Synthesis of Poly(styrene-vinyl sodium sulfonate-butyl acrylate-ethyl methacrylate) and Its Blocking Mechanism as a Nanometer Material in Water-Based Drilling Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Nanomaterials
2.3. Blocking Performance Test
2.3.1. Filtration Test with a Simulated Mud Cake
2.3.2. Simulated Rock Core Permeation Experiment
3. Results and Discussion
3.1. Characterization
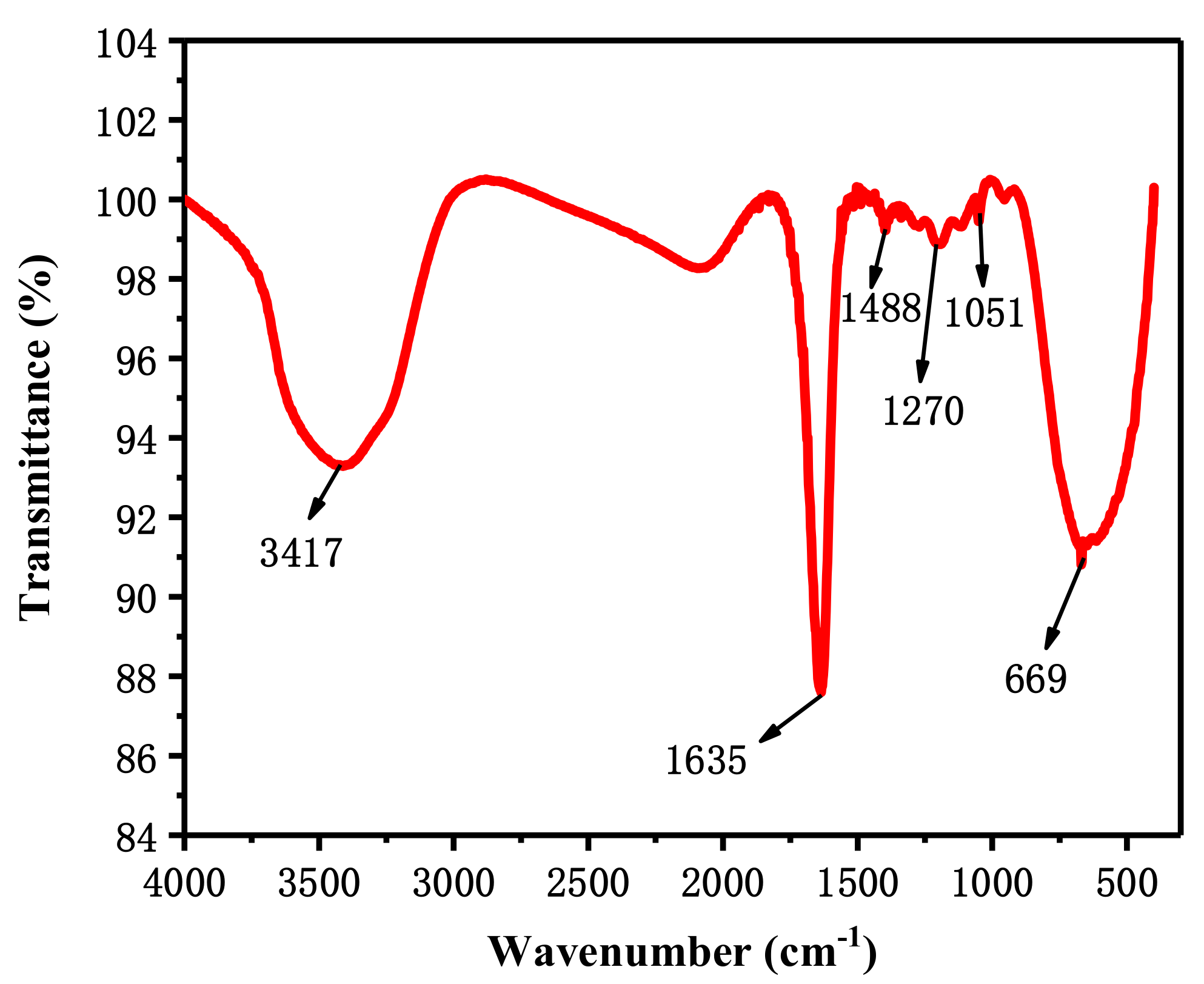
3.1.1. Infrared Spectrum
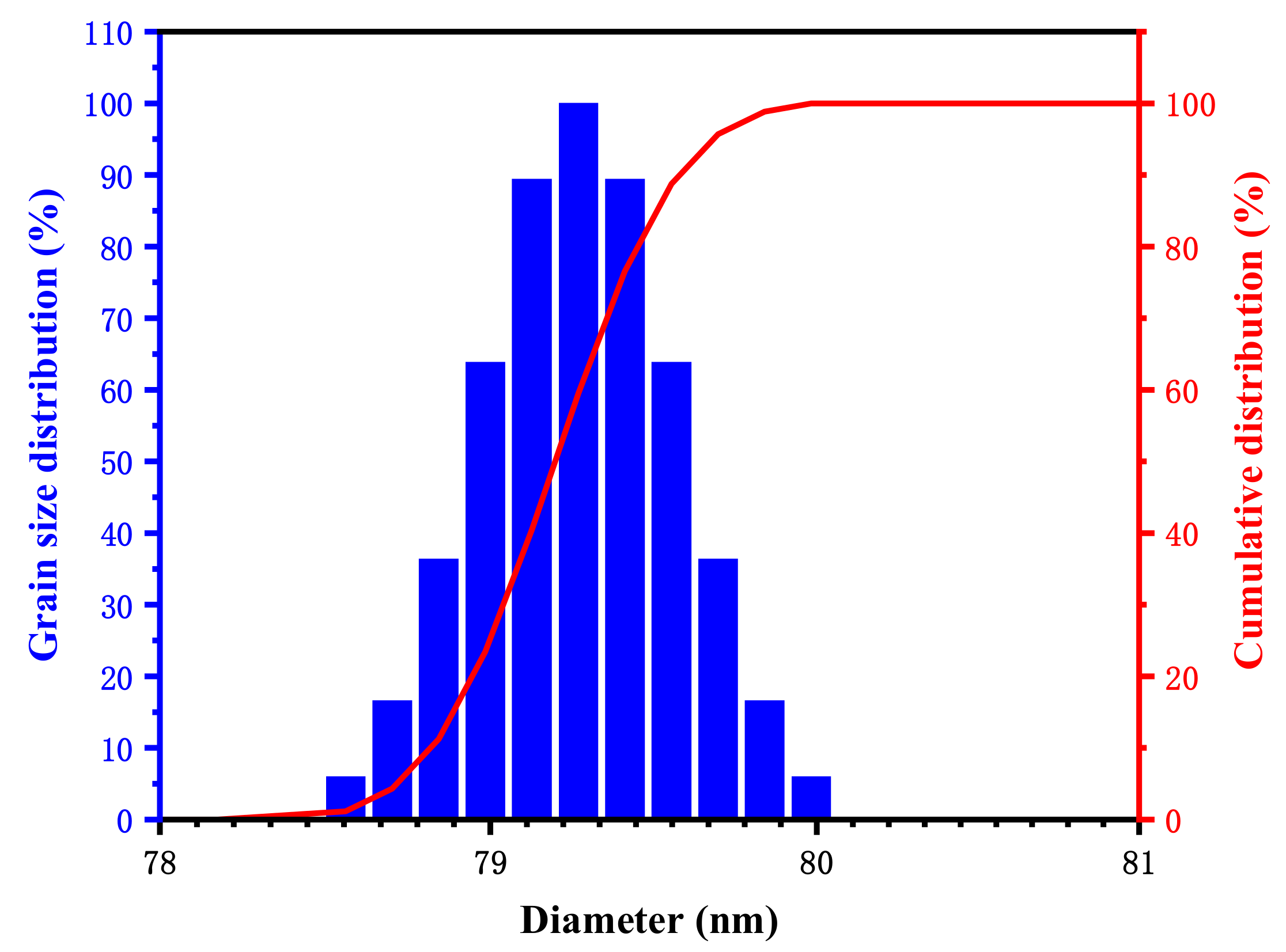
3.1.2. Grain Size Distribution of Poly(ST-VS-B-E) at Room Temperature
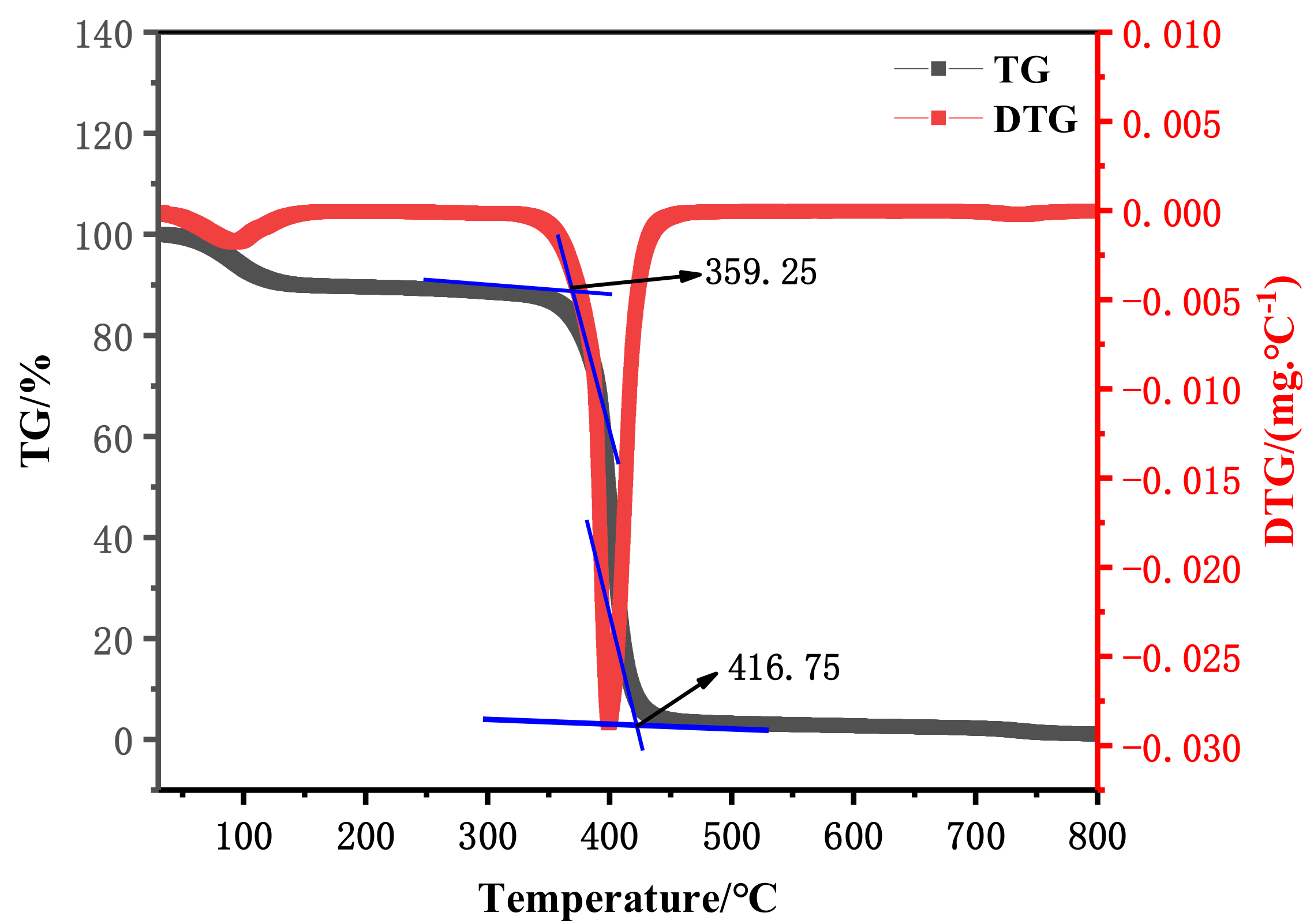
3.1.3. Thermogravimetric Analysis
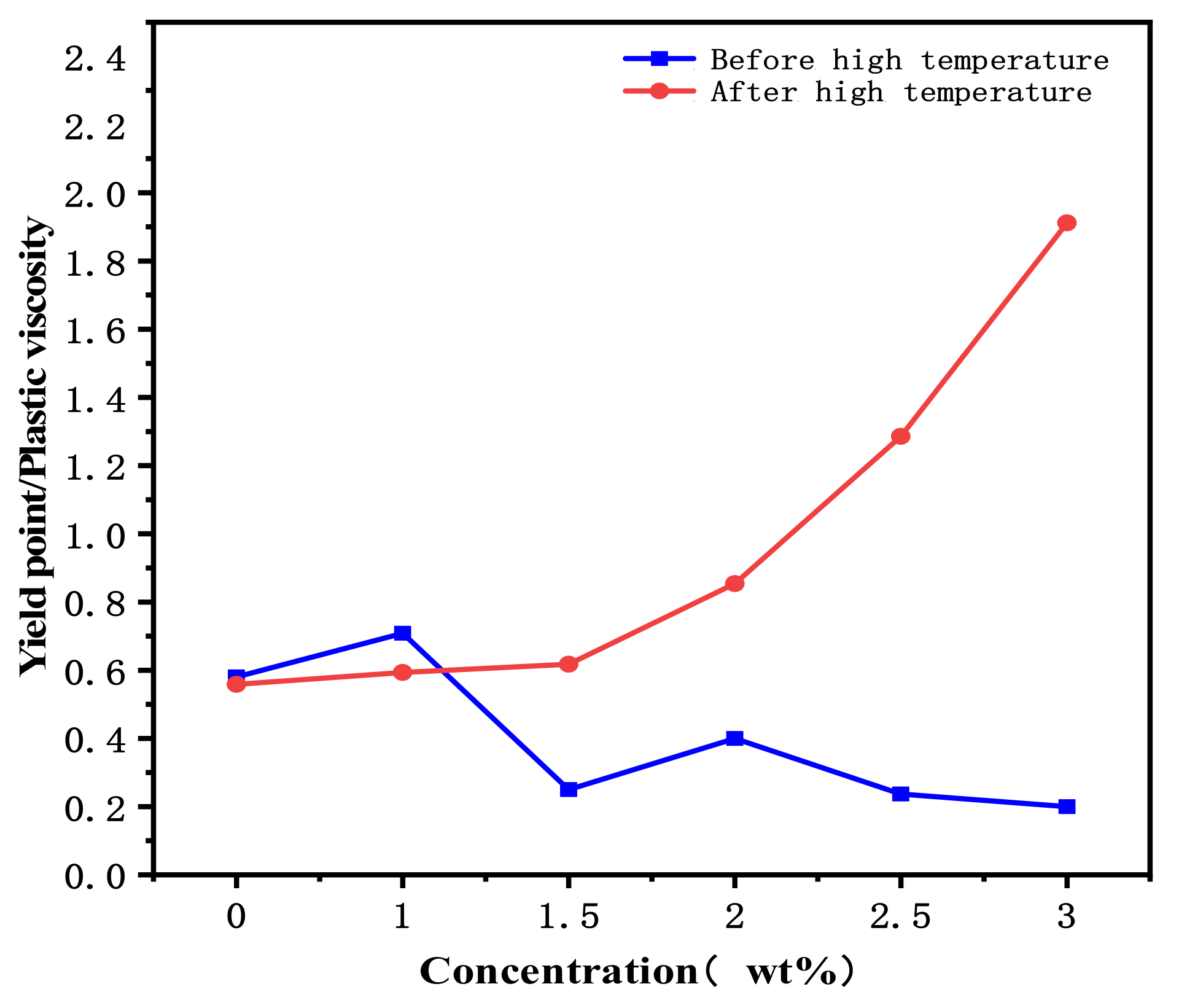
3.2. Evaluation of Water-Based Drilling Fluid Performance
