewater. In Biorefinery of Inorganics; John Wiley & Sons, Ltd.: Ghent, Belgium, 2020; pp. 107–119. ISBN 978-1-118-92148-7. [Google Scholar]
- Koyuncu, S.; Arıman, S. Domestic wastewater treatment by real-scale electrocoagulation process. Water Sci. Technol. 2020, 81, 656–667. [Google Scholar] [CrossRef]
- Hug, A.; Udert, K.M. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2013, 47, 289–299. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Teymouri, A.; Herring, A.M.; Greenlee, L.F. Electrochemical removal and recovery of phosphorus as struvite in an acidic environment using pure magnesium vs. the AZ31 magnesium alloy as the anode. Chem. Eng. J. 2020, 380, 122480. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Abolhassani, M.; Sultana, R.; Anari, Z.; Brye, K.R.; Pollet, B.G.; Greenlee, L.F. The effect of anode degradation on energy demand and production efficiency of electrochemically precipitated struvite. J. Appl. Electrochem. 2022, 52, 205–215. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Moore, J.P.; Abolhassani, M.; Attarzadeh, F.; Hestekin, J.A.; Greenlee, L.F. The Passivating Layer Influence on Mg-Based Anode Corrosion and Implications for Electrochemical Struvite Precipitation. J. Electrochem. Soc. 2019, 166, E358–E364. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Abolhassani, M.; Perez Bakovic, S.I.; Anari, Z.; Moore, J.P., II; Pollet, B.G.; Greenlee, L.F. Electroless Production of Fertilizer (Struvite) and Hydrogen from Synthetic Agricultural Wastewaters. J. Am. Chem. Soc. 2020, 142, 18844–18858. [Google Scholar] [CrossRef] [PubMed]
- Rajaniemi, K.; Hu, T.; Nurmesniemi, E.-T.; Tuomikoski, S.; Lassi, U. Phosphate and Ammonium Removal from Water through Electrochemical and Chemical Precipitation of Struvite. Processes 2021, 9, 150. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; English, L.; Anari, Z.; Abolhassani, M.; Pollet, B.G.; Popp, J.; Greenlee, L.F. Electrochemical nutrient removal from natural wastewater sources and its impact on water quality. Water Res. 2022, 210, 118001. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fu, R.; Lv, H.; Zhu, G.; Lu, B.; Zhou, Z.; Wu, X.; Chen, H. Phosphate Recovery from Swine Wastewater by a Struvite Precipitation Electrolyzer. Sci. Rep. 2019, 9, 8893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, I.; Teymouri, A.; Park, R.; Greenlee, L.F.; Herring, A.M. Simultaneous Electrochemical Nutrient Recovery and Hydrogen Generation from Model Wastewater Using a Sacrificial Magnesium Anode. J. Electrochem. Soc. 2019, 166, E576–E583. [Google Scholar] [CrossRef]
- Cai, Y.; Han, Z.; Lin, X.; Du, J.; Lei, Z.; Ye, Z.; Zhu, J. Mechanisms of releasing magnesium ions from a magnesium anode in an electrolysis reactor with struvite precipitation. J. Environ. Chem. Eng. 2022, 10, 106661. [Google Scholar] [CrossRef]
- Song, Y.-H.; Hidayat, S.; Effendi, A.J.; Park, J.-Y. Simultaneous hydrogen production and struvite recovery within a microbial reverse-electrodialysis electrolysis cell. J. Ind. Eng. Chem. 2021, 94, 302–308. [Google Scholar] [CrossRef]
- Arvidsson, R.; Tillman, A.; Sandén, B.A.; Janssen, M.; Nordelöf, A.; Kushnir, D.; Molander, S. Environmental Assessment of Emerging Technologies: Recommendations for Prospective LCA. J. Ind. Ecol. 2018, 22, 1286–1294. [Google Scholar] [CrossRef] [Green Version]
- Thonemann, N.; Schulte, A.; Maga, D. How to Conduct Prospective Life Cycle Assessment for Emerging Technologies? A Systematic Review and Methodological Guidance. Sustainability 2020, 12, 1192. [Google Scholar] [CrossRef] [Green Version]
- Pre Consultants SimaPro 9.1 2022; Pre Consultants: Amersfoort, The Netherlands, 2022.
