Mechanical Strength of Saline Sandy Soils Stabilized with Alkali-Activated Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Characterization of Microstructure
2.3. Binders and Alkali Activator Characterization
2.4. Sample Preparation
2.5. Unconfined Compressive Strength (UCS) Test
3. Results
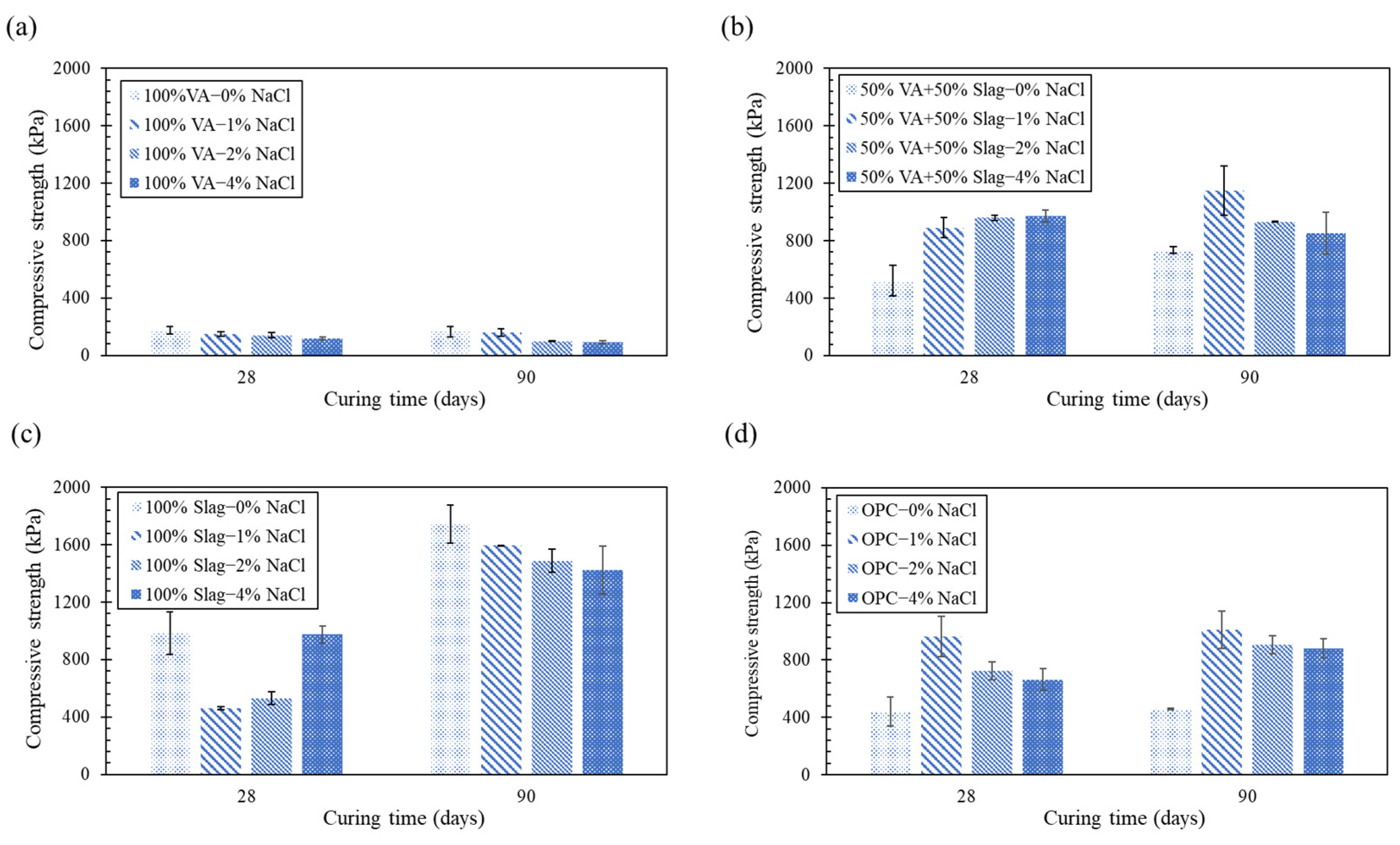
3.1. Unconfined Compressive Strength (UCS) Tests
