Removal of Hexavalent Chromium by Electrospun Silicon Dioxide Nanofibers Embedded with Copper-Based Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
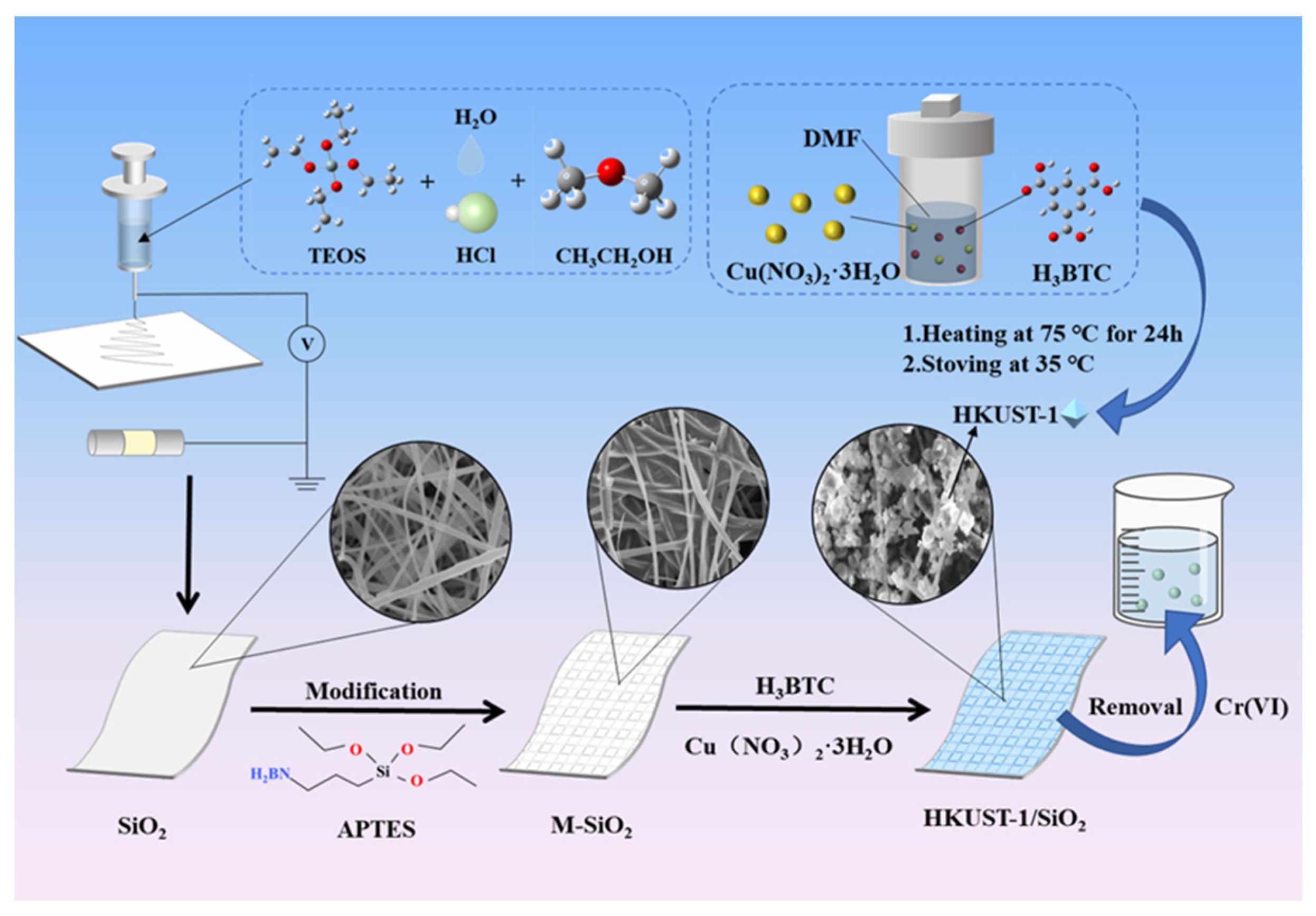
2.2. Preparation of HKUST-1/SiO2 Composite Nanofibers
2.2.1. The Synthesis of HKUST-1
2.2.2. Preparation of SiO2 Nanofibers
2.2.3. Preparation of HKUST-1/SiO2 Composite Nanofibers
2.3. Characterization of the Samples
2.4. Adsorption Experiment
2.5. Adsorbent Regeneration Experiment
2.6. Real Sample Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of HKUST-1/SiO2 Composite Nanofibers
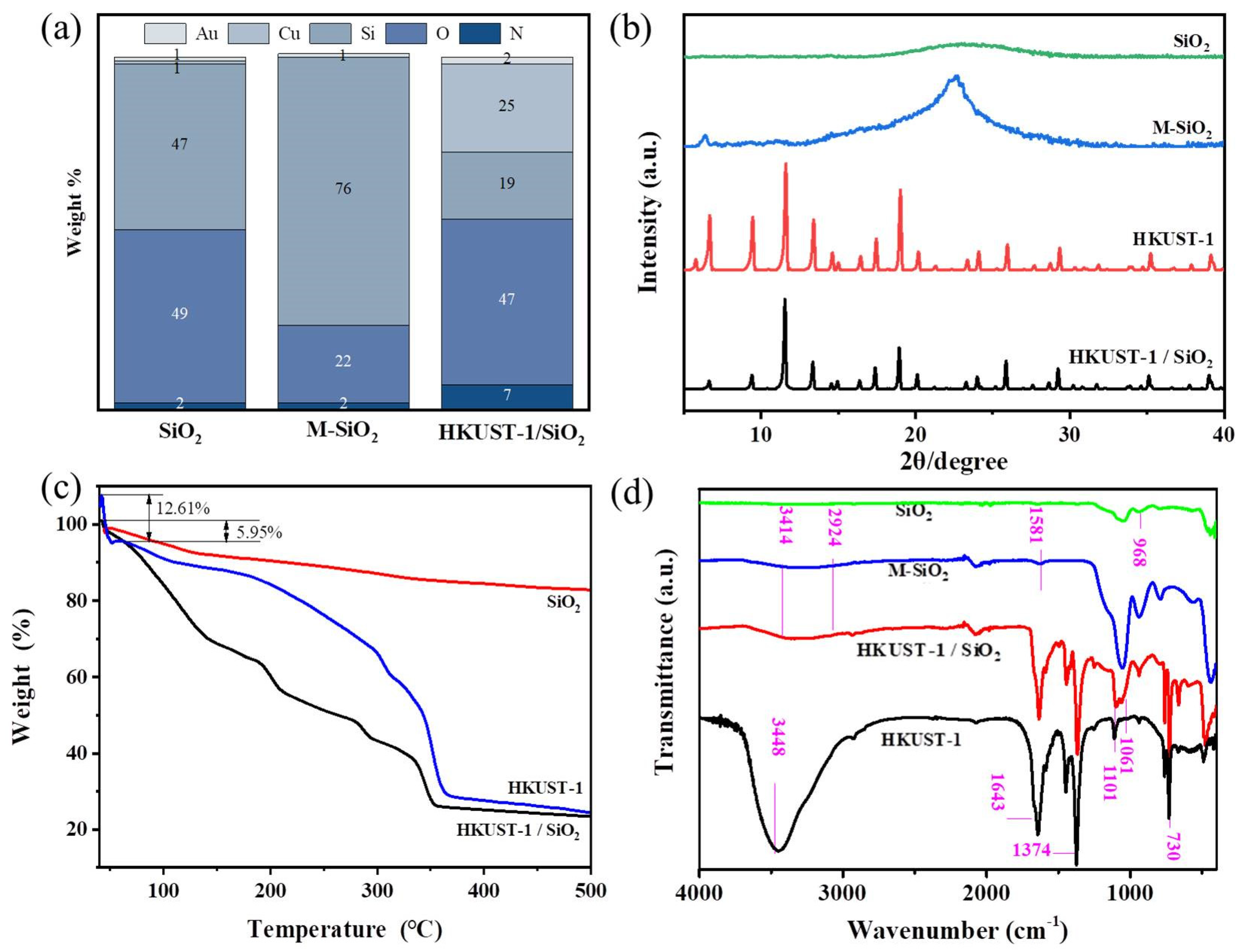
3.1.1. Morphological Characteristics Analysis of Composite Fibers
3.1.2. Analysis of Crystal Structure Characteristics of Composite Fibers
3.1.3. Thermal Stability Analysis of Composite Fibers
3.1.4. Structural Analysis of Functional Groups of Composite Fibers
3.2. Adsorption Performance of Composite Fibers
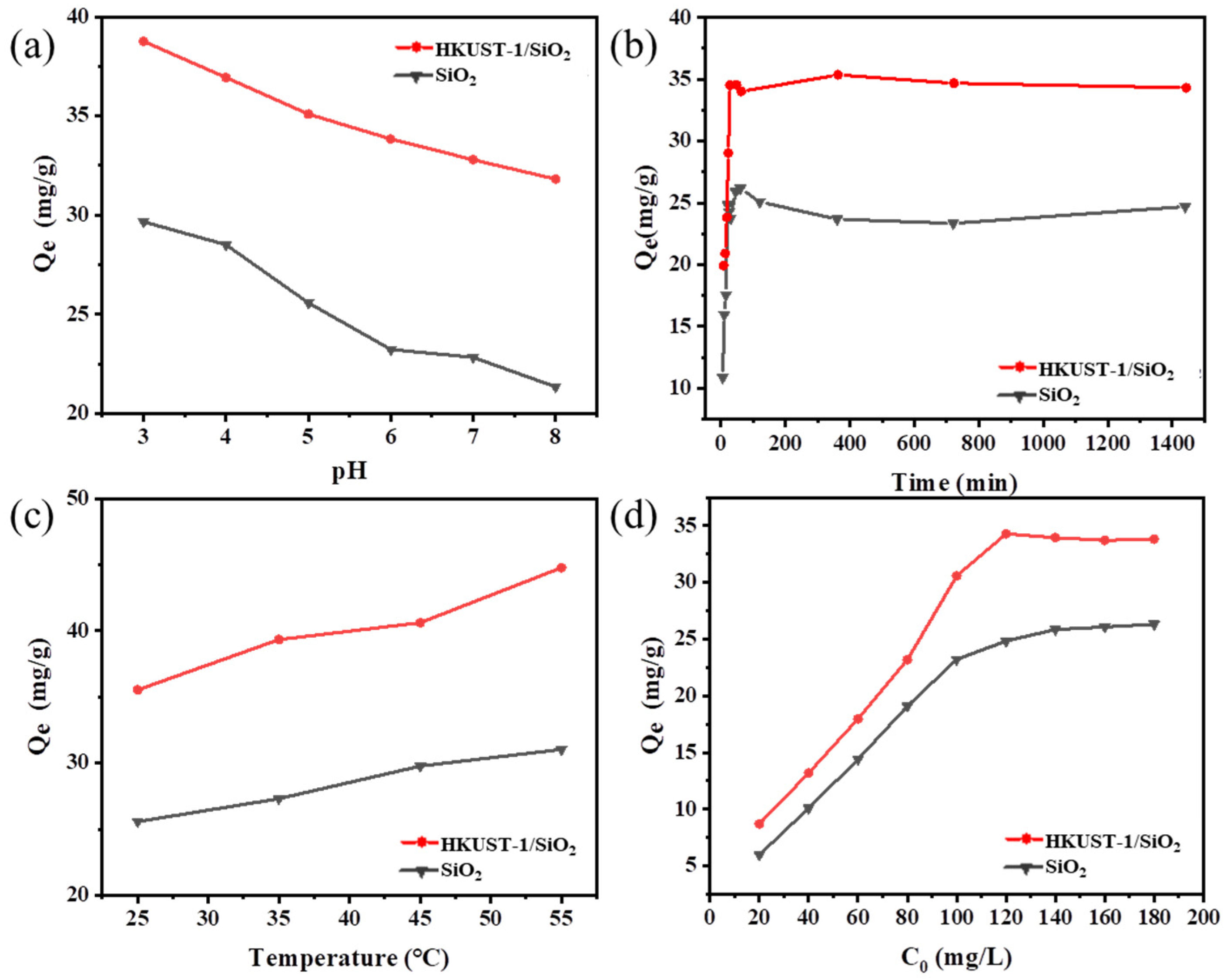
3.2.1. Effect of pH on Cr (VI) Adsorption
3.2.2. Effect of Contact Time on Cr (VI) Adsorption
3.2.3. Effect of Temperature on Cr (VI) Adsorption
3.2.4. Effect of the Initial Concentration on Cr (VI) Adsorption
3.2.5. Adsorption Kinetics of Cr (VI)
3.2.6. Adsorption Isothermal of Cr (VI)
3.2.7. Adsorption Thermodynamics of Cr (VI)
3.3. Mechanism of Adsorption of Cr (VI)
3.4. Regeneration Property Analysis of Composite Fiber
3.5. Application Analysis of Composite Fiber in Real Samples
3.6. Comparative Analysis of Composite Fiber and Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, F.; Othmani, A.; Kesraoui, A.; Seffen, M. Coupling alternating current and biosorption for the removal of hexavalent chromium. Chem. Eng. Technol. 2021, 44, 339–348. [Google Scholar] [CrossRef]
- Li, S.; Hu, Z.; Xie, S.; Liu, H.; Liu, J. Removal of Cr(VI) From electroplating industry effluent via electrochemical reduction. Int. J. Electrochem. Sci. 2018, 13, 655–663. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]