- Bulle, C.; Margni, M.; Patouillard, L.; Boulay, A.-M.; Bourgault, G.; De Bruille, V.; Cao, V.; Hauschild, M.; Henderson, A.; Humbert, S.; et al. IMPACT World+: A globally regionalized life cycle impact assessment method. Int. J. Life Cycle Assess. 2019, 24, 1653–1674. [Google Scholar] [CrossRef]
- Jolliet, O.; Margni, M.; Charles, R.; Humbert, S.; Payet, J.; Rebitzer, G.; Rosenbaum, R. IMPACT 2002+: A new life cycle impact assessment methodology. Int. J. Life Cycle Assess. 2003, 8, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Bertanza, G.; Boiocchi, R. Interpreting per capita loads of organic matter and nutrients in municipal wastewater: A study on 168 Italian agglomerations. Sci. Total Environ. 2022, 819, 153236. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Stensel, H.D.; Burton, F.L.; Metcalf & Eddy Inc. Wastewater Engineering: Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2003; ISBN 0-07-041878-0. [Google Scholar]
- Bradford-Hartke, Z.; Lane, J.; Lant, P.; Leslie, G. Environmental Benefits and Burdens of Phosphorus Recovery from Municipal Wastewater. Environ. Sci. Technol. 2015, 49, 8611–8622. [Google Scholar] [CrossRef] [PubMed]
- Corominas, L.; Foley, J.; Guest, J.; Hospido, A.; Larsen, H.; Morera, S.; Shaw, A. Life cycle assessment applied to wastewater treatment: State of the art. Water Res. 2013, 47, 5480–5492. [Google Scholar] [CrossRef] [PubMed]
- Doka, G. A Model for Composition-Specific Life Cycle Inventories of Regionalised Wastewater Fates; Swiss Federal Office for the Environment (FOEN): Berne, Switzerland; Zurich, Switzerland, 2021. [Google Scholar]
- Whiting, A.; Azapagic, A. Life cycle environmental impacts of generating electricity and heat from biogas produced by anaerobic digestion. Energy 2014, 70, 181–193. [Google Scholar] [CrossRef]
- Doka, G. Calculation Manual for LCI Calculation Tools for Regionalised Waste Treatment; Doka Life Cycle Assessments: Zurich, Switzerland, 2021. [Google Scholar]
- Lundin, M.; Bengtsson, M.; Molander, S. Life Cycle Assessment of Wastewater Systems: Influence of System Boundaries and Scale on Calculated Environmental Loads. Environ. Sci. Technol. 2000, 34, 180–186. [Google Scholar] [CrossRef]
- Pryce, D.; Memon, F.A.; Kapelan, Z. Life cycle analysis approach to comparing environmental impacts of alternative materials used in the construction of small wastewater treatment plants. Environ. Adv. 2021, 4, 100065. [Google Scholar] [CrossRef]
- Nagels, M.; Verhoeven, B.; Larché, N.; Dewil, R.; Rossi, B. Comparative life cycle cost assessment of (lean) duplex stainless steel in wastewater treatment environments. J. Environ. Manag. 2022, 306, 114375. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis–A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Gay, S.W.; Knowlton, K.F. Ammonia Emissions and Animal Agriculture. In Virginia Cooperative Extension; Virginia Polytechnic Institute and State University, College of Agriculture and Life Sciences: Blacksburg, VA, USA, 2009. [Google Scholar]
- Hacatoglu, K.; Rosen, M.A.; Dincer, I. Comparative life cycle assessment of hydrogen and other selected fuels. Int. J. Hydrog. Energy 2012, 37, 9933–9940. [Google Scholar] [CrossRef]
- Granovskii, M.; Dincer, I.; Rosen, M.A. Life cycle assessment of hydrogen fuel cell and gasoline vehicles. Int. J. Hydrog. Energy 2006, 31, 337–352. [Google Scholar] [CrossRef]
- Darrow, K.; Tidball, R.; Wang, J.; Hampson, A. Catalog of CHP Technologies; U.S. Environmental Protection Agency, Combined Heat and Power Partnership: Washington, DC, USA, 2017.