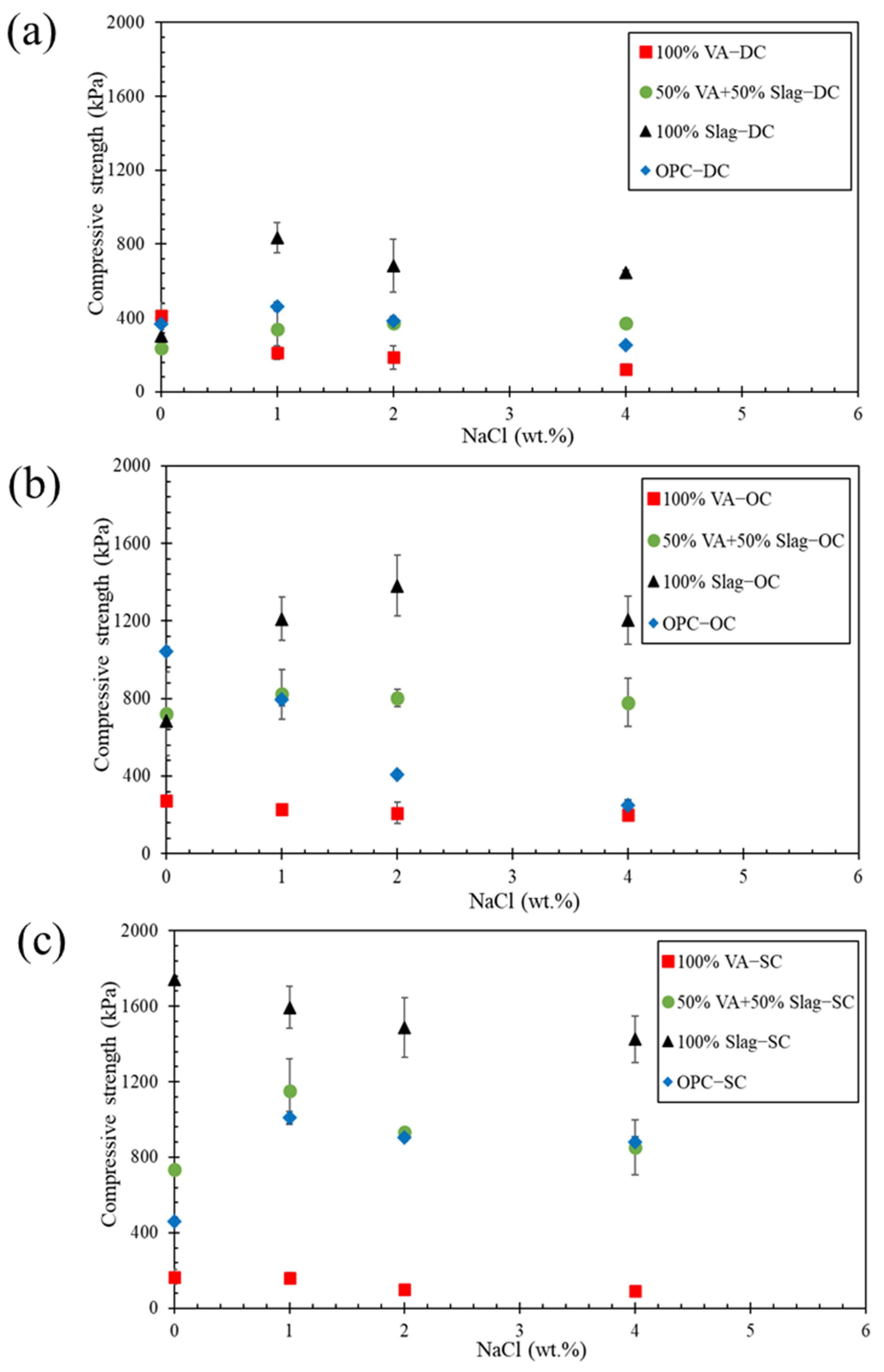
3.1.1. DC Condition
3.1.2. OC Condition
3.1.3. SC Condition
3.1.4. The Effect of Curing Condition on UCS
3.2. Microstructural Analysis
3.2.1. XRD Analysis
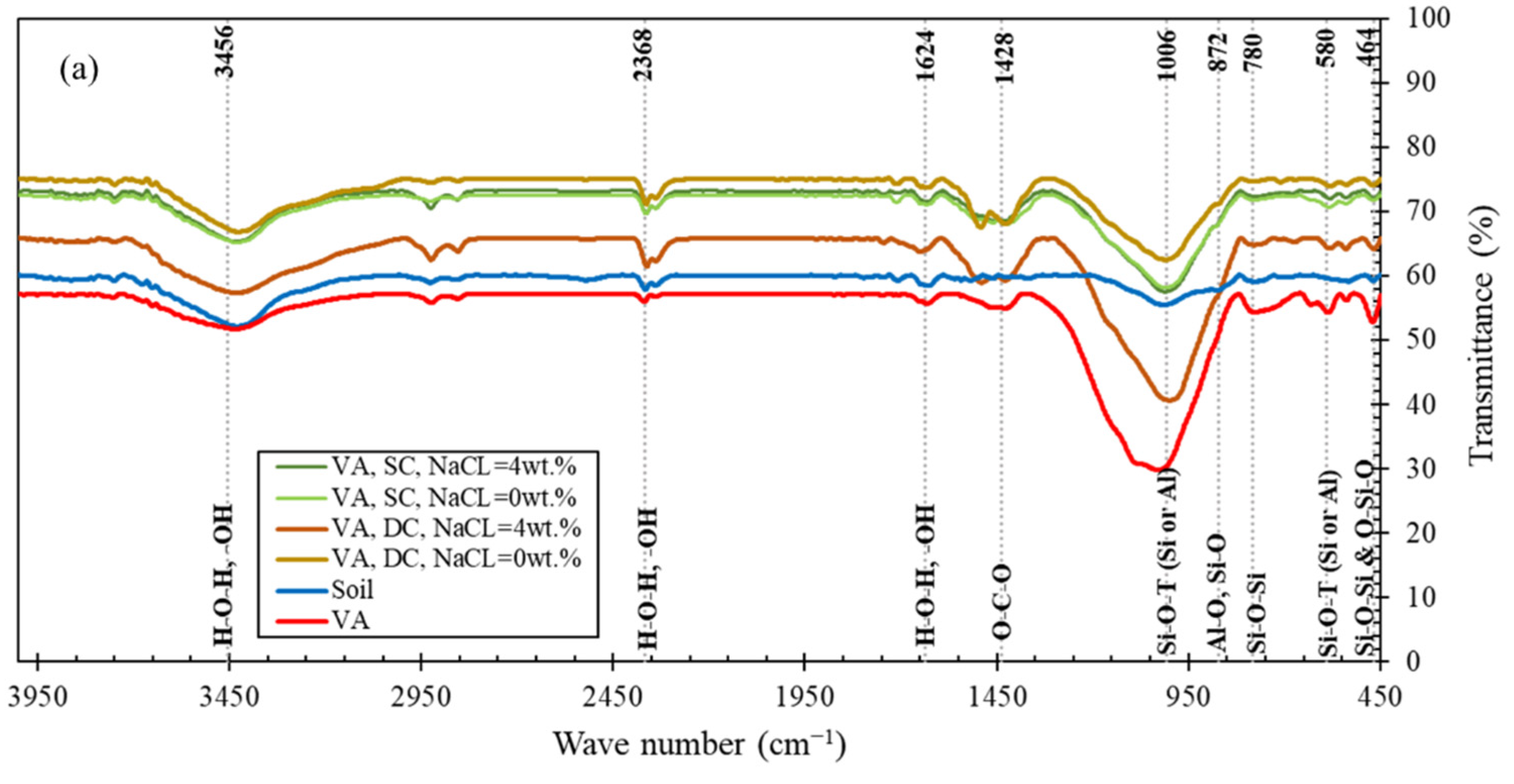
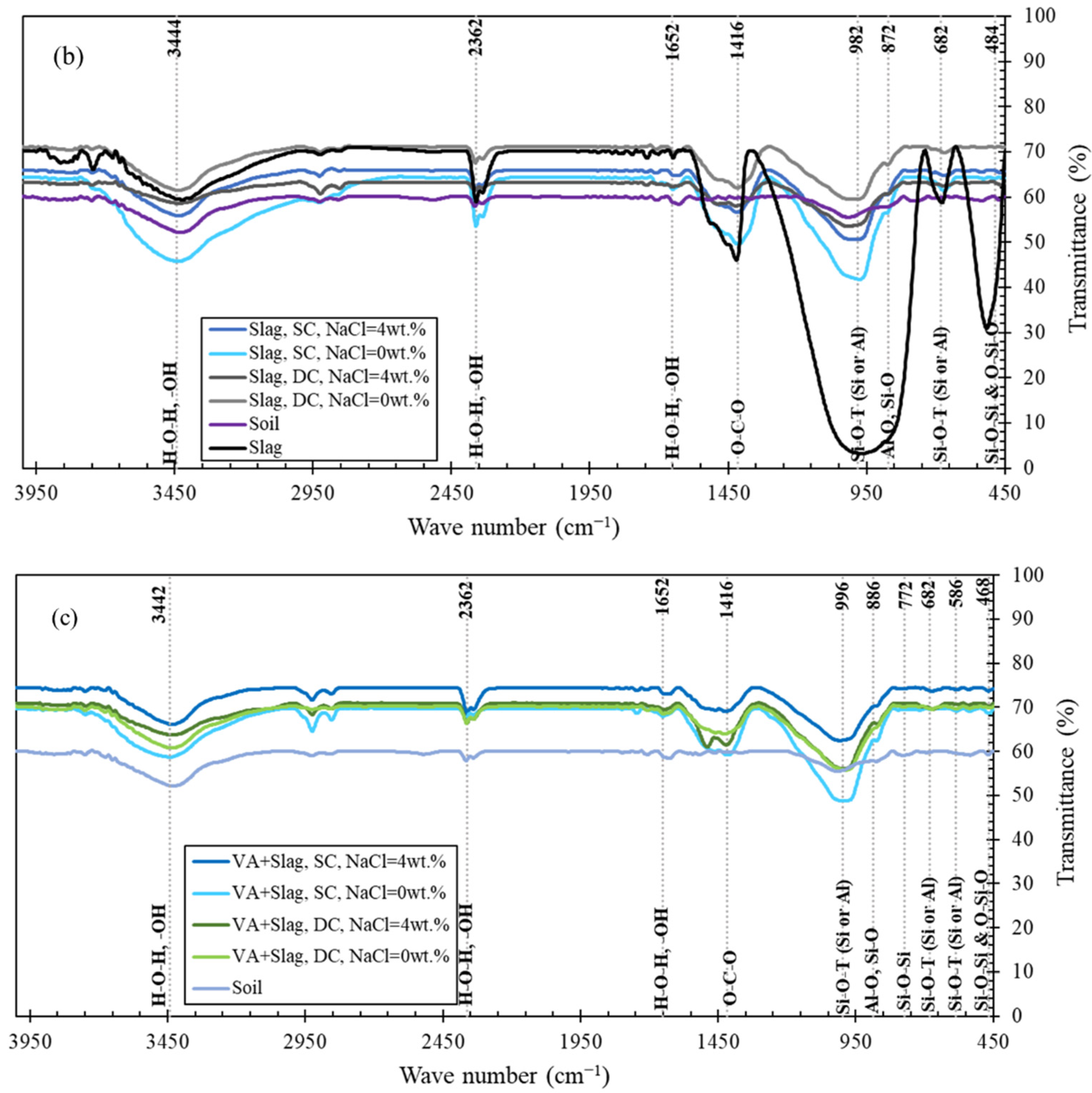
3.2.2. FTIR Analysis
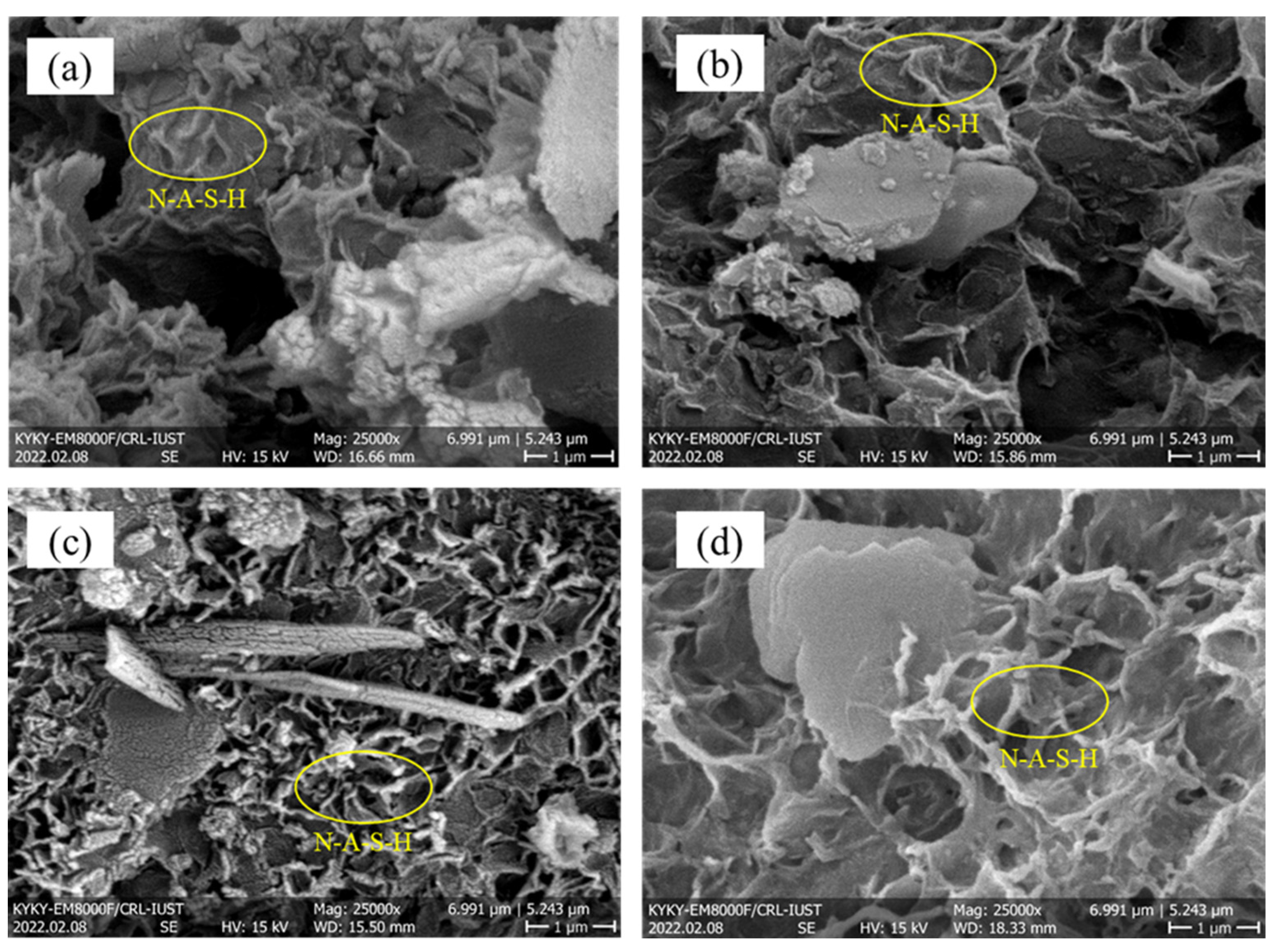