- Ma, B.; Yao, J.; Chen, Z.; Liu, B.; Kim, J.; Zhao, C.; Zhu, X.; Mihucz, V.G.; Minkina, T.; Knudsen, T.Š. Superior elimination of Cr(VI) using polydopamine functionalized attapulgite supported nZVI composite: Behavior and mechanism. Chemosphere 2022, 287, 131970. [Google Scholar] [CrossRef]
- Petrinic, I.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.; Bhatnagar, A. Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: Mechanisms, methods, and prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of hexavalent chromium-industry treated water and wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Ediati, R.; Setyani, M.A.; Sulistiono, D.O.; Santoso, E.; Hartanto, D.; Abdullah, M.M.A.B. Optimization of the use of mother liquor in the synthesis of HKUST-1 and their performance for removal of chromium (VI) in aqueous solutions. J. Water Process Eng. 2021, 39, 101670. [Google Scholar] [CrossRef]
- Raganati, F.; Gargiulo, V.; Ammendola, P.; Alfe, M.; Chirone, R. CO2 capture performance of HKUST-1 in a sound assisted fluidized bed. Chem. Eng. J. 2014, 239, 75–86. [Google Scholar] [CrossRef]
- Hoang, L.P.; Nguyen TM, P.; Van, H.T.; Hoang TK, D.; Vu, X.H.; Nguyen, T.V.; Ca, N.X. Cr(VI) Removal from aqueous solution using a magnetite snail shell. Water Air Soil Pollut. 2020, 231, 28. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Valentín-Reyes, J.; García-Reyes, R.B.; García-González, A.; Soto-Regalado, E.; Cerino-Córdova, F. Adsorption mechanisms of hexavalent chromium from aqueous solutions on modified activated carbons. J. Environ. Manag. 2019, 236, 815–822. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wang, A. Enhanced adsorption of methylene blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 2010, 22, 486–493. [Google Scholar] [CrossRef]
- Lis, M.J.; Caruzi, B.B.; Gil, G.A.; Samulewski, R.B.; Bail, A.; Scacchetti, F.A.P.; Moisés, M.P.; Maestá Bezerra, F. In-situ direct synthesis of HKUST-1 in wool fabric for the improvement of antibacterial properties. Polymers 2019, 11, 713. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, W.; Zhu, X.; Ma, H.; Wang, P.; Shang, J.; Luo, P. In situ growth of UIO-66-NH2 on thermally stabilized electrospun polyacrylonitrile nanofibers for visible-light driven Cr (VI) photocatalytic reduction. J. Solid State Chem. 2022, 307, 122836. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.G. Progresses on electrospun metal–organic frameworks nanofibers and their wastewater treatment applications. Mater. Today Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Sun, Z.; Yang, C.; Tang, D. Effective strategy to fabricate ZIF-8@ZIF-8/polyacrylonitrile nanofibers with high loading efficiency and improved removing of Cr(VI). Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125292. [Google Scholar] [CrossRef]
- Li, L.-L.; Feng, X.-Q.; Han, R.-P.; Zang, S.-Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Liu, X.; Chu, C.; Yao, D.; Mao, S. Modification strategies on 2D Ni-Fe MOF-based catalysts in peroxydisulfate activation for efficient organic pollutant removal. Chin. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Geng, H.; Hu, B.; Song, G.; Xu, Z. A MOF/graphite oxide hybrid (MOF: HKUST-1) material for the adsorption of methylene blue from aqueous solution. J. Mater. Chem. A 2013, 35, 10292–10299. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Geng, H.Y.; Hu, B.; Song, G.W.; Xu, Z.S. Preparation and characterization of APTES-assisted Fe3O4/CNTs composites with high decontamination and high magnetic recovery capacity. Surf. Interfaces 2022, 31, 102017. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Sun, H.; Periasamy, V.; Tadé, M.O.; Wang, S. One-pot synthesis of binary metal organic frameworks (HKUST-1 and UiO-66) for enhanced adsorptive removal of water contaminants. J. Colloid Interface Sci. 2017, 490, 685–694. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, F.; Zhang, C.; Shen, S.; Su, L.; Zhao, S. Synthesis of magnetic porous γ-Fe2O3/C@HKUST-1 composites for efficient removal of dyes and heavy metal ions from aqueous solution. RSC Adv. 2015, 7, 5164–5172. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, D.; Wu, C.; Chen, S.; Wang, Y.; Chen, C. Magnetic metal organic frameworks/graphene oxide adsorbent for the removal of U(VI) from aqueous solution. Applied radiation and isotopes: Including data, instrumentation and methods for use in agriculture. Ind. Med. 2020, 162, 109160. [Google Scholar]