- Nielsen, M.; Nielsen, O.-K.; Plejdrup, M.S. Danish Emission Inventories for Stationary Combustion Plants; Aarhus University, DCE–Danish Centre for Environment and Energy: Aarhus, Denmark, 2018. [Google Scholar]
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental impact of biogas: A short review of current knowledge. J. Environ. Sci. Health Part A 2018, 53, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, R.; Benisch, M.; Clark, D.; Sprick, R.G. Struvite Control–A Common and Nuisance. Proc. Water Environ. Fed. 2002, 2002, 480–495. [Google Scholar] [CrossRef]
- Parsons, S.; Doyle, J. Struvite scale formation and control. Water Sci. Technol. 2004, 49, 177–182. [Google Scholar] [CrossRef]
- Abbasi, N.; Ahmadi, M.; Naseri, M. Quality and cost analysis of a wastewater treatment plant using GPS-X and CapdetWorks simulation programs. J. Environ. Manag. 2021, 284, 111993. [Google Scholar] [CrossRef]
- Arif, A.U.A.; Sorour, M.T.; Aly, S.A. Cost analysis of activated sludge and membrane bioreactor WWTPs using CapdetWorks simulation program: Case study of Tikrit WWTP (middle Iraq). Alex. Eng. J. 2020, 59, 4659–4667. [Google Scholar] [CrossRef]
- Duan, M.; O’Dwyer, E.; Stuckey, D.C.; Guo, M. Wastewater to Resource: Design of a Sustainable Phosphorus Recovery System. ChemistryOpen 2019, 8, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.; (Beshears Construction, Fort Smith, AR, USA). Personal Communication, 2022.
- U.S. Bureau of Labor Statistics Sebastian County 2021 Annual Average Weekly Wage for NAICS 1012 Construction. Available online: https://data.bls.gov/cew/apps/data_views/data_views.htm#tab=Tables (accessed on 28 June 2022).
- The City of Fort Smith. 2022 Proposed Operating Budget. Available online: https://www.fortsmithar.gov/index.php?option=com_jdownloads&view=category&catid=56&Itemid=566 (accessed on 21 September 2022).
- Alameda County Water District. Authorization of Agreement for the Purchase of Ferric Chloride for Water Treatment. Available online: https://www.acwd.org/AgendaCenter/ViewFile/Minutes/_07222021-1076#:~:text=District%20staff%20received%20one%20response,price%20of%20%24823%2Fdry%20ton (accessed on 21 September 2022).
- Bray, E.L. Mineral Commodity Summaries 2022–Magnesium Metal; U.S. Geological Survey: Reston, VA, USA, 2022; p. 2.
- Damodaran, A. Margins by Sector (US). Available online: https://pages.stern.nyu.edu/~adamodar/New_Home_Page/datafile/margin.html (accessed on 28 June 2022).
- U.S. Bureau of Labor Statistics. Producer Price Index–Metals and Metal Products: Mid–Atlantic Information Office: U.S. Bureau of Labor Statistics. Available online: https://www.bls.gov/regions/mid-atlantic/data/producerpriceindexmetals_us_table.htm (accessed on 28 June 2022).
- U.S. Energy Information Administration. Electric Power Monthly–U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/electricity/monthly/epm_table_grapher.php (accessed on 28 June 2022).
- U.S. Energy Information Administration. Arkansas Natural Gas Prices. Available online: https://www.eia.gov/dnav/ng/NG_PRI_SUM_DCU_SAR_M.htm (accessed on 2 September 2022).
- Larson, W. New Estimates of Value of Land of the United States; U.S. Department of Commerce, Bureau of Economic Analysis: Washington, DC, USA, 2015; p. 30.
- U.S. Federal Housing Finance Agency. All-Transactions House Price Index for Arkansas. Available online: https://fred.stlouisfed.org/series/ARSTHPI (accessed on 28 June 2022).
- Key, J.; P Street Wastewater Treatment Plant, Fort Smith, AR, USA. Personal Communication, 2021.
- City of Fort Smith: Solid Waste Services. Available online: https://solidwaste.fortsmithar.gov/landfill/ (accessed on 2 September 2022).
- CapdetWorks, version 4.0; Wastewater Modeling Software; Hydromantis Environmental Software Solutions, Inc.: Hamilton, ON, Canada, 2018.