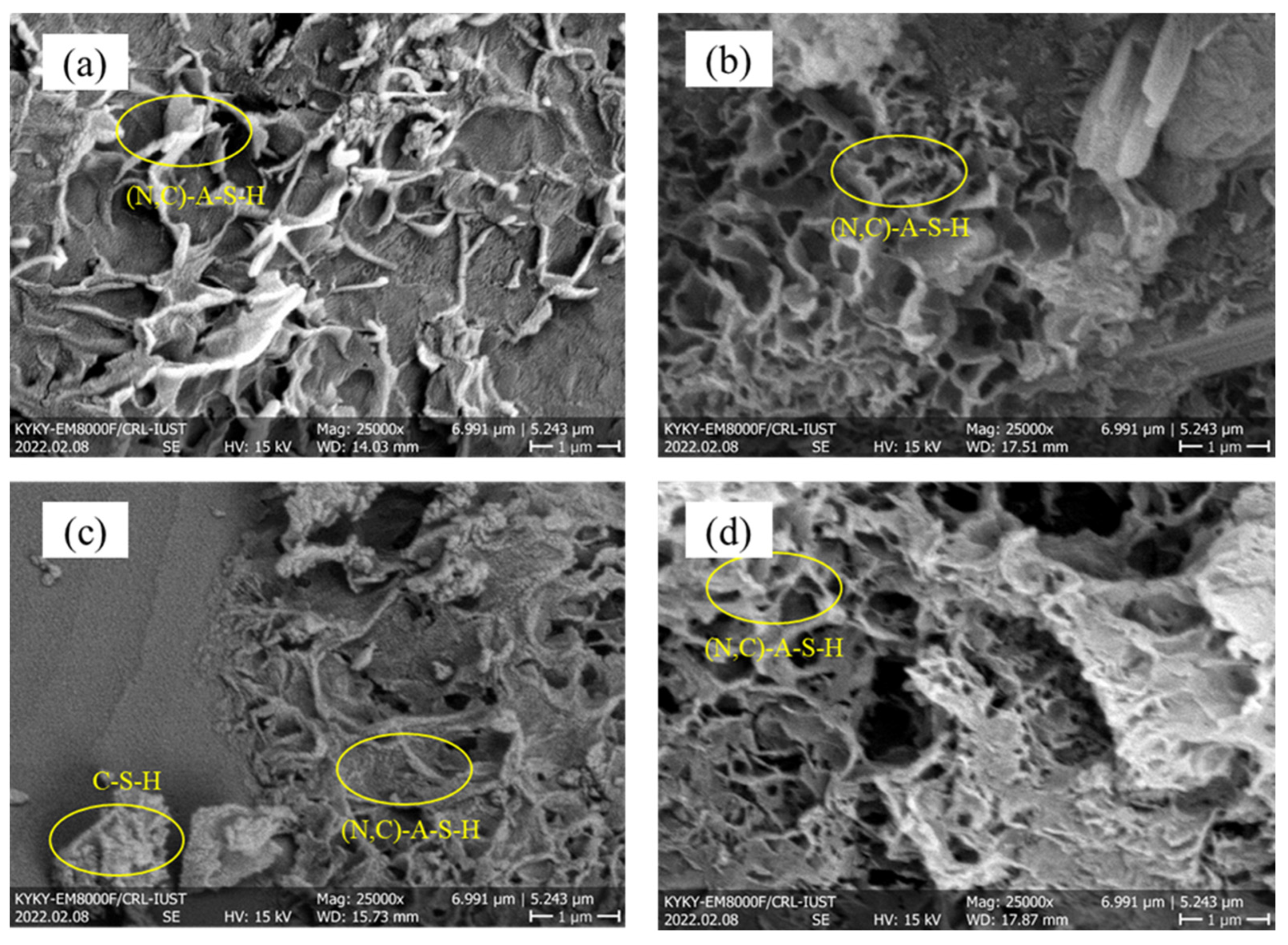
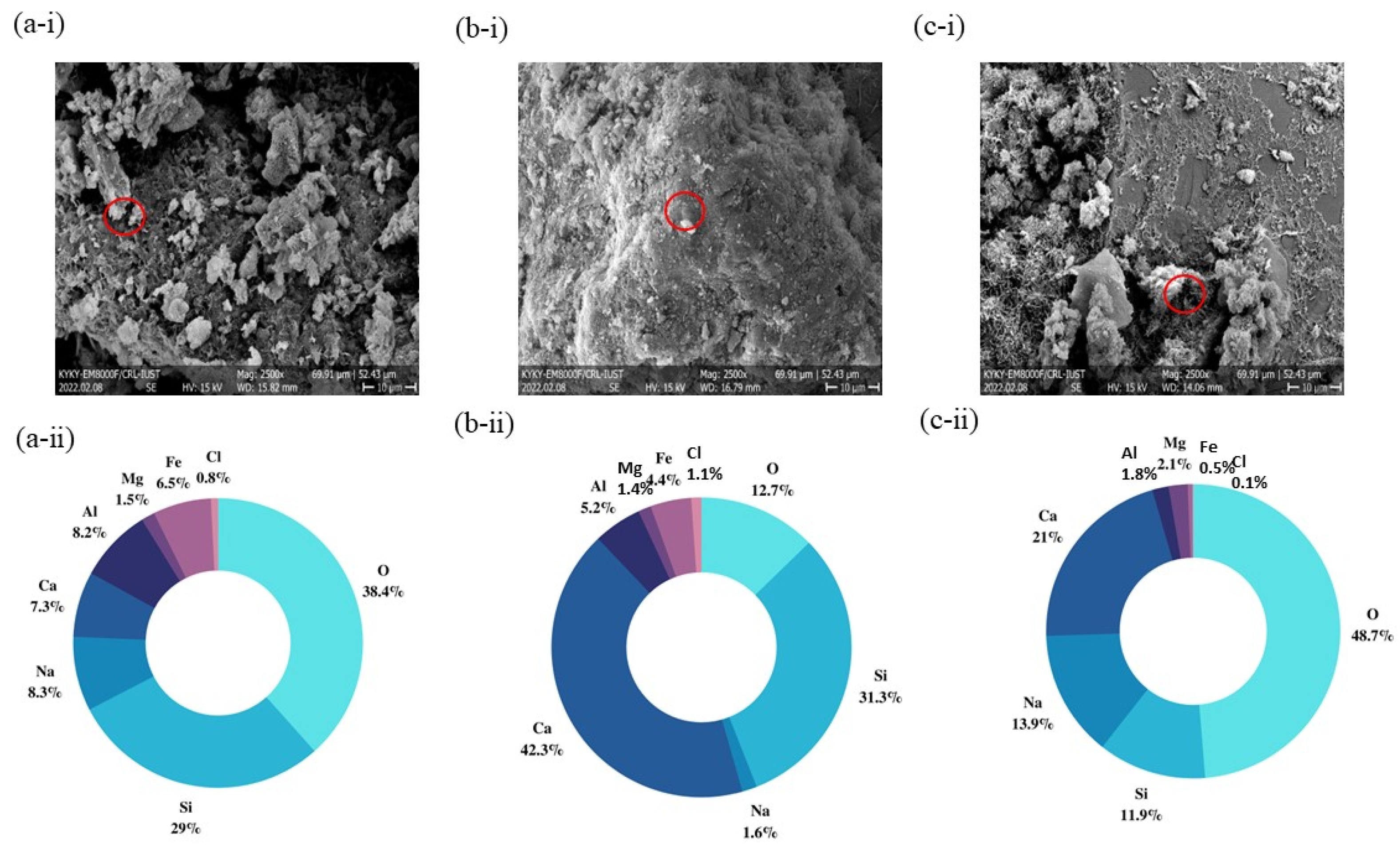
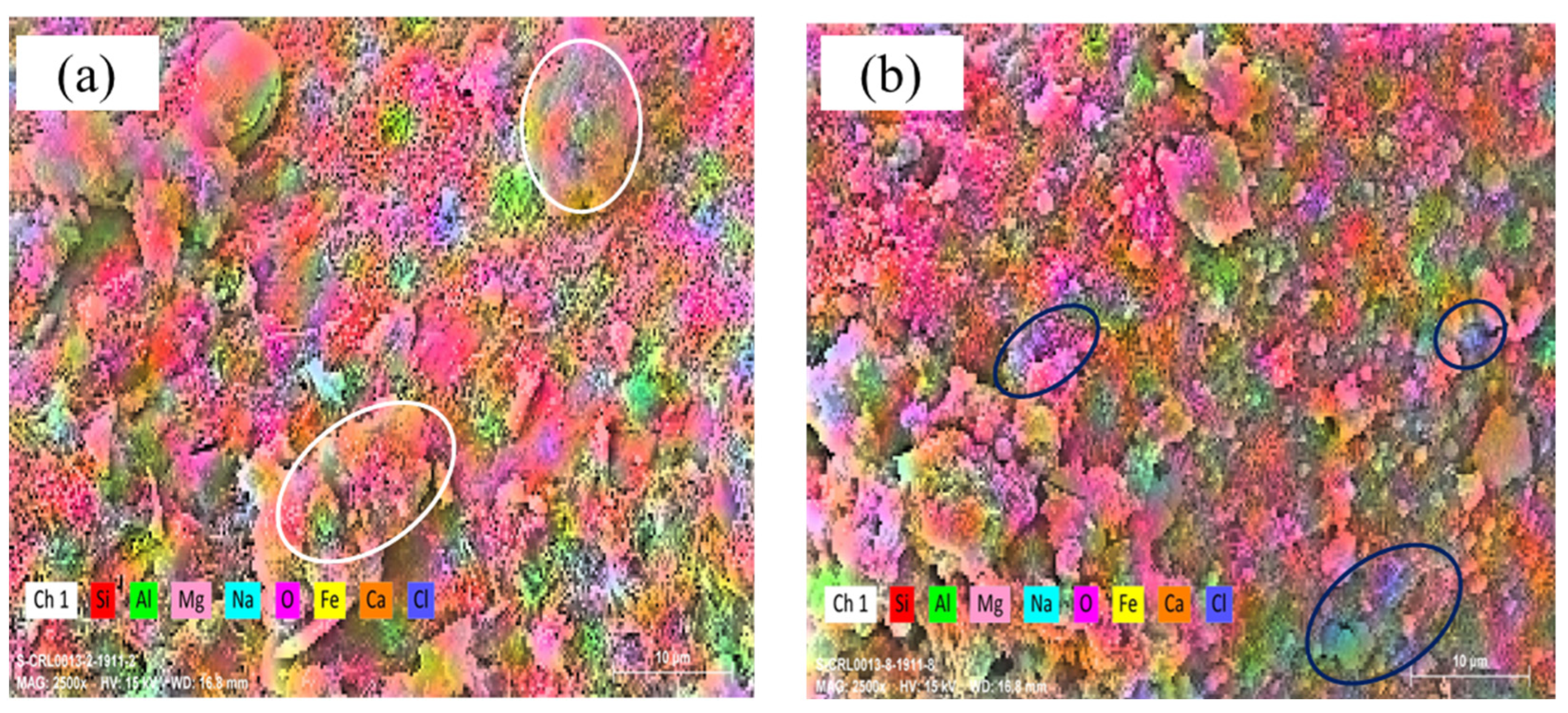
3.2.3. SEM–EDS Mapping Analysis
4. Conclusions
- In soil samples stabilized with alkali-activated volcanic ash, adding sodium chloride from 0 to 4 wt.% decreased the long-term (90 days) strength up to 70% in all three curing conditions. Meanwhile, the strength of soil specimens stabilized with alkali-activated slag increased up to 244% when the sodium chloride content was raised by 1 wt.%, and adding more sodium chloride content (up to 4 wt.%) had a nuanced effect on the strength under all curing conditions.
- In soil samples stabilized with alkali-activated volcanic ash, the density of N-A-S-H geopolymer gel in specimens with sodium chloride was higher than specimens without sodium chloride, which was consistent with the results of the XRD and FTIR tests.
- In stabilized soil samples containing slag and volcanic ash, the surface adsorption mechanism of chloride ion by N-A-S-H gel and the chemical encapsulation mechanism of chloride ion by (N, C)-A-S-H and C-S-H gels occurred simultaneously.