- Zhao, L.; Duan, X.; Azhar, M.R.; Sun, H.; Fang, X.; Wang, S. Selective adsorption of rare earth ions from aqueous solution on metal-organic framework HKUST-1. Chem. Eng. J. Adv. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and Characterization of an SWCNT@HKUST-1 Composite: Enhancing the CO2 Adsorption Properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef]
- Hasan, Z.; Cho, J.; Rinklebe, J.; Ok, Y.S.; Cho, D.W.; Song, H. Metal organic framework derived Cu-carbon composite: An efficient non-noble metal catalyst for reduction of hexavalent chromium and pendimethalin(Article). J. Ind. Eng. Chem. 2017, 52, 331–337. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, J.; Fu, Y.; Zhao, P.; Zhang, Y.; He, B.; He, P. Underwater superoleophobic APTES-SiO2/PVA organohydrogel for low-temperature tolerant, self-healing, recoverable oil/water separation mesh. Chem. Eng. J. 2020, 382, 122925. [Google Scholar] [CrossRef]
- Conde-Gonzlez, J.E.; Pea-Mndez, E.M.; Rybkov, S.; Pasn, J.; Ruiz-Prez, C.; Havel, J. Adsorption of silver nanoparticles from aqueous solution on copper-based metal organic frameworks (HKUST-1). Chemosphere 2016, 150, 659–666. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.; Xie, X.; Liu, P.; Huq, M.E. Enhanced visible light photocatalytic degradation of dyes in aqueous solution activated by HKUST-1: Performance and mechanism. RSC Adv. 2020, 10, 37028–37034. [Google Scholar] [CrossRef]
- Yuan, B.; Yin, X.-Q.; Liu, X.-Q.; Li, X.-Y.; Sun, L.-B. Enhanced hydrothermal stability and catalytic performance of HKUST-1 by incorporating carboxyl-functionalized attapulgite. ACS Appl. Mater. Interfaces 2016, 8, 16457–16464. [Google Scholar] [CrossRef]
- Goyal, P.; Paruthi, A.; Menon, D.; Behara, R.; Jaiswal, A.; V, K.; Kumar, A.; Krishnan, V.; Misra, S.K. Fe doped bimetallic HKUST-1 MOF with enhanced water stability for trapping Pb(II) with high adsorption capacity. Chem. Eng. J. 2022, 430, 133088. [Google Scholar] [CrossRef]