- CapdetWorks, version 4.0; State-of-the-Art Software for the Design and Cost Estimation of Wastewater Treatment Plants, User’s Guide; Hydromantis Environmental Solutions, Inc.: Hamilton, ON, Canada, 2018.
- Ostara, Inc. Nutrient Recovery Technology Customized to Meet Your Needs. Available online: http://ostara.com/wp-content/uploads/2018/08/Ostara_Pearl_-Handout-180424.pdf (accessed on 21 September 2022).
- Seymour, D. Can Nutrient Recovery Be a Financially Sustainable Development Objective? In Proceedings of the Annual Conference, Pacific Northwest Clean Water Association, Boise, ID, USA, 15 September 2009. [Google Scholar]
- Khan, M.A.; Young, C.; MacKinnon, C.; Layzell, D.B. Technical Brief: The Techno-Economics of Hydrogen Compression; The Transition Accelerator: Calgary, AB, Canada, 2021. [Google Scholar]
- Rustagi, N.; Elgowairy, A.; Gupta, E. DOE Hydrogen and Fuel Cells Program Record–Hydrogen Delivery Cost Projections–2015; Department of Energy, United States of America: Washington, DC, USA, 2016. [Google Scholar]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Zahid, W.M. Cost Analysis of Trickling-Filtration and Activated-Sludge Plants for the Treatment of Municipal Wastewater. In Proceedings of the 7th Saudi engineering conference, Riyadh, Saudi Arabia, 2–5 December 2007; Volume 25. [Google Scholar]
- Quinn, R. Most Fertilizer Prices Continue to Move Lower. Available online: https://www.dtnpf.com/agriculture/web/ag/news/crops/article/2022/06/22/fertilizer-prices-continue-move (accessed on 28 June 2022).
- McKinney, J.; Bond, E.; Crowell, M.; Odufuwa, E. Joint Agency Staff Report on Assembly Bill 8: 2021 Annual Assessment of Time and Cost Needed to Attain 100 Hydrogen Refueling Stations in California; California Energy Commission: Sacramento, CA, USA, 2015; p. 121. [Google Scholar]
- Baronas, J.; Achtelik, G. Joint Agency Staff Report on Assembly Bill 8: 2019 Annual Assessment of Time and Cost Needed to Attain 100 Hydrogen Refueling Stations in California; California Energy Commission: Sacramento, CA, USA, 2019; p. 98. [Google Scholar]
- Benckiser, G.; Eilts, R.; Linn, A.; Lorch, H.-J.; Sümer, E.; Weiske, A.; Wenzhöfer, F. N2O emissions from different cropping systems and from aerated, nitrifying and denitrifying tanks of a municipal waste water treatment plant. Biol. Fertil. Soils 1996, 23, 257–265. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Ilhan, F.; Kocak, E.; Akbin, H.M. Feasibility of struvite recovery process for fertilizer industry: A study of financial and economic analysis. J. Clean. Prod. 2017, 152, 88–102. [Google Scholar] [CrossRef]
- Sena, M.; Rodriguez Morris, M.; Seib, M.; Hicks, A. An exploration of economic valuation of phosphorus in the environment and its implications in decision making for resource recovery. Water Res. 2020, 172, 115449. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Unit | Value |
|---|---|---|
| Average Flow | m3/day | 45,425 |
| TSS 1 | g/m3 | 215 |
| cBOD5 2 | g/m3 | 250 |
| COD 1 | g/m3 | 431 |
| Ammonia N | g/m3 | 11.0 |
| Nitrite N 2 | g/m3 | 0 |
| Nitrate N 2 | g/m3 | 0 |
| TKN 1 | g/m3 | 15.7 |
| Soluble PO4-P 1 | g P/m3 | 4.8 |
| TP | g P/m3 | 6.0 |
| Total Alkalinity 2 | g CaCO3/m3 | 250 |
| Soluble Mg 2 | g/m3 | 50 |