3.3. Evaluation of Blocking Performance
3.3.1. Sealing Performance Evaluation of a Simulated Mud Cake
3.3.2. Sealing Performance Evaluation of a Simulated Core
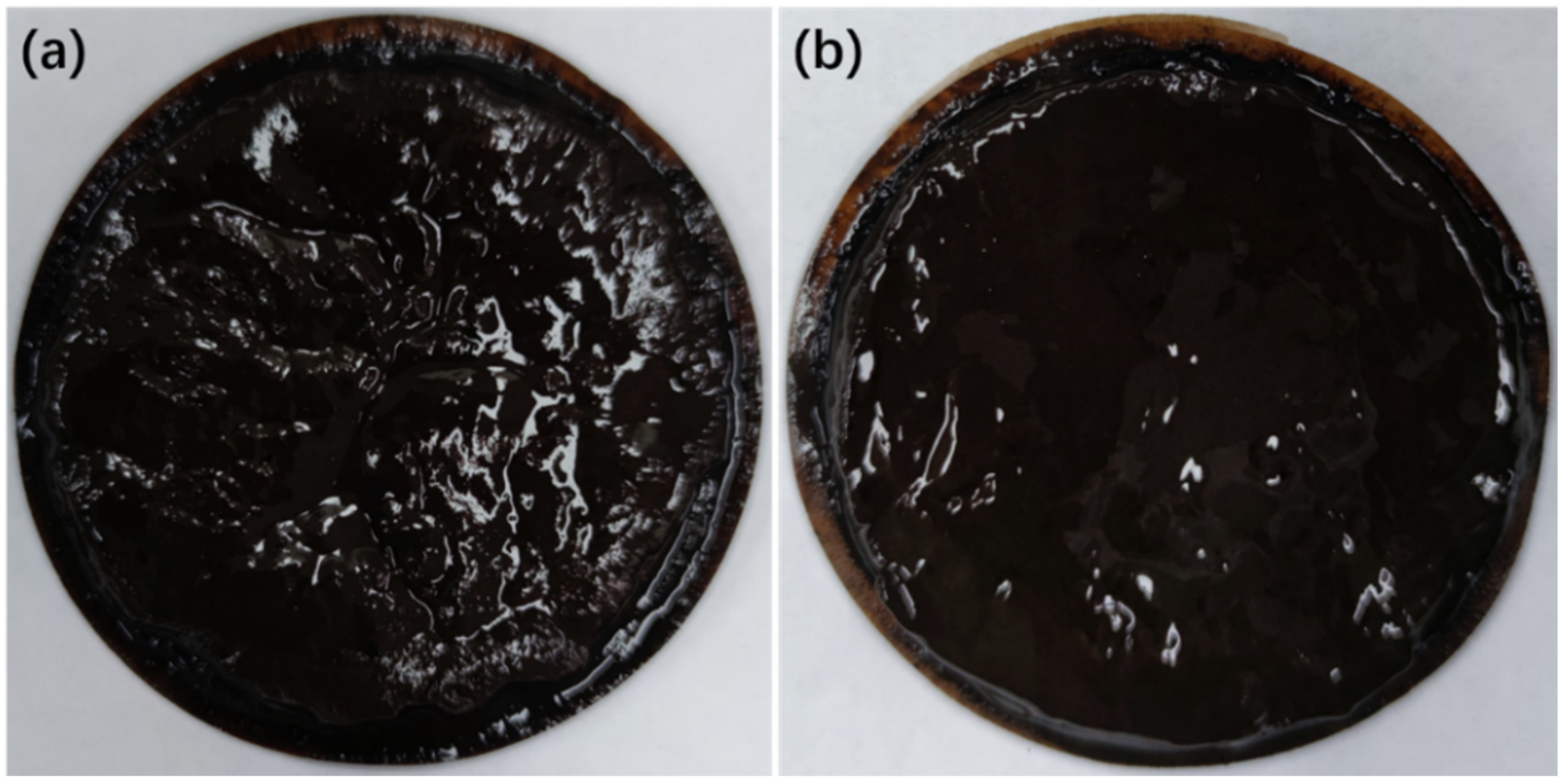
3.4. Simulation of the Microscopic Morphology of Mud Cake
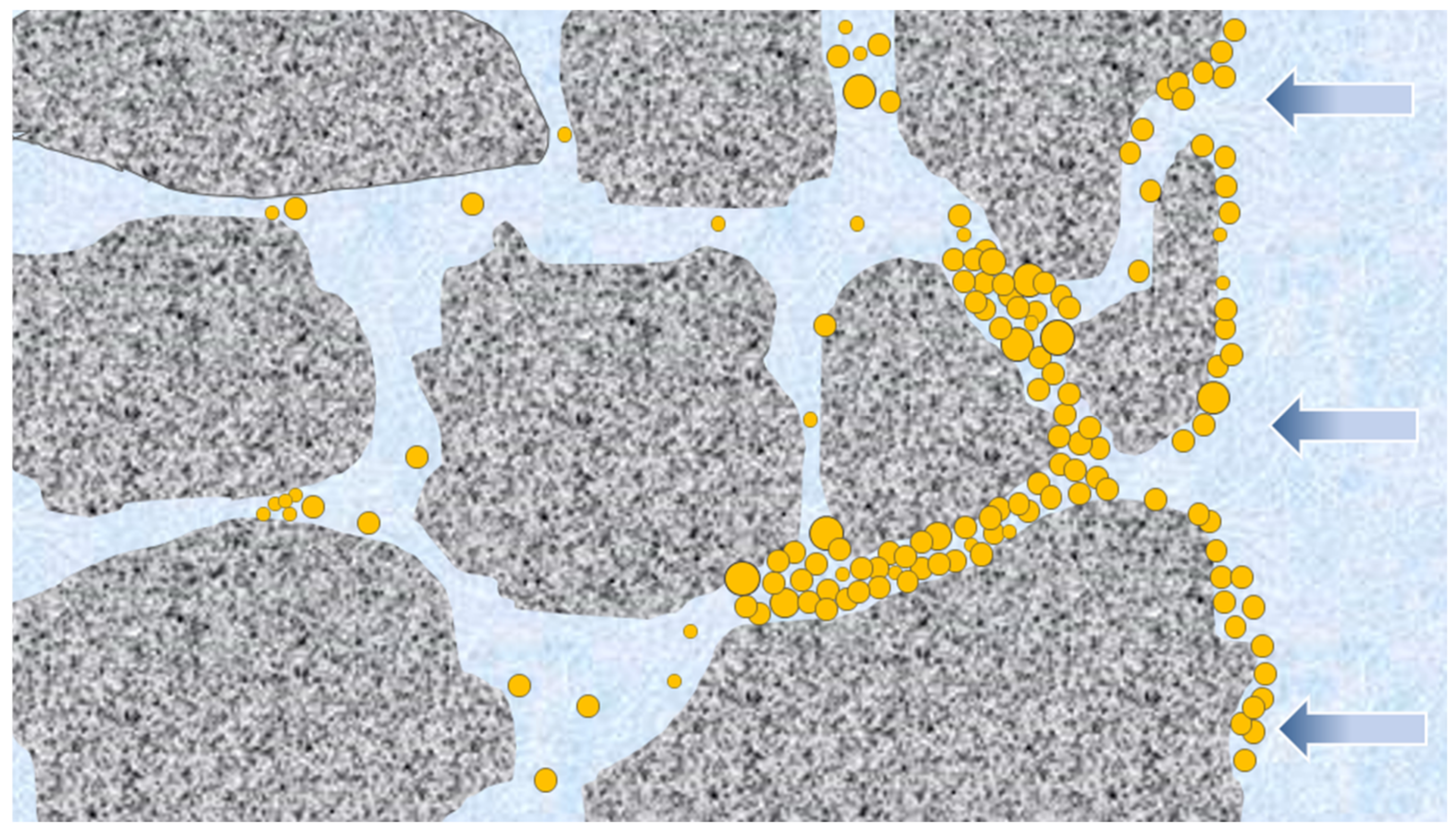
3.5. Research on the Nano-Blocking Mechanism of Poly(ST-VS-B-E)
4. Conclusions
- (1)
- Poly(ST-VS-B-E) was synthesized from styrene, vinyl sodium sulfonate, ethyl methacrylate, and butyl acrylate. The median particle size of the poly(ST-VS-B-E) nanometer blocking materials was 79.15 nm, which could resist a high temperature of 359.25 °C and had almost no effect on water-based drilling fluid performance. The apparent viscosity of the water-based drilling fluid with 2.5% poly(ST-VS-B-E) blocking material added was 48 mPa·s, the plastic viscosity was 21 mPa·s, the yield point was 27 Pa, and the fluid loss was 3.1 mL. With the increase in the amount of poly(ST-VS-B-E), the rock-carrying ability and blocking performance of the water-based drilling fluid were improved to a certain extent.