- Tian, N.; Gao, Y.; Wu, J.; Luo, S.; Dai, W. HKUST-1 water-resistant functionalized with polydimethylsiloxane for rubidium ion efficient capture. New J. Chem. 2019, 43, 15539–15547. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, W.; Li, X.; Wang, X.; Zheng, Y.; Yan, B.; Wu, C. Water-stable composite of HKUST-1 with its pyrolysis products for enhanced CO2 capture capacity. Inorg. Chem. Commun. 2022, 146, 110063. [Google Scholar] [CrossRef]
- Xu, C.H.; Zhu, L.J.; Wang, X.H.; Lin, S.; Chen, Y.M. Fast and highly efficient removal of chromate from aqueous solution using nanoscale zero-valent iron/activated carbon (NZVI/AC). Water Air Soil Pollut. 2014, 225, 1845. [Google Scholar] [CrossRef]
- Gasemloo, S.K.; Morteza1, S.; Mahmoud, R.D.; Siavoush, G. Response surface methodology (RSM) modeling to improve removal of Cr (VI) ions from tannery wastewater using sulfated carboxymethyl cellulose nanofilter (Article). J. Clean. Prod. 2019, 208, 736–742. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, L.; Ouyang, D.; Chen, Y.; Han, L.; Chen, M. Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization. Chemosphere 2019, 221, 683–692. [Google Scholar] [CrossRef]
- Mirabedini, M.; Kassaee, M.; Poorsadeghi, S. Novel Magnetic Chitosan Hydrogel Film, Cross-Linked with Glyoxal as an Efficient Adsorbent for Removal of Toxic Cr (VI) from Water. Arab. J. Sci. Eng. 2017, 1, 115–124. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2732. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Zhang, X.; Guo, H.; Cao, X.; Lou, Z.; Zhang, W.; Wang, C. A novel lignin hydrogel supported nZVI for efficient removal of Cr (VI). Chemosphere 2022, 301, 134781. [Google Scholar] [CrossRef]
- Kong, A.; Sun, Y.; Peng, M.; Gu, H.; Fu, Y.; Zhang, J.; Li, W. Amino-functionalized MXenes for efficient removal of Cr (VI). Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126388. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water-A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Mallik, A.K.; Moktadir, A.; Rahman, A.; Shahruzzaman; Rahman, M.M. Progress in surface-modified silicas for Cr(VI) adsorption: A review. J. Hazard Mater. 2022, 423, 127041. [Google Scholar] [CrossRef]