| pH 2 | - | 7.0 |
| DO 2 | g O2/m3 | 0 |
| Liquid Temperature | °C | 20 |
| Unit Process | Design Parameters | Values |
|---|---|---|
| Grit chamber | Grit production per flow (m3/L) Dry solid content of grit (%) | 20 98 |
| Anoxic reactors (Bioselectors) | Tank depth (m) Maximum volume (m3) | 4 2650 1 |
| Plug-flow contact basins | Number of reactors in series Tank depth (m) Maximum volume (m3) | 3 4 6321 |
| Plug-flow stabilization basins | Number of reactors in series Tank depth (m) Maximum volume (m3) | 3 4 3142 |
| Secondary clarifiers | Surface area (m2) Tank depth (m) RAS/WAS 3 pumped flow (m3/day) | 2917.2 2 3 22,712 |
| RAS/WAS pumping station | WAS pumped flow (m3/day) | 378.5 |
| Dewatering | Underflow solids (mg/L) Solids removal efficiency (%) | 200,000 95 |
| Disinfection | Volume (m3) Chlorine dosage (mg/L) | 844 6.0 |
| Gravity thickener 4 | Surface area (m2) Tank depth (m) Underflow solids (mg/L) Removal efficiency (%) | 154.2 3 48,000 90 |
| Anaerobic digester 4 | Maximum volume (m3) Temperature (°C) | 1500 35 |
| Electrochemical struvite Reactor 5 | Section S1a–d | - |
| Solids separation 5 | Pumped flow (m3/day) Struvite removal efficiency (%) | 1 99 |
| Scenario | Description | % Struvite Yield | H2 Capture | Anaerobic Digestor |
|---|---|---|---|---|
| B1 | Existing treatment scheme in Ft. Smith | 0 | No | No |
| B2-45 | Existing treatment scheme with struvite production | 45 | No | No |
| B2-90 | Existing treatment scheme with struvite production | 90 | No | No |
| B3-45 | Existing treatment scheme with struvite production and H2 capture | 45 | Yes | No |
| B3-90 | Existing treatment scheme with struvite production and H2 capture | 90 | Yes | No |
| A1 | Existing treatment scheme with added anaerobic digestor | 0 | No | Yes |
| A2-45 | Added anaerobic digestor with struvite recovery | 45 | No | Yes |
| A3-90 | Added anaerobic digestor with struvite recovery | 90 | No | Yes |
| A3-45 | Added anaerobic digestor with struvite recovery and H2 capture | 45 | Yes | Yes |
| A3-90 | Added anaerobic digestor with struvite recovery and H2 capture | 90 | Yes | Yes |
| Cost Parameters | Value | Units | Source |
|---|---|---|---|
| Reactor | 1.73 | $ | [74,75] |
| Building Space | 0.43 | $ | [74,75] |
| Labor | 0.006 | h | [74,75] |
| Maintenance | 0.23 | $ | [74,75] |
| Mg (99.9%) | 3.24 | $ | [63,64,65] |
| Energy | 0.29 | $ | [66] |
| By-Product | B1 | B2-45 | B2-90 | B3-45 | B3-90 | A1 | A2-45 | A2-90 | A3-45 | A3-90 |
|---|---|---|---|---|---|---|---|---|---|---|
| Struvite (kg/day) | - | 0.013 | 0.026 | 0.013 | 0.026 | 0 | 389 | 702 | 389 | 702 |
| Hydrogen gas (L/day) | - | - | - | 1.81 | 3.59 | - | - | - | 52,330 | 94,500 |
| Electricity, combustion of biogas (kWh) | - | - | - | - | - | 89.1 | 88.3 | 89.7 | 88.3 | 89.7 |
| Heat, combustion of biogas (kWh) | - | - | - | - | - | 124.4 | 123.2 | 125.3 | 123.2 | 125.3 |
| Phosphorus recovered in struvite (%) | - | ~0 | ~0 | ~0 | ~0 | - | 18 | 33 | 18 | 33 |
| Cost Category | B1 | B2-45 | B2-90 | B3-45 | B3-90 | A1 | A2-45 | A2-90 | A3-45 | A3-90 |
|---|---|---|---|---|---|---|---|---|---|---|
| Capital Costs | ||||||||||
| Standard Processes | $3,688,420 | $3,688,420 | $3,688,420 | $3,688,420 | $3,688,420 | $3,785,057 | $3,607,614 | $3,605,763 | $3,605,787 | $3,604,067 |