- (2)
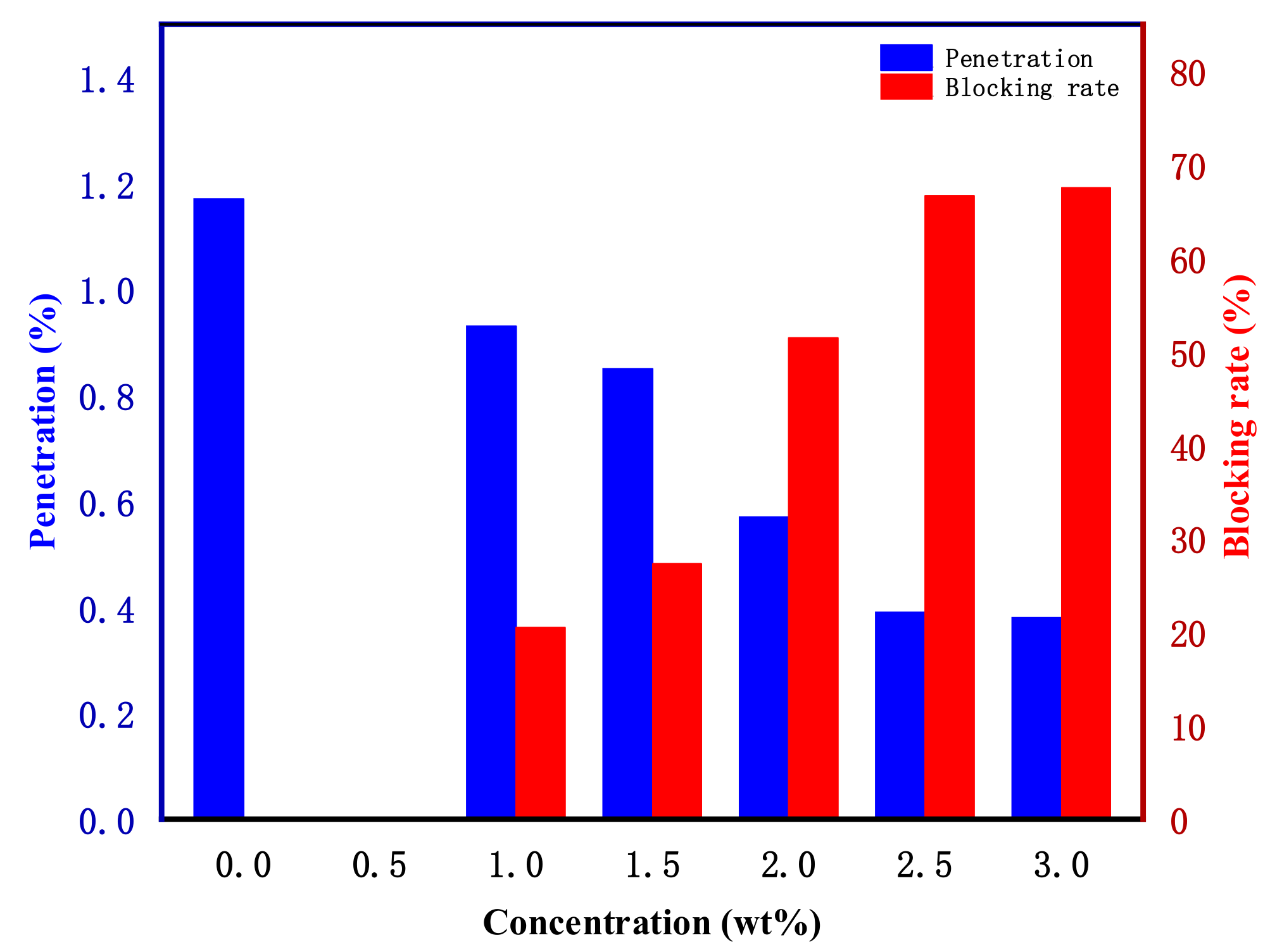
- The plugging performance of water-based drilling fluid increased with the increase in poly(ST-VS-B-E) content. When the amount of poly(ST-VS-B-E) was 2.5 wt%, the blocking rate of the simulated mud cake could reach 66.67%. When the amount of poly(ST-VS-B-E) was 2.5 wt%, the blocking rate of the artificial core was up to 93.33%. The water-based drilling fluid with poly(ST-VS-B-E) nano-blocking material not only had stable performance, but also had excellent plugging performance in the simulated mud cake filtration test and the simulated core permeability test.