- It can be concluded that alkali-activated slag cement can be a sustainable alternative to Portland cement for saline soil stabilization.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuisinier, O.; Le Borgne, T.; Deneele, D.; Masrouri, F. Quantification of the effects of nitrates, phosphates and chlorides on soil stabilization with lime and cement. Eng. Geol. 2011, 117, 229–235. [Google Scholar] [CrossRef]
- Ying, Z.; Cui, Y.-J.; Benahmed, N.; Duc, M. Salinity assessment for salted soil considering both dissolved and precipitated salts. Geotech. Test. J. 2020, 44, 9-p. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. Influence of metakaolin and limestone on chloride binding of slag activated by mixed magnesium oxide and sodium hydroxide. Cem. Concr. Compos. 2021, 127, 104397. [Google Scholar] [CrossRef]
- Ghavami, S.; Jahanbakhsh, H.; Saeedi Azizkandi, A.; Moghadas Nejad, F. Influence of sodium chloride on cement kiln dust-treated clayey soil: Strength properties, cost analysis, and environmental impact. Environ. Dev. Sustain. 2021, 23, 683–702. [Google Scholar] [CrossRef]
- Mishra, P.N.; Scheuermann, A.; Bore, T.; Li, L. Salinity effects on soil shrinkage characteristic curves of fine-grained geomaterials. J. Rock Mech. Geotech. Eng. 2019, 11, 181–191. [Google Scholar] [CrossRef]
- Armistead, S.J.; Smith, C.C.; Staniland, S.S. Sustainable biopolymer soil stabilization in saline rich, arid conditions: A ‘micro to macro’approach. Sci. Rep. 2022, 12, 2880. [Google Scholar] [CrossRef] [PubMed]
- Khoshsirat, V.; Bayesteh, H.; Sharifi, M. Effect of high salinity in grout on the performance of cement-stabilized marine clay. Constr. Build. Mater. 2019, 217, 93–107. [Google Scholar] [CrossRef]
- Barman, D.; Dash, S.K. Stabilization of expansive soils using chemical additives: A review. J. Rock Mech. Geotech. Eng. 2022, 14, 1319–1342. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Yang, C.; Chu, C.; Tan, X. Effect of chloride attack on strength and leaching properties of solidified/stabilized heavy metal contaminated soils. Eng. Geol. 2018, 246, 28–35. [Google Scholar] [CrossRef]
- Ying, Z.; Cui, Y.-J.; Benahmed, N.; Duc, M. Salinity effect on the compaction behaviour, matric suction, stiffness and microstructure of a silty soil. J. Rock Mech. Geotech. Eng. 2021, 13, 855–863. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, M.S.; Mondal, B.C.; Das, A. Strength behavior of mortar using slag with cement in sea water environment. J Civ Eng-IEB 2009, 37, 111–122. [Google Scholar]
- Kaushik, S.; Islam, S. Suitability of sea water for mixing structural concrete exposed to a marine environment. Cem. Concr. Compos. 1995, 17, 177–185. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Weiss, J. Damage in cement pastes exposed to NaCl solutions. Constr. Build. Mater. 2018, 171, 120–127. [Google Scholar] [CrossRef]
- Xing, H.; Yang, X.; Xu, C.; Ye, G. Strength characteristics and mechanisms of salt-rich soil–cement. Eng. Geol. 2009, 103, 33–38. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Z.; Fan, L.; Liu, S.; Liu, W. Evaluation of the influence of salt concentration on cement stabilized clay by electrical resistivity measurement method. Eng. Geol. 2014, 170, 80–88. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phojan, W.; Suddeepong, A.; Chinkulkijniwat, A.; Liu, M.D. Strength development in blended cement admixed saline clay. Appl. Clay Sci. 2012, 55, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Dingwen, Z.; Libin, F.; Songyu, L.; Yongfeng, D. Experimental investigation of unconfined compression strength and stiffness of cement treated salt-rich clay. Mar. Georesources Geotechnol. 2013, 31, 360–374. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Miranda, T.; Oliveira, D.; Silva, R. Soil stabilisation using alkaline activation of fly ash for self compacting rammed earth construction. Constr. Build. Mater. 2012, 36, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Modmoltin, C.; Voottipruex, P. Influence of salts on strength of cement-treated clays. Proc. Inst. Civ. Eng. -Ground Improv. 2009, 162, 15–26. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Hasanzadehshooiili, H.; Ghadir, P.; Saeidi, F.; Moharami, F. Compressive strength of sandy soils stabilized with alkali-activated volcanic ash and slag. J. Mater. Civ. Eng. 2021, 33, 04021295. [Google Scholar] [CrossRef]
- Araújo, N.; Corrêa-Silva, M.; Miranda, T.; Topa Gomes, A.; Castro, F.; Teixeira, T.; Cristelo, N. Unsaturated response of clayey soils stabilised with alkaline cements. Molecules 2020, 25, 2533. [Google Scholar] [CrossRef] [PubMed]
- Žurinskas, D.; Vaičiukynienė, D.; Stelmokaitis, G.; Doroševas, V. Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing. Minerals 2020, 10, 1076. [Google Scholar] [CrossRef]
- Mohebbi, H.R.; Javadi, A.A.; Saeedi Azizkandi, A. The Effects of Soil Porosity and Mix Design of Volcanic Ash-Based Geopolymer on the Surface Strength of Highly Wind Erodible Soils. Minerals 2022, 12, 984. [Google Scholar] [CrossRef]
- Şahin, E.; Çiftçioğlu, M. Monetite promoting effect of NaCl on brushite cement setting kinetics. J. Mater. Chem. B 2013, 1, 2943–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odeh, N.A.; Al-Rkaby, A.H. Strength, Durability, and Microstructures characterization of sustainable geopolymer improved clayey soil. Case Stud. Constr. Mater. 2022, 16, e00988. [Google Scholar] [CrossRef]
- Ngo, T.-P.; Bui, Q.-B.; Phan, V.T.-A.; Tran, H.-B. Durability of geopolymer stabilised compacted earth exposed to wetting–drying cycles at different conditions of pH and salt. Constr. Build. Mater. 2022, 329, 127168. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Strength and Durability of Granular Soil Stabilized with FA-GGBS Geopolymer. J. Mater. Civ. Eng. 2021, 33, 06021003. [Google Scholar] [CrossRef]
- ASTM D2487-17e1; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2017.
- Konyushkova, M.; Alavipanah, S.; Heidari, A.; Kozlov, D.; Yu, M.; Semenkov, I. Spatial and seasonal salt translocation in the young soils at the coastal plains of the Caspian Sea. Quat. Int. 2021, 590, 15–25. [Google Scholar] [CrossRef]
- ASTM D422-63; Standard Test Method for Particle-Size Analysis of Soils. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2002.
- ASTM D7928-21e1; Standard Test Method for Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedimentation (Hydrometer) Analysis. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2021.
