Hydrogen Purification by Pressure Swing Adsorption: High-Pressure PSA Performance in Recovery from Seasonal Storage
Abstract
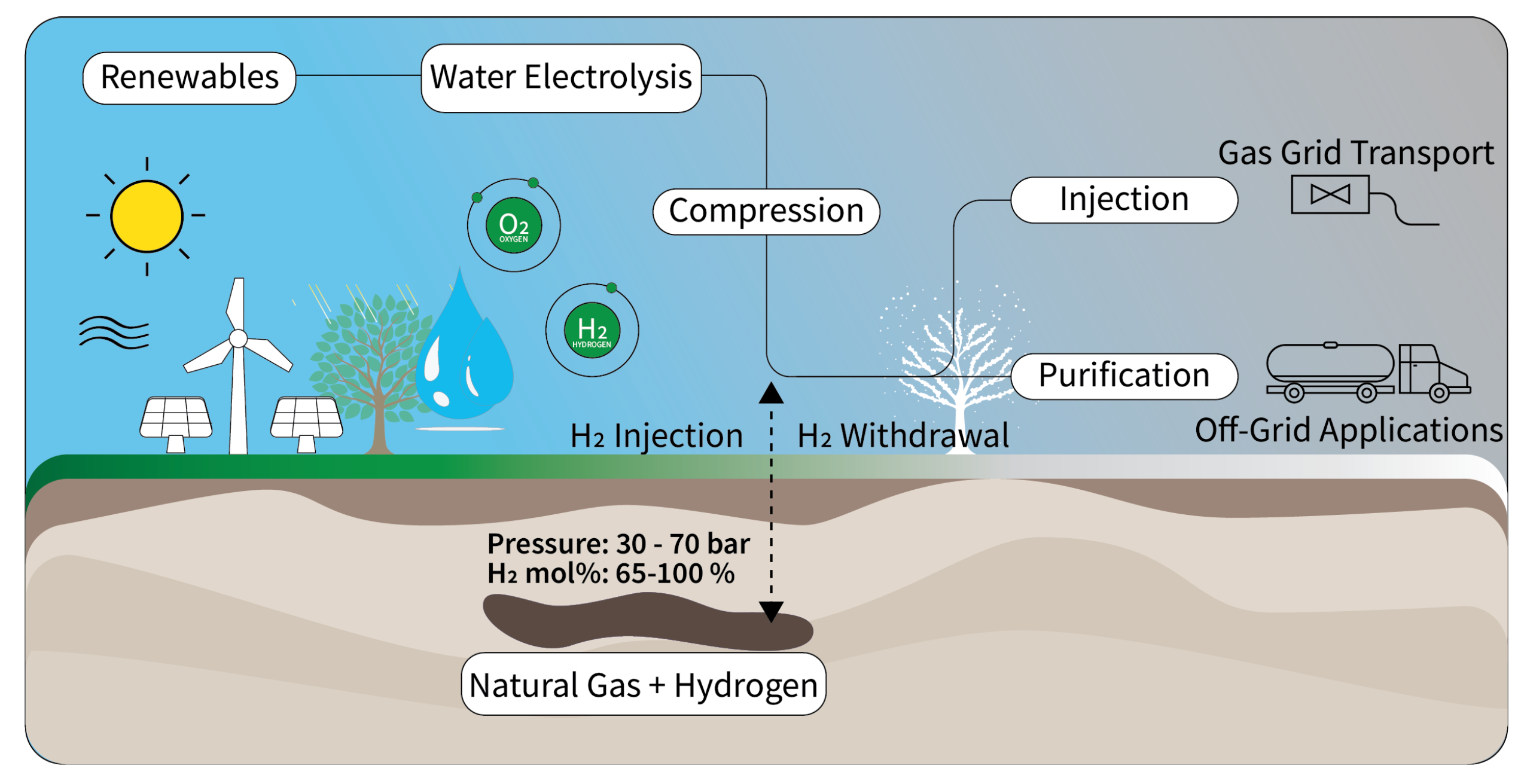
1. Introduction
2. Materials and Methods
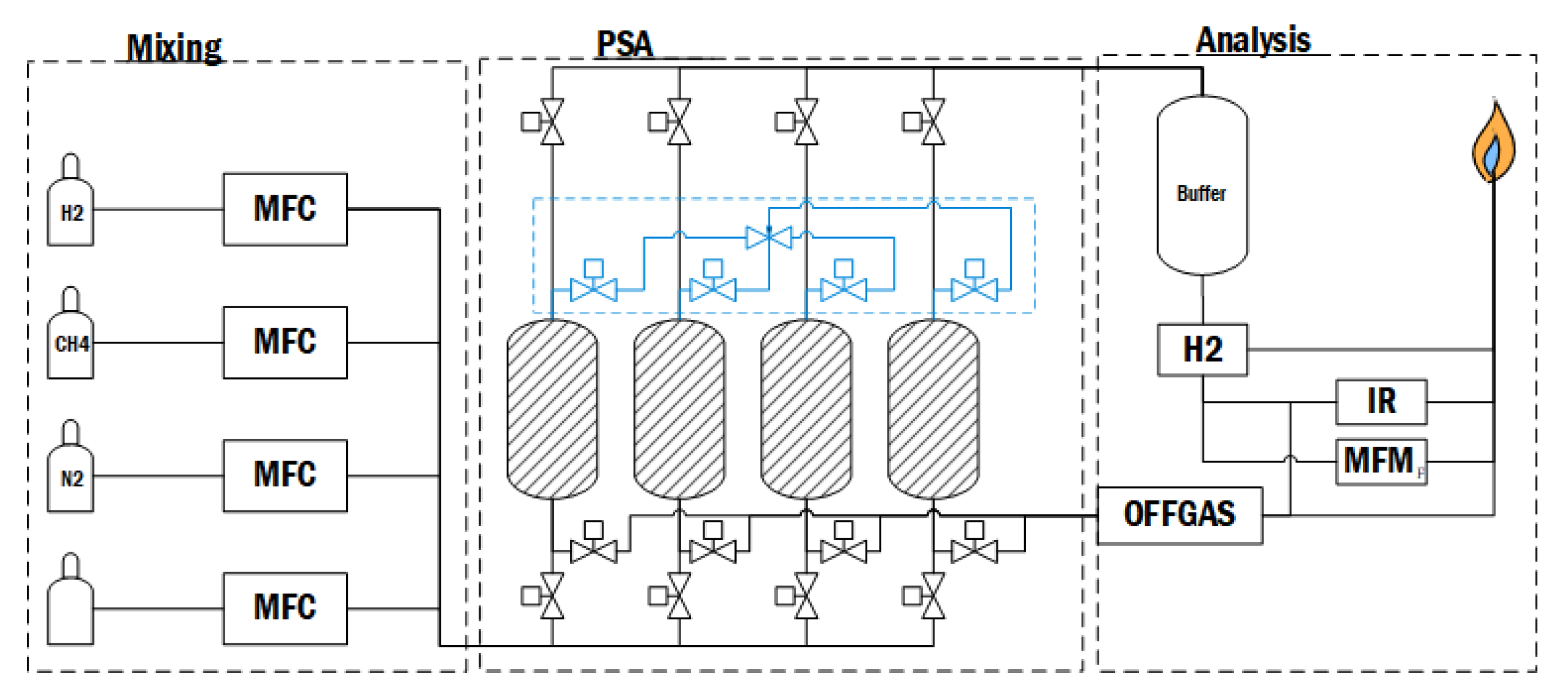
- The mixing station includes the gas cylinders inside a ventilated cabinet, the feed lines and a set of mass flow controllers (MFCs). Here, the gas mixture can be tailored to the experiment. Hydrogen and methane were used, and nitrogen was connected on the third line for flushing and inertization of the system.
- The pressure swing adsorption unit consists of four carbon-filled pressure vessels, each with a set of valves for feed, offgas and product. All columns are connected to the crossflow panel for the interconnection of adsorbers in the depressurization, purging and pressurization steps. The central point of this panel is the manual needle valve, which allows for gradual pressure equalization. Each adsorber is equipped with a pressure sensor, filter and safety valves. This subunit of the system was scaled up to a pilot-sized plant, the correlation and scaling property being interstitial velocity.
- The analysis section starts with an empty adsorber column on the product line, which serves as a buffer to protect the sensitive equipment from surges of flow or pressure. The product and offgas lines are equipped with pressure sensors and PID-controlled control valves to limit the flowrate. Then, both lines are routed to the flare under a closed fume hood to neutralize dangerous gases on atmospheric pressures. There are three routes for the gases to take to the flare: direct route, through the mass flow meter or the gas analyzer unit with gas sensors. An infrared sensor measures the methane, and the hydrogen is measured through temperature conductive capacity in the same unit.