- Jia, D.; Jing, Z.; Duan, Y.; Li, J. Ultrafast removal of Cr (VI) ions using polyamine modified carbon nanotubes. J. Taiwan Inst. Chem. Eng. 2022, 133, 104265. [Google Scholar] [CrossRef]
- Taha, A.A.; Wu, Y.-N.; Wang, H.; Li, F. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr (VI) ion removal from aqueous solution. J. Environ. Manag. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Vazquez-Velez, E.; Lopez-Zarate, L.; Martinez-Valencia, H. Electrospinning of polyacrylonitrile nanofibers embedded with zerovalent iron and cerium oxide nanoparticles, as Cr (VI) adsorbents for water treatment. J. Appl. Polym. Sci. 2020, 137, 48663. [Google Scholar] [CrossRef]
- Wu, S.; Ge, Y.; Wang, Y.; Chen, X.; Li, F.; Xuan, H.; Li, X. Adsorption of Cr(VI) on nano Uio-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948. [Google Scholar] [CrossRef]
- Bo, S.; Ren, W.; Lei, C.; Xie, Y.; Cai, Y.; Wang, S.; Gao, J.; Ni, Q.; Yao, J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr (IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Maleki, A.; Hayati, B.; Naghizadeh, M.; Joo, S.W. Adsorption of hexavalent chromium by metal organic frameworks from aqueous solution. J. Ind. Eng. Chem. 2015, 28, 211–216. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Avcı, A.; Pehlivan, E. Electrospinning of polymeric nanofiber (nylon 6,6/graphene oxide) for removal of Cr (VI): Synthesis and adsorption studies. J. Anal. Sci. Technol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Shao, Q.; Peng, X.; Guo, Z. Magnetic nanocarbon adsorbents with enhanced hexavalent chromium removal: Morphology dependence of fibrillar vs particulate structures. Ind. Eng. Chem. Res. 2017, 56, 10689–10701. [Google Scholar] [CrossRef]
- Jin, X.; Wang, H.; Jin, X.; Wang, H.; Chen, L.; Wang, W.; Lin, T.; Zhu, Z. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr(VI). Sci. Total Environ. 2020, 710, 135546. [Google Scholar] [CrossRef]