- ASTM D4253-16e1; Standard Test Methods for Maximum Index Density and Unit Weight of Soils Using a Vibratory Table. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2019.
- ASTM D4254-16; Standard Test Methods for Minimum Index Density and Unit Weight of Soils and Calculation of Relative Density. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2016.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2016.
- Sun, J.; Huang, Y. Modeling the Simultaneous Effects of Particle Size and Porosity in Simulating Geo-Materials. Materials 2022, 15, 1576. [Google Scholar] [CrossRef]
- ASTM D2166-16; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2016.
- Ghadir, P.; Zamanian, M.; Mahbubi-Motlagh, N.; Saberian, M.; Li, J.; Ranjbar, N. Shear strength and life cycle assessment of volcanic ash-based geopolymer and cement stabilized soil: A comparative study. Transp. Geotech. 2021, 31, 100639. [Google Scholar] [CrossRef]
- Miraki, H.; Shariatmadari, N.; Ghadir, P.; Jahandari, S.; Tao, Z.; Siddique, R. Clayey soil stabilization using alkali-activated volcanic ash and slag. J. Rock Mech. Geotech. Eng. 2021, 14, 576–591. [Google Scholar] [CrossRef]
- Van Deventer, J.; Provis, J.; Duxson, P.; Lukey, G. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Pourakbar, S.; Huat, B.B.; Asadi, A.; Fasihnikoutalab, M.H. Model study of alkali-activated waste binder for soil stabilization. Int. J. Geosynth. Ground Eng. 2016, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; van Deventer, J.S. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspars. Colloids Surf. A: Physicochem. Eng. Asp. 2003, 216, 27–44. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated slag concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Mayhoub, O.A.; Mohsen, A.; Alharbi, Y.R.; Abadel, A.A.; Habib, A.; Kohail, M. Effect of curing regimes on chloride binding capacity of geopolymer. Ain Shams Eng. J. 2021, 12, 3659–3668. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Maheri, M.R.; Mehrali, M. Hot-pressed geopolymer. Cem. Concr. Res. 2017, 100, 14–22. [Google Scholar] [CrossRef]
- Aldaood, A.; Bouasker, M.; Al-Mukhtar, M. Impact of wetting–drying cycles on the microstructure and mechanical properties of lime-stabilized gypseous soils. Eng. Geol. 2014, 174, 11–21. [Google Scholar] [CrossRef]
- Farnam, Y.; Dick, S.; Wiese, A.; Davis, J.; Bentz, D.; Weiss, J. The influence of calcium chloride deicing salt on phase changes and damage development in cementitious materials. Cem. Concr. Compos. 2015, 64, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al(OH)6(Cl, OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- De Weerdt, K.; Orsáková, D.; Geiker, M.R. The impact of sulphate and magnesium on chloride binding in Portland cement paste. Cem. Concr. Res. 2014, 65, 30–40. [Google Scholar] [CrossRef]
- Hirao, H.; Yamada, K.; Takahashi, H.; Zibara, H. Chloride binding of cement estimated by binding isotherms of hydrates. J. Adv. Concr. Technol. 2005, 3, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Hooton, R.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Yoon, S.; Ha, J.; Chae, S.R.; Kilcoyne, D.A.; Jun, Y.; Oh, J.E.; Monteiro, P.J. Phase changes of monosulfoaluminate in NaCl aqueous solution. Materials 2016, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Kayali, O.; Khan, M.; Ahmed, M.S. The role of hydrotalcite in chloride binding and corrosion protection in concretes with ground granulated blast furnace slag. Cem. Concr. Compos. 2012, 34, 936–945. [Google Scholar] [CrossRef]
- Du, Y.-J.; Wei, M.-L.; Reddy, K.R.; Liu, Z.-P.; Jin, F. Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef]
- Du, Y.-J.; Bo, Y.-L.; Jin, F.; Liu, C.-Y. Durability of reactive magnesia-activated slag-stabilized low plasticity clay subjected to drying–wetting cycle. Eur. J. Environ. Civ. Eng. 2016, 20, 215–230. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A. The effect of heat-curing on transport properties of low-calcium fly ash-based geopolymer concrete. Constr. Build. Mater. 2016, 112, 464–477. [Google Scholar] [CrossRef]