2.1. Experimental Work
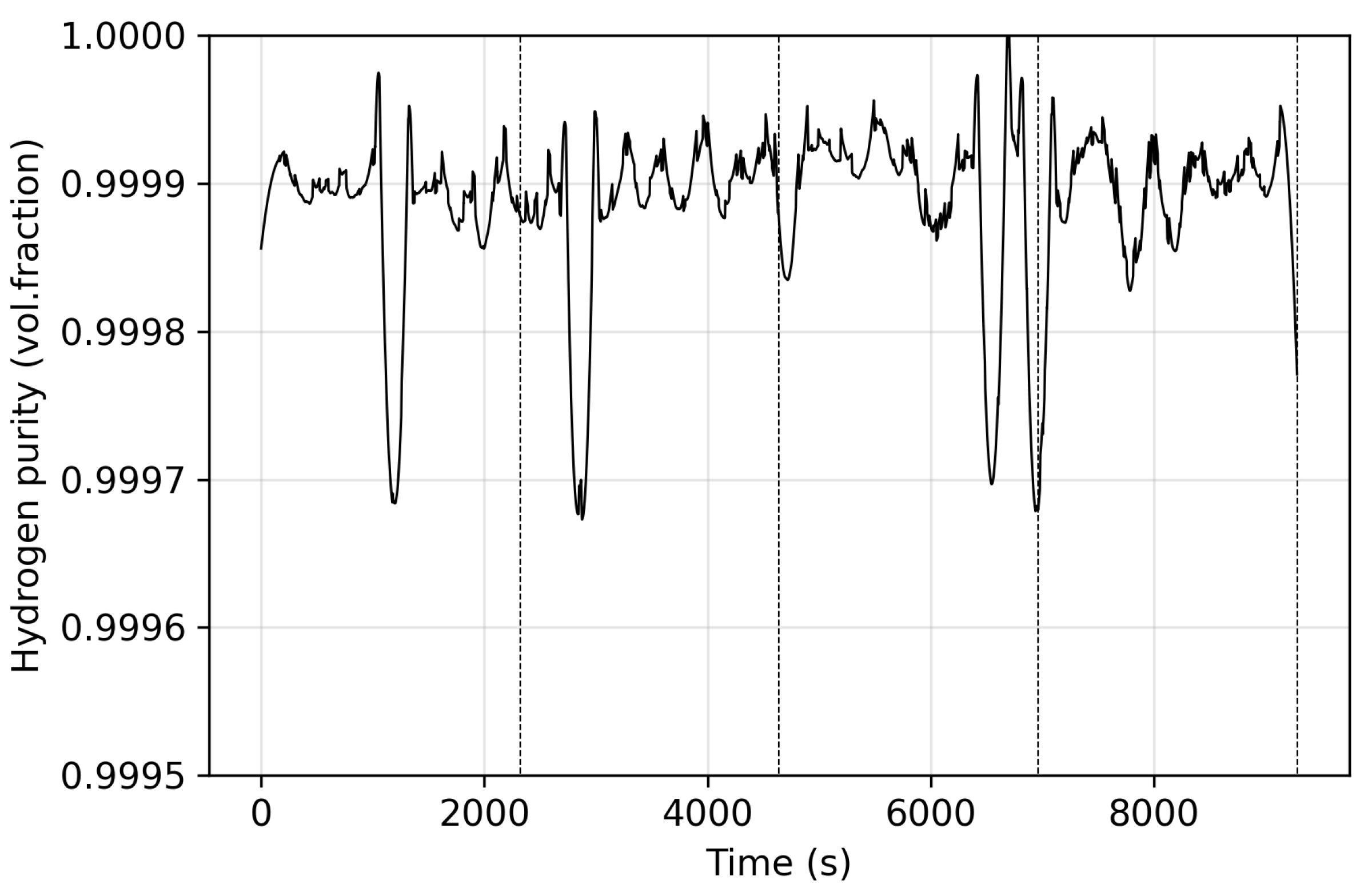
2.2. Product Purity
2.3. Hydrogen Recovery
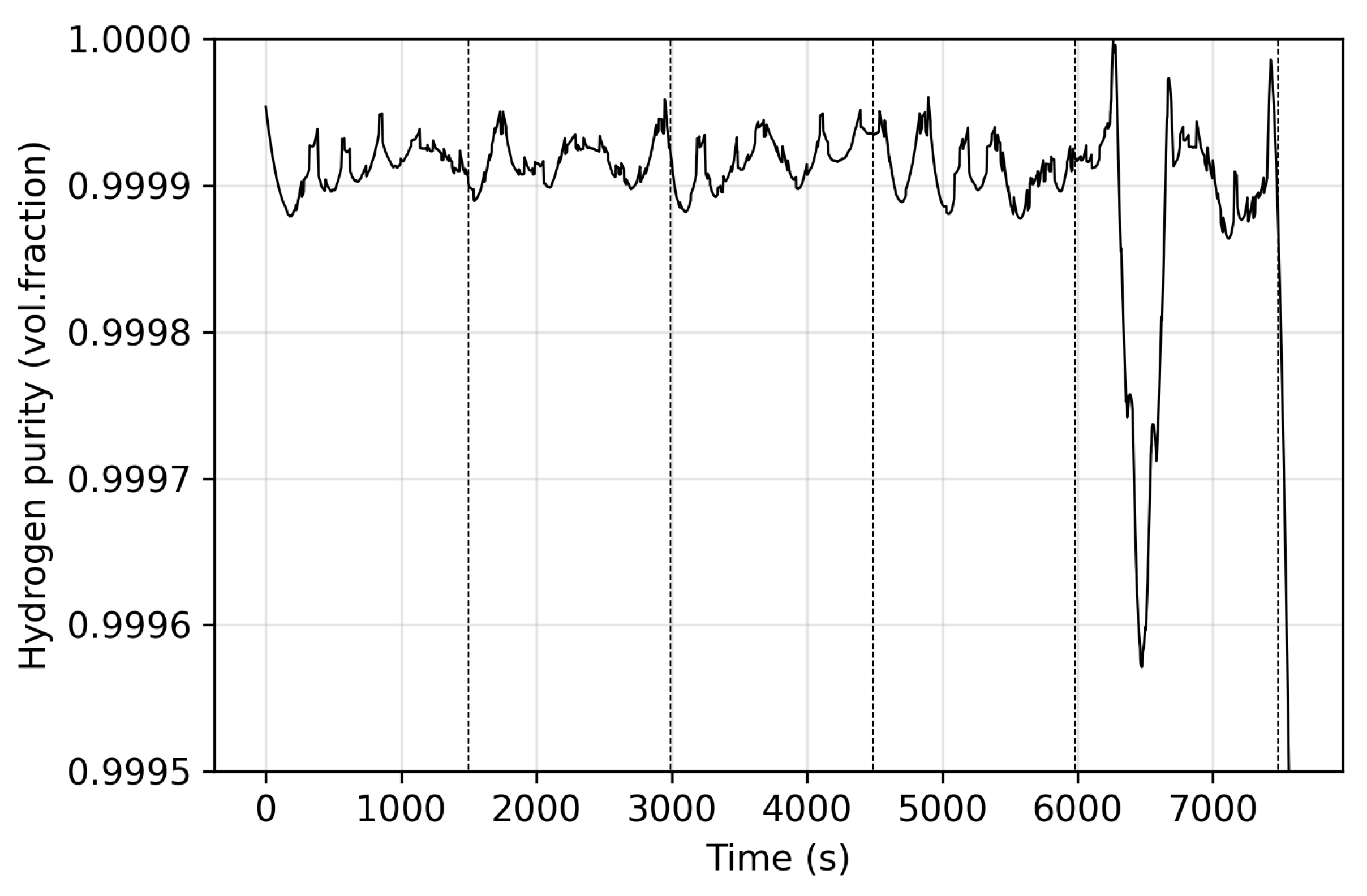
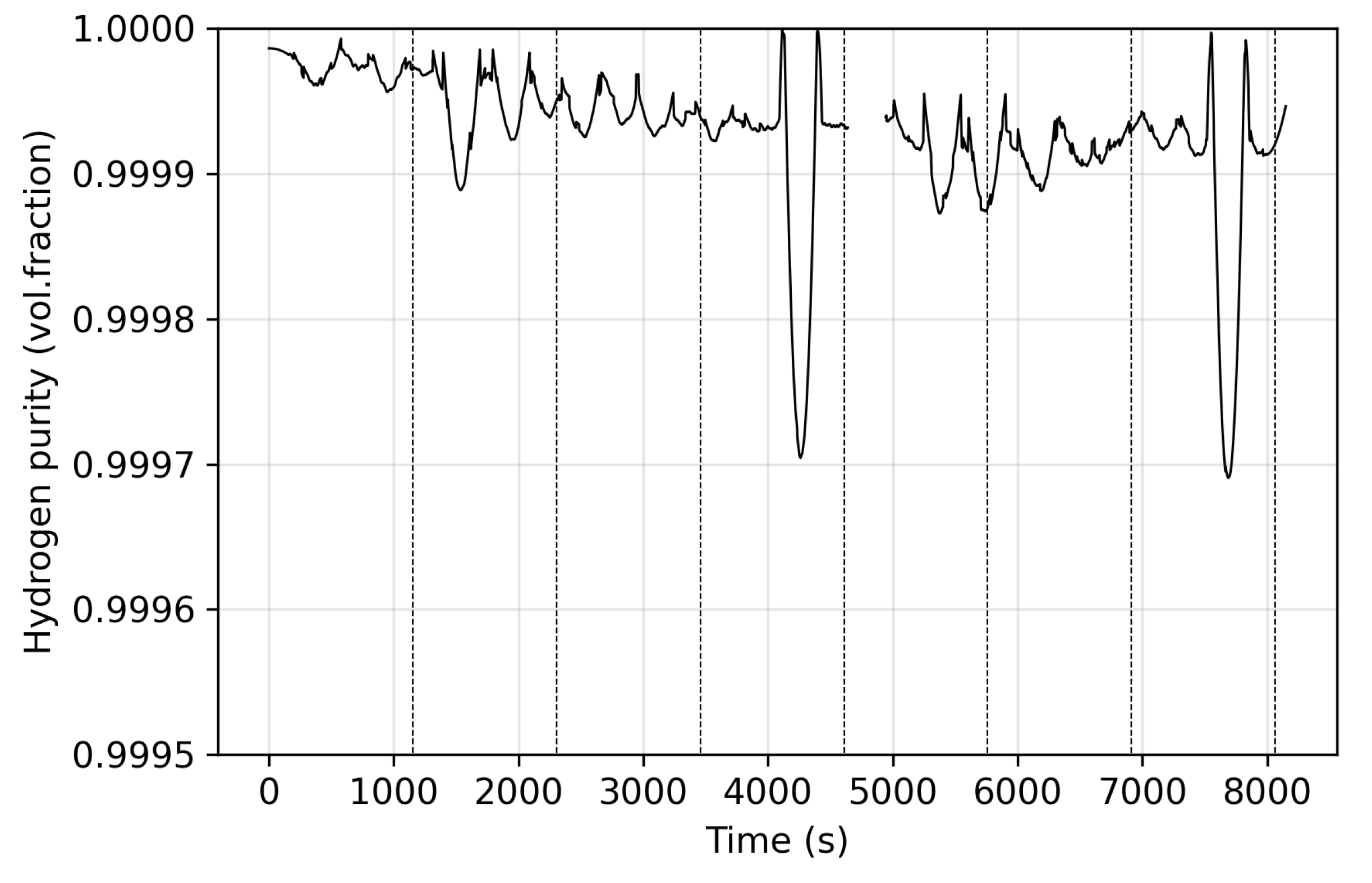
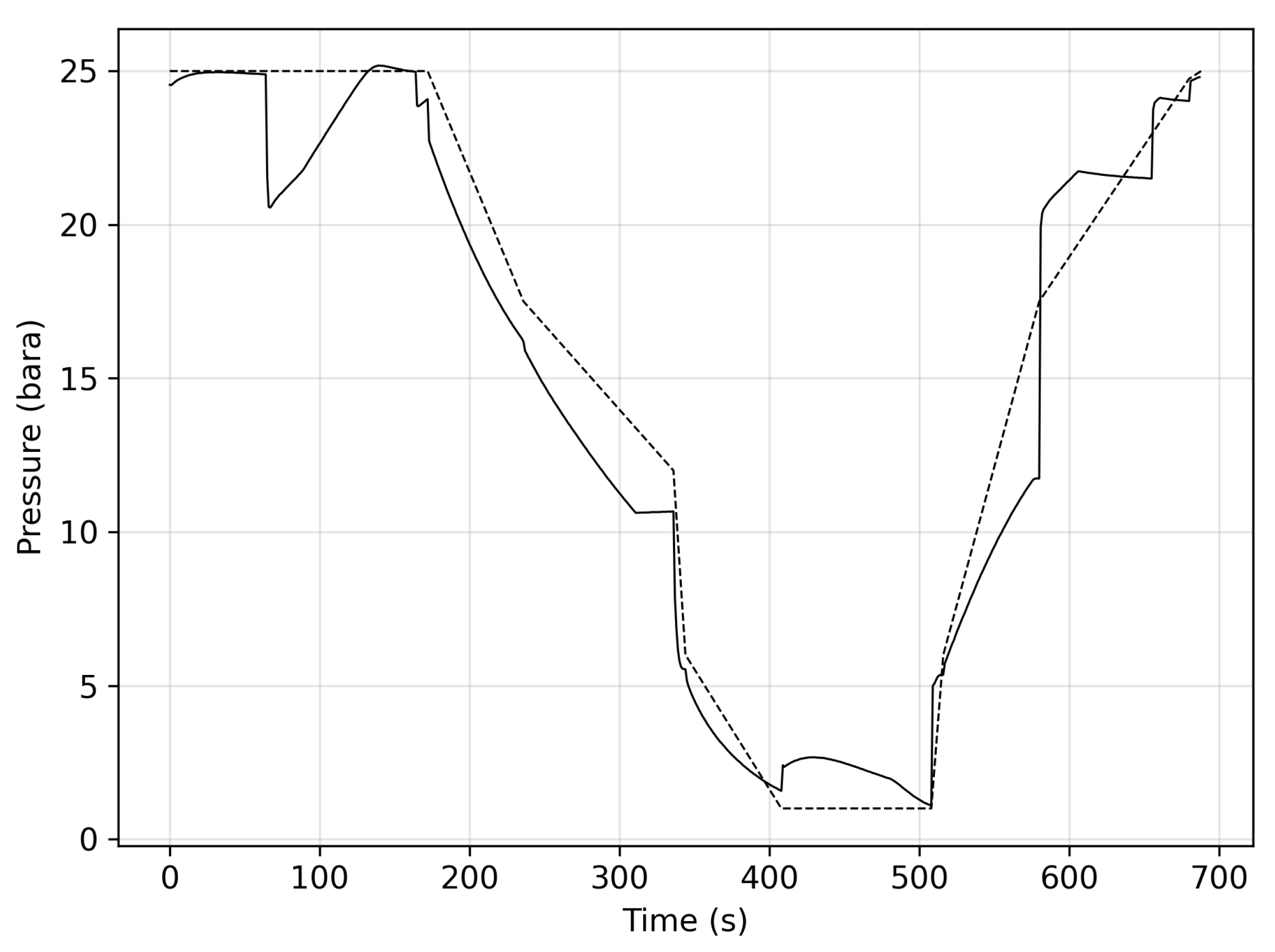
3. Results
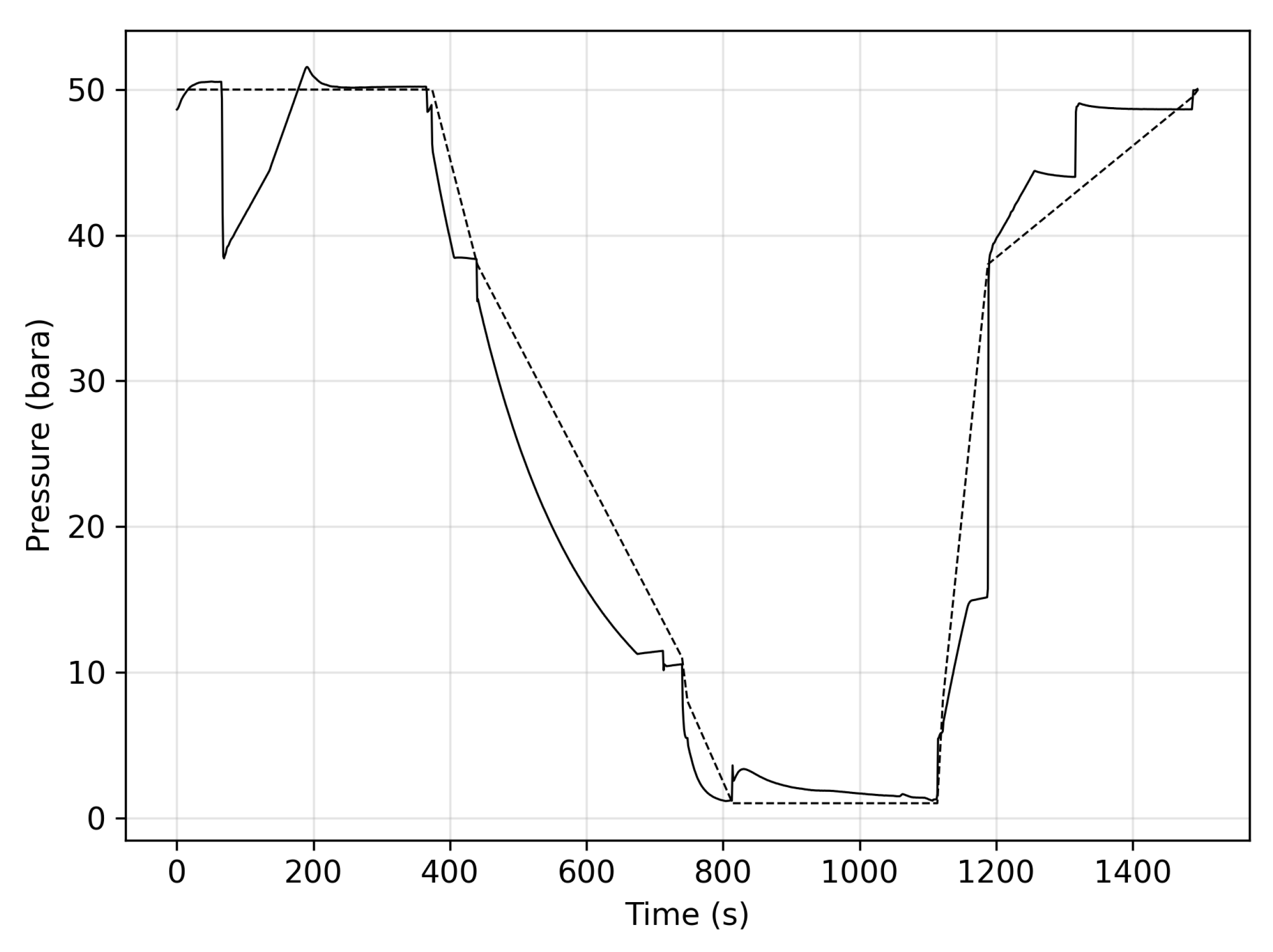
3.1. Stage One
3.2. Stage Two
3.3. Stage Three
3.4. Stage Four
3.5. Recovery and Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EU | European Union |
| GHG | Greenhouse gas |
| IR | Infrared |
| MFC | Mass flow controller |
| MFM | Mass flow meter |
| PSA | Pressure swing adsorption |
| PLC | Programmable logic controller |
| SMR | Steam methane reforming |
| UGS | Underground storage |
References
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Our World Data 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 18 August 2022).
- European Commission, Directorate-General for Communication. The European Green Deal; COM/2019/640 Final; Publications Office of the European Union: Luxembourg, 2019. [CrossRef]
- European Commission, Directorate-General for Energy. A Hydrogen Strategy for Climate Neutral Europe; COM/2020/301 Final; Publications Office of the European Union: Luxembourg, 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0301 (accessed on 20 October 2022).