| Sludge Management | $60,357 | $60,357 | $60,357 | $60,357 | $60,357 | $46,189 | $44,797 | $44,797 | $44,797 | $44,797 |
| Anaerobic Digestion | $0 | $0 | $0 | $0 | $0 | $144,679 | $124,041 | $124,041 | $124,041 | $124,041 |
| ECST Reactor | $0 | $11 | $21 | $11 | $21 | $0 | $307,108 | $554,500 | $307,108 | $554,500 |
| H2 Compression | $0 | $0 | $0 | $119 | $162 | $0 | $0 | $0 | $13,401 | $17,590 |
| H2 Storage | $0 | $0 | $0 | $0 | $1 | $0 | $0 | $0 | $11,114 | $20,066 |
| Total Capital Cost | $3,748,777 | $3,748,788 | $3,748,798 | $3,748,907 | $3,748,961 | $3,975,925 | $4,083,560 | $4,329,101 | $4,106,248 | $4,365,062 |
| Operational Costs | ||||||||||
| O&M | $485,400 | $485,402 | $485,403 | $485,447 | $485,465 | $540,000 | $560,570 | $599,696 | $565,684 | $606,408 |
| Materials | $598,063 | $598,596 | $599,483 | $598,596 | $599,483 | $530,248 | $493,634 | $482,719 | $495,763 | $485,559 |
| Chemicals | $141,000 | $141,016 | $141,031 | $141,016 | $141,031 | $317,015 | $598,327 | $968,341 | $598,327 | $968,341 |
| Energy | $202,720 | $204,248 | $203,786 | $204,248 | $203,786 | $171,036 | $212,991 | $240,074 | $213,828 | $241,583 |
| Total Operational Cost | $1,427,183 | $1,429,261 | $1,429,704 | $1,429,306 | $1,429,766 | $1,558,299 | $1,865,522 | $2,290,829 | $1,873,603 | $2,301,891 |
| Potential Revenues | ||||||||||
| Struvite Fertilizer a | $0 | $6 | $11 | $6 | $11 | $0 | $163,627 | $295,438 | $163,627 | $295,438 |
| H2 Gas b | $0 | $0 | $0 | $0 | $1 | $0 | $0 | $0 | $12,624 | $22,793 |
| Total Revenue | $0 | $6 | $11 | $6 | $12 | $0 | $163,627 | $295,438 | $176,251 | $318,230 |
| Net Cost | ||||||||||
| $/year | $5,175,960 | $5,178,043 | $5,178,491 | $5,178,207 | $5,178,715 | $5,534,224 | $5,785,455 | $6,324,493 | $5,803,600 | $6,348,722 |
| $/m3 | $0.31 | $0.31 | $0.31 | $0.31 | $0.31 | $0.33 | $0.35 | $0.38 | $0.35 | $0.38 |
| Struvite 1 | Hydrogen 2 | |
|---|---|---|
| ($/kg) | ($/kg) | |
| CAPEX | 2.16 | 13.22 |
| O&M | 0.34 | 2.36 |
| Magnesium Metal (99.9%) | 3.24 | - |
| Energy (electricity) | 0.29 | 0.01 |
| Break-Even Price | 6.03 | 15.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrissey, K.G.; English, L.; Thoma, G.; Popp, J. Prospective Life Cycle Assessment and Cost Analysis of Novel Electrochemical Struvite Recovery in a U.S. Wastewater Treatment Plant. Sustainability 2022, 14, 13657. https://doi.org/10.3390/su142013657
Morrissey KG, English L, Thoma G, Popp J. Prospective Life Cycle Assessment and Cost Analysis of Novel Electrochemical Struvite Recovery in a U.S. Wastewater Treatment Plant. Sustainability. 2022; 14(20):13657. https://doi.org/10.3390/su142013657
Chicago/Turabian StyleMorrissey, Karla G., Leah English, Greg Thoma, and Jennie Popp. 2022. "Prospective Life Cycle Assessment and Cost Analysis of Novel Electrochemical Struvite Recovery in a U.S. Wastewater Treatment Plant" Sustainability 14, no. 20: 13657. https://doi.org/10.3390/su142013657
APA StyleMorrissey, K. G., English, L., Thoma, G., & Popp, J. (2022). Prospective Life Cycle Assessment and Cost Analysis of Novel Electrochemical Struvite Recovery in a U.S. Wastewater Treatment Plant. Sustainability, 14(20), 13657. https://doi.org/10.3390/su142013657