- (3)
- Poly(ST-VS-B-E), as a nano-blocking material, can accumulate at a certain distance from shale surface cracks under formation pressure and form a bridge to create an effective sealing structure. In addition, poly(ST-VS-B-E) has a strong adsorption effect. Poly(ST-VS-B-E) adsorbed on the inner wall of shale pore layers can effectively prevent water-based drilling fluid filtrate from infiltrating a shale formation. Poly(ST-VS-B-E) is expected to be a potential new nano-blocking material for shale formations with wellbore instability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Peng, Y.-M.; Gao, B.; Zhang, J.-C.; Cai, D.-S.; Xue, G.; Bao, S.-J.; Xu, Z.-Y.; Tang, X.-L.; Dahdah, N. Geology and shale gas resource potentials in the Sichuan Basin. Energy Explor. Exploit. 2016, 34, 689–710. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-J.; Ma, F.; Tong, X.-G.; Liu, Z.D.; Zhang, X.-S.; Wu, Z.-Z.; Li, D.-H.; Wang, B.; XIE, Y.-F.; Yang, L.-Y. Assessment of global unconventional oil and gas resources. Pet. Explor. Dev. 2016, 43, 925–940. [Google Scholar] [CrossRef]
- Chen, S.-S.; Yu, H.-Y.; Lu, M.-H.; Lebedev, M.-X.; Li, X.-L.; Yang, Z.; Chen, W.; Yuan, Y.-J.; Ding, S.-W.; Johnson, L.-M. A New Approach To Calculate Gas Saturation in Shale Reservoirs. Energy Fuels 2022, 36, 1904–1915. [Google Scholar] [CrossRef]
- Miao, Y.-N.; Zhao, C.-J.; Wu, K.-L.; Li, X.-F. Analysis of production prediction in shale reservoirs: Influence of water film in inorganic matter. J. Nat. Gas Sci. Eng. 2019, 63, 1–9. [Google Scholar] [CrossRef]
- Salem, A.-C.; Naruk, S.-J.; Solum, J.-G. Impact of natural fractures on production from an unconventional shale: The Delaware Basin Wolfcamp shale. AAPG Bull 2022, 106, 1–20. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, G.-J.; Wang, W.-Z.; Zhang, S.-J.; Yuan, L.-L. Characteristics of pore-fissure and permeability of shales in the Longmaxi Formation in southeastern Sichuan Basin. J. China Coal Soc. 2013, 38, 772–777. [Google Scholar]
- Li, B.-Y.; Pang, X.-Q.; Dong, Y.-X.; Peng, J.-W.; Gao, P.; Wu, H.; Huang, C.; Shao, X.-H. Lithofacies and pore characterization in an argillaceous-siliceous-calcareous shale system: A case study of the Shahejie Formation in Nanpu Sag, Bohai Bay Basin, China. J. Pet. Sci. Eng. 2019, 173, 804–819. [Google Scholar] [CrossRef]
- Zhang, P.-X.; He, X.-P.; Gao, Q.-F.; Gao, Y.-Q.; Sun, B.; Cai, X.; He, G.-S.; Zhang, Z.-P.; Liu, N.-N. Geological characteristics and enrichment pattern of Permian Mao 1 Member shale gas reservoirs at the southeastern margin of Sichuan Basin. Oil Gas Geol. 2021, 42, 146–157. [Google Scholar]
- Kerunwa, A.; Ekwueme, S.-T. An Approach to Curbing Wellbore Instability in Shales through Nanoparticles-Augmented Water-Based Drilling Muds. Pet. Coal 2020, 62, 1465–1473. [Google Scholar]
- Yan, X.-P.; Kang, Y.-L.; You, L.-J. Wellbore Instability Induced by the Coupling of High-pH Fluid–Shale Reaction and Fracture Surface Sliding in Shale Gas Wells: Experimental and Field Studies. Energy Fuels 2020, 34, 5578–5588. [Google Scholar] [CrossRef]
- Hany, G.; Salaheldin, E.; Salem, B.; Abdulaziz, A. Effect of pH on Rheological and Filtration Properties of Water-Based Drilling Fluid Based on Bentonite. Sustainability 2019, 11, 6714. [Google Scholar]
- Ding, L.-Q.; Wang, Z.-Q.; Liu, B.-L.; Lv, J.-G. Assessing borehole stability in bedding-parallel strata: Validity of three models. J. Pet. Sci. Eng. 2019, 173, 690–704. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.-S.; Shen, F.; Li, W.; Zhang, W.-Z. Mechanism of wellbore instability in continental shale gas horizontal sections and its water-based drilling fluid countermeasures. Nat. Gas Ind. B 2020, 7, 680–688. [Google Scholar] [CrossRef]