- European Commission, Directorate-General for Communication. REPowerEU, Joint European Action for More Affordable, Secure and Sustainable Energy; COM/2022/230 Final; Publications Office of the European Union: Luxembourg, 2022. [CrossRef]
- Gatzen, C. Green gas supply for Germany & Europe—A technology neutral approach. In Proceedings of the 5th European Gas Technology Conference, Hamburg, Germany, 14–15 June 2022. [Google Scholar]
- Tarkowski, R.; Uliasz-Misiak, B.; Tarkowski, P. Storage of hydrogen, natural gas, and carbon dioxide—Geological and legal conditions. Int. J. Hydrogen Energy 2021, 46, 20010–20022. [Google Scholar] [CrossRef]
- Hévin, G. Underground storage of Hydrogen in salt caverns. In Proceedings of the European Workshop on Underground Energy Storage, Paris, France, 7–8 November 2019. [Google Scholar]
- Portarapillo, M.; Di Benedetto, A. Risk Assessment of the Large-Scale Hydrogen Storage in Salt Caverns. Energies 2021, 14, 2856. [Google Scholar] [CrossRef]
- Netherlands Enterprise Agency. The Effects of Hydrogen Injection in Natural Gas Networks for the Dutch Underground Storages; Technical Report; DBI Gas- und Umwelttechnik GmbH: Leipzig, Germany, 2017.
- Sambo, C.; Dudun, A.; Samuel, S.A.; Esenenjor, P.; Muhammed, N.S.; Haq, B. A review on worldwide underground hydrogen storage operating and potential fields. Int. J. Hydrogen Energy 2022, 47, 22840–22880. [Google Scholar] [CrossRef]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Amid, A.; Mignard, D.; Wilkinson, M. Seasonal storage of hydrogen in a depleted natural gas reservoir. Int. J. Hydrogen Energy 2016, 41, 5549–5558. [Google Scholar] [CrossRef]
- Hemme, C.; Van Berk, W. Hydrogeochemical modeling to identify potential risks of underground hydrogen storage in depleted gas fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Cihlar, J.; Mavins, D.; van der Leun, K. Picturing the Value of Underground Gas Storage to the European Hydrogen System; Technical Report; Gas Infrastructure Europe: Utrecht, The Netherlands, 2021. [Google Scholar]
- Perez, A.; Pérez, E.; Dupraz, S.; Bolcich, J. Patagonia Wind—Hydrogen Project: Underground Storage and Methanation. In Proceedings of the 21st World Hydrogen Energy Conference, Zaragoza, Spain, 13–16 June 2016. [Google Scholar]
- Underground Sun Storage Website. 2017. Available online: https://www.underground-sun-storage.at/en/ (accessed on 11 August 2022).
- Underground Sun Storage 2030 Website. 2021. Available online: https://www.uss-2030.at/en/ (accessed on 11 August 2022).
- Yang, R.T. Gas Separation by Adsorption Processes; Butterworth Publishers: Stoneham, MA, USA, 1987. [Google Scholar]
- Skarstrom, C.W. Method and Apparatus for Fractionating Gaseous Mixtures by Adsorption. US2944627A, 12 July 1960. [Google Scholar]
- Montgareuil, P.G.D.; Domine, D. Process for Separating a Binary Gaseous Mixture by Adsorption. US3155468A, 3 November 1964. [Google Scholar]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1984. [Google Scholar]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering; McGraw-Hill, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Ruthven, D.M.; Farooq, S.; Knaebel, K.S. Pressure Swing Adsorption; VCH Publishers, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Voss, C. Applications of pressure swing adsorption technology. Adsorption 2005, 11, 527–529. [Google Scholar] [CrossRef]
- Malek, A.; Farooq, S. Hydrogen purification from refinery fuel gas by pressure swing adsorption. AIChE J. 1998, 44, 1985–1992. [Google Scholar] [CrossRef]
- Sircar, S.; Waldron, W.; Rao, M.; Anand, M. Hydrogen production by hybrid SMR–PSA–SSF membrane system. Sep. Purif. Technol. 1999, 17, 11–20. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.N.; Cho, S.H. Performance analysis of four-bed H2 PSA process using layered beds. AIChE J. 2000, 46, 790–802. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, M.K.; Lee, D.G.; Ahn, H.; Kim, M.J.; Lee, C.H. Heat-exchange pressure swing adsorption process for hydrogen separation. AIChE J. 2008, 54, 2054–2064. [Google Scholar] [CrossRef]
- Lopes, F.V.S. New Pressure Swing Adsorption Cycles for Hydrogen Purification from Stem Reforming Off-Gases. Ph.D. Thesis, University of Porto, Porto, Portugal, 2010. [Google Scholar]