- Ghadir, P.; Razeghi, H.R. Effects of sodium chloride on the mechanical strength of alkali activated volcanic ash and slag pastes under room and elevated temperatures. Constr. Build. Mater. 2022, 344, 128113. [Google Scholar] [CrossRef]
- Khan, M.; Kayali, O.; Troitzsch, U. Chloride binding capacity of hydrotalcite and the competition with carbonates in ground granulated blast furnace slag concrete. Mater. Struct. 2016, 49, 4609–4619. [Google Scholar] [CrossRef]
- Song, H.-W.; Saraswathy, V. Studies on the corrosion resistance of reinforced steel in concrete with ground granulated blast-furnace slag—An overview. J. Hazard. Mater. 2006, 138, 226–233. [Google Scholar] [CrossRef]
- Lee, N.; Jang, J.G.; Lee, H.-K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Wang, W.-C.; Wang, H.-Y.; Lo, M.-H. The fresh and engineering properties of alkali activated slag as a function of fly ash replacement and alkali concentration. Constr. Build. Mater. 2015, 84, 224–229. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.-G.; Shi, C.-J. Mechanical properties of alkali-activated concrete: A state-of-the-art review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Bernal, S.A.; Provis, J.L. Chloride binding capacity of synthetic C-(A)-SH type gels in alkali-activated slag simulated pore solutions. In Proceedings of the 1st International Conference on Construction Materials for Sustainable Future, Zadar, Croatia, 19–21 April 2017. [Google Scholar]
- Zhu, Y.; Wan, X.; Han, X.; Ren, J.; Luo, J.; Yu, Q. Solidification of chloride ions in alkali-activated slag. Constr. Build. Mater. 2022, 320, 126219. [Google Scholar] [CrossRef]
- Li, F.; Liu, L.; Yang, Z.; Li, S. Physical and mechanical properties and micro characteristics of fly ash-based geopolymer paste incorporated with waste Granulated Blast Furnace Slag (GBFS) and functionalized Multi-Walled Carbon Nanotubes (MWCNTs). J. Hazard. Mater. 2020, 401, 123339. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. Self-cementation solidification of heavy metals in lead-zinc smelting slag through alkali-activated materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
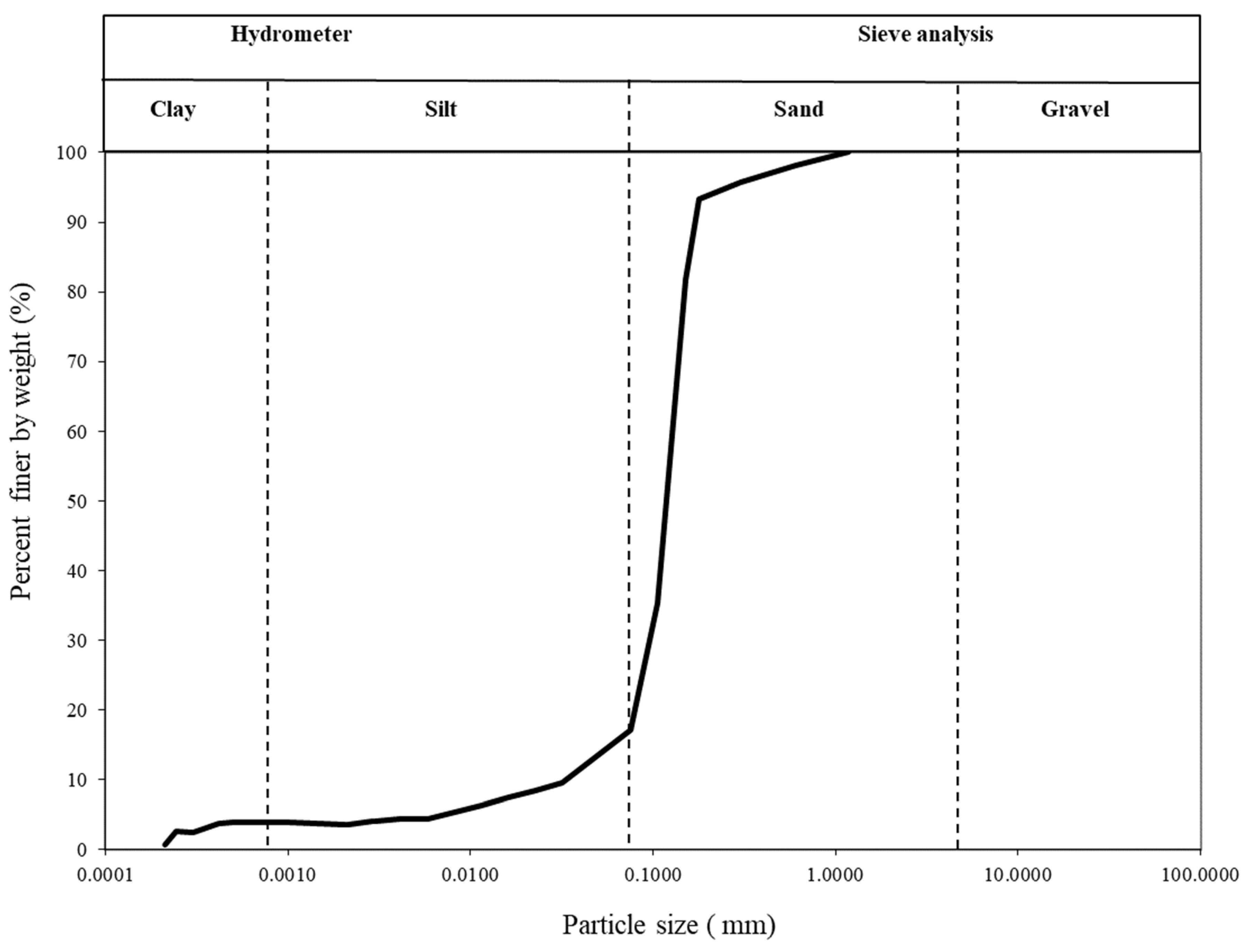







| Soil Classification | Grain Fractions (%) | D10 (μm) | D30 (μm) | D60 (μm) | Cu | Cc | Gs | ɤd−max (kN/m3) | ɤd−min (kN/m3) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | |||||||||
| SM | 4 | 13 | 83 | 37.6 | 99.8 | 132.7 | 3.52 | 1.99 | 2.66 | 16 | 12.5 |
| Oxide Composition | SiO2 | CaO | Al2O3 | Fe2O3 | K2O | Na2O | MgO | TiO2 | SrO | SO3 | P2O5 | MnO | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA [wt.%] | 53.89 | 8.96 | 20.31 | 3.44 | 1.91 | 5.15 | 1.42 | 0.50 | 0.07 | 0.26 | 0.22 | - | 3.79 |
| Slag [wt.%] | 34.86 | 36.59 | 13.93 | 0.20 | 1.01 | - | 6.04 | 1.91 | 0.08 | 2.70 | - | 1.45 | 1.19 |
| OPC [wt.%] | 18.42 | 61.46 | 5.23 | 3.60 | 0.88 | - | 2.73 | 0.36 | 0.09 | 4.10 | - | 0.19 | 2.90 |
| Soil [wt.%] | 45.87 | 15.52 | 15.54 | 6.13 | 2.04 | 4.17 | 2.88 | 0.63 | 0.1 | 0.35 | 0.24 | 0.12 | 9.30 |
| Series | Binder Type | Activator Type | Binder/Soil | Activator/Binder | Initial Water (wt.% of Soil) |
|---|---|---|---|---|---|
| Alkali-activated | VA/Slag | NaOH (8 M) | 0.3 | 1 | 10 |
| Portland cement | OPC | H2O | 0.3 | 1 | 10 |
| Material Sets | Binder Type | Slag Replacement (%) | Curing Time [day] | Curing Condition | NaCl Content (wt.%) |
|---|---|---|---|---|---|
| Set 1 | Alkali-activated | 0, 50, 100 | 28, 90 | DC *, OC ¥, SC ђ | 0, 1, 2, 4 |
| Set 2 | OPC | _ | 28, 90 | DC, OC, SC | 0, 1, 2, 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razeghi, H.R.; Ghadir, P.; Javadi, A.A. Mechanical Strength of Saline Sandy Soils Stabilized with Alkali-Activated Cements. Sustainability 2022, 14, 13669. https://doi.org/10.3390/su142013669
Razeghi HR, Ghadir P, Javadi AA. Mechanical Strength of Saline Sandy Soils Stabilized with Alkali-Activated Cements. Sustainability. 2022; 14(20):13669. https://doi.org/10.3390/su142013669
Chicago/Turabian StyleRazeghi, Hamid Reza, Pooria Ghadir, and Akbar A. Javadi. 2022. "Mechanical Strength of Saline Sandy Soils Stabilized with Alkali-Activated Cements" Sustainability 14, no. 20: 13669. https://doi.org/10.3390/su142013669