- Salaheldin, E.; Hany, G.; Abdulmalek, A.; Pranjal, S.; Shiv, S.; Maryam, A. A Novel Solution for Severe Loss Prevention While Drilling Deep Wells. Sustainability 2020, 12, 1339. [Google Scholar]
- Yu, X.-A.; Guan, C.-J.; You, R.-Q.; Qing, Y.-G. Plugging Agent of Shale Base on Nano Flexible Polymer. Appl. Mech. Mater. 2016, 835, 15–19. [Google Scholar]
- Xu, J.-G.; Qiu, Z.-S.; Zhao, X.; Zhong, H.-Y.; Li, G.-R.; Huang, W.-A. Synthesis and characterization of shale stabilizer based on polyethylene glycol grafted nano-silica composite in water-based drilling fluids. J. Pet. Sci. Eng. 2018, 163, 371–377. [Google Scholar] [CrossRef]
- Li, X.-L.; Jiang, G.-C.; Shen, X.-L.; Li, G.-R. Poly-I-arginine as a High-Performance and Biodegradable Shale Inhibitor in Water-Based Drilling Fluids for Stabilizing Wellbore. ACS Sustain. Chem. Eng. 2020, 8, 1899–1907. [Google Scholar] [CrossRef]
- Reza, L.-K.; Nezhad, T.; Alireza, S.; Husein, M.-M. Shape Memory Polyurethane as a Drilling Fluid Lost Circulation Material. Macromol. Mater. Eng 2021, 306, 2100354. [Google Scholar]
- Li, W.-Q.; Jiang, G.-C.; Ni, X.-X.; Li, Y.-Y.; Wang, X.-Z.; Luo, X.-G. Styrene butadiene resin/nano-SiO2 composite as a water-and-oil-dispersible plugging agent for oil-based drilling fluid. Colloids Surf. A Phys. Eng. Asp. 2020, 606, 125245. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.-C.; Wang, K.; Wang, X. Laponite nanoparticle as a multi-functional additive in water-based drilling fluids. J. Mater. Sci 2017, 52, 12266–12278. [Google Scholar] [CrossRef]
- Li, P.-X. Preparation of a novel nano polymer emulsion blocking agent using in drilling fluids for shale gas exploration. Fresenius Environ. Bull. 2020, 29, 1798–1803. [Google Scholar]
- Xie, G.; Luo, P.-Y.; Deng, M.-Y.; Wang, Z. Nanoblocking Performance of Hyperbranched Polyamine as Nanoblocking Agent in Oil-Based Drilling Fluid. J. Nanomater. 2015, 2015, 821910. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Xiao, Y.-R.; Deng, M.-Y.; Luo, Y.-J.; Luo, P.-Y. Low Molecular Weight Branched Polyamine as a Clay Swelling Inhibitor and Its Inhibition Mechanism: Experiment and Density Functional Theory Simulation. Energy Fuels 2020, 34, 2169–2177. [Google Scholar] [CrossRef]




| Name | Permeability after Blocking | Blocking Rate |
|---|---|---|
| /10−3 mD | /wt% | |
| Clearwater | 5.85 | - |
| 2.5 wt% poly(ST-VS-B-E) + Clearwater | 0.39 | 93.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Chen, Y.; Li, J.; Sheng, C.; Sheng, J.; Wu, Z.; Zhou, Y.; Li, X.; Xie, G. Synthesis of Poly(styrene-vinyl sodium sulfonate-butyl acrylate-ethyl methacrylate) and Its Blocking Mechanism as a Nanometer Material in Water-Based Drilling Fluid. Sustainability 2022, 14, 13861. https://doi.org/10.3390/su142113861
Yu J, Chen Y, Li J, Sheng C, Sheng J, Wu Z, Zhou Y, Li X, Xie G. Synthesis of Poly(styrene-vinyl sodium sulfonate-butyl acrylate-ethyl methacrylate) and Its Blocking Mechanism as a Nanometer Material in Water-Based Drilling Fluid. Sustainability. 2022; 14(21):13861. https://doi.org/10.3390/su142113861
Chicago/Turabian StyleYu, Jiantao, Yu Chen, Jingying Li, Chen Sheng, Jiaxun Sheng, Zhengtian Wu, Ye Zhou, Xiaodong Li, and Gang Xie. 2022. "Synthesis of Poly(styrene-vinyl sodium sulfonate-butyl acrylate-ethyl methacrylate) and Its Blocking Mechanism as a Nanometer Material in Water-Based Drilling Fluid" Sustainability 14, no. 21: 13861. https://doi.org/10.3390/su142113861