- Lopes, F.V.; Grande, C.A.; Rodrigues, A.E. Activated carbon for hydrogen purification by pressure swing adsorption: Multicomponent breakthrough curves and PSA performance. Chem. Eng. Sci. 2011, 66, 303–317. [Google Scholar] [CrossRef]
- Shi, W.; Yang, H.; Shen, Y.; Fu, Q.; Zhang, D.; Fu, B. Two-stage PSA/VSA to produce H2 with CO2 capture via steam methane reforming (SMR). Int. J. Hydrogen Energy 2018, 43, 19057–19074. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Burgers, I.A.E. Novel Technology for Hydrogen Separation from Natural Gas Using Pressure Swing Adsorption. Master’s Thesis, TU Delft, Delft, The Netherlands, 2020. [Google Scholar]
- Luberti, M.; Ahn, H. Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]






| Location | Operator | Pressure | Energy |
|---|---|---|---|
| Clemens Dome (USA, TX) | Conoco Philips | 70–135 bar | 92 GWh |
| Moss Bluff (USA, TX) | Praxair | 55–152 bar | 120 GWh |
| Spindletop (USA, TX) | Air Liquide | 68–202 bar | 270 GWh |
| Teesside (UK) | Sabic | 45 bar | 25 GWh |
| Stage | Adsorption Pressure (bar) | Mole Fraction Ratio (H2:CH4) |
|---|---|---|
| Stage 1 | 60 | 98:2 |
| Stage 2 | 50 | 90:10 |
| Stage 3 | 35 | 75:25 |
| Stage 4 | 25 | 65:35 |
| Experiment Number | Feed H2/CH4 [mol%] | Adsorption Pressure [bara] | Cycle Time [sec] | P/F Ratio 1 |
|---|---|---|---|---|
| Run#06 (Stage 1) | 98.0/2.0 | 60 | 2520 | 0.18 |
| Run#09 (Stage 1) | 98.0/2.0 | 60 | 1512 | 0.18 |
| Run#13 (Stage 1) | 98.0/2.0 | 60 | 2320 | 0.12 |
| Run#07 (Stage 2) | 90.0/10.0 | 50 | 1496 | 0.38 |
| Run#10 (Stage 2) | 90.1/9.9 | 50 | 896 | 0.30 |
| Run#14 (Stage 2) | 90.1/9.9 | 50 | 1496 | 0.20 |
| Run#01 (Stage 3) | 80.1/19.9 | 35 | 1152 | 0.30 |
| Run#03 (Stage 3) | 80.1/19.9 | 35 | 1152 | 0.40 |
| Run#04 (Stage 3) | 80.1/19.9 | 35 | 1152 | 0.40 |
| Run#11 (Stage 3) | 80.1/19.9 | 35 | 692 | 0.40 |
| Run#15 (Stage 3) | 80.1/19.9 | 35 | 1152 | 0.30 |
| Run#02 (Stage 4) | 70.1/29.9 | 25 | 688 | 0.50 |
| Run#05 (Stage 4) | 70.2/29.8 | 25 | 688 | 0.60 |
| Run#08 (Stage 4) | 70.1/29.9 | 25 | 492 | 0.50 |
| Run#12 (Stage 4) | 70.1/29.9 | 25 | 492 | 0.30 |
| Run#16 (Stage 4) | 70.2/29.8 | 25 | 688 | 0.30 |
| Bed | Step Sequence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | ADS | EQ1− | PPG | EQ2− | BD | PG | EQ1+ | EQ2+ | PR | |||
| B | EQ1+ | PR | ADS | EQ1− | PPG | EQ2− | BD | PG | EQ2+ | |||
| C | BD | PG | EQ2+ | EQ1+ | PR | ADS | EQ1− | PPG | EQ2− | |||
| D | EQ1− | PPG | EQ2− | BD | PG | EQ2+ | EQ1+ | PR | ADS | |||
| Component | Content |
|---|---|
| Hydrogen purity (mole fraction) | 99.97% |
| Total non-hydrogen gases | 300 ppm |
| Water (H2O) | 5 ppm |
| Methane (CH4) | 100 ppm |
| Non-methane hydrocarbons | 2 ppm |
| Oxygen (O2) | 5 ppm |
| Helium (He) | 300 ppm |
| Nitrogen (N2) | 300 ppm |
| Argon (Ar) | 300 ppm |
| Carbon-dioxide (CO2) | 2 ppm |
| Carbon-monoxide (CO) | 20 ppm |
| Hydrogen-sulfide (H2S) | 0.004 ppm |
| Formaldehyde (HCHO) | 0.2 ppm |
| Formic acid (HCOOH) | 0.2 ppm |
| Ammonia (NH3) | 0.1 ppm |
| Total halide ion | 0.05 ppm |
| Maximum particulate matter | 1 mg/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalman, V.; Voigt, J.; Jordan, C.; Harasek, M. Hydrogen Purification by Pressure Swing Adsorption: High-Pressure PSA Performance in Recovery from Seasonal Storage. Sustainability 2022, 14, 14037. https://doi.org/10.3390/su142114037
Kalman V, Voigt J, Jordan C, Harasek M. Hydrogen Purification by Pressure Swing Adsorption: High-Pressure PSA Performance in Recovery from Seasonal Storage. Sustainability. 2022; 14(21):14037. https://doi.org/10.3390/su142114037
Chicago/Turabian StyleKalman, Viktor, Johannes Voigt, Christian Jordan, and Michael Harasek. 2022. "Hydrogen Purification by Pressure Swing Adsorption: High-Pressure PSA Performance in Recovery from Seasonal Storage" Sustainability 14, no. 21: 14037. https://doi.org/10.3390/su142114037
APA StyleKalman, V., Voigt, J., Jordan, C., & Harasek, M. (2022). Hydrogen Purification by Pressure Swing Adsorption: High-Pressure PSA Performance in Recovery from Seasonal Storage. Sustainability, 14(21), 14037. https://doi.org/10.3390/su142114037