- Haso, H.W.; Dubale, A.A.; Chimdesa, M.A.; Wang, W.Y.; Lin, T.; Zhu, Z.T. High performance copper based metal organic framework for removal of heavy metals from wastewater. Front. Mater. 2022, 9, 840806. [Google Scholar] [CrossRef]



| Absorbents | Qm (mg/g) | References |
|---|---|---|
| HKUST-1(S0) | 90.9 | 11 |
| HKUST-1(S5) | 100 | 11 |
| ZIF-8@ZIF-8/PAN | 39.68 | 20 |
| silver-triazolate MOF | 37.0 | 21 |
| FCA/SiO2 membranes | 19.45 | 48 |
| PAN-CeO2 NP NFs | 28.00 | 49 |
| Uio-66-NH2 | 32.26 | 50 |
| ZIF-8@CA | 41.800 | 51 |
| Cu-BTC | 48.000 | 52 |
| nylon 6,6/graphene oxide nanofiber | 47.17 | 53 |
| fibrillar magnetic carbon | 43.17 | 54 |
| keratin/PET nanofiber | 75.860 | 55 |
| Cu-DPA MOF | 3.21 | 56 |
| SiO2 nanofibers | 47.391 | This work |
| HKUST-1/SiO2 composite nanofibers | 62.380 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Ni, J.; Li, S.; Cao, X.; Gao, J.; Zhang, W.; Chen, F.; Huang, R.; Zhang, Y.; Feng, S. Removal of Hexavalent Chromium by Electrospun Silicon Dioxide Nanofibers Embedded with Copper-Based Organic Frameworks. Sustainability 2022, 14, 13780. https://doi.org/10.3390/su142113780
Feng S, Ni J, Li S, Cao X, Gao J, Zhang W, Chen F, Huang R, Zhang Y, Feng S. Removal of Hexavalent Chromium by Electrospun Silicon Dioxide Nanofibers Embedded with Copper-Based Organic Frameworks. Sustainability. 2022; 14(21):13780. https://doi.org/10.3390/su142113780
Chicago/Turabian StyleFeng, Shanshan, Jie Ni, Shouzhu Li, Xun Cao, Jingshuai Gao, Wenyang Zhang, Feng Chen, Rouxue Huang, Yao Zhang, and Sheng Feng. 2022. "Removal of Hexavalent Chromium by Electrospun Silicon Dioxide Nanofibers Embedded with Copper-Based Organic Frameworks" Sustainability 14, no. 21: 13780. https://doi.org/10.3390/su142113780
APA StyleFeng, S., Ni, J., Li, S., Cao, X., Gao, J., Zhang, W., Chen, F., Huang, R., Zhang, Y., & Feng, S. (2022). Removal of Hexavalent Chromium by Electrospun Silicon Dioxide Nanofibers Embedded with Copper-Based Organic Frameworks. Sustainability, 14(21), 13780. https://doi.org/10.3390/su142113780




