Posidonia-Based Compost and Dredged Sediment in Growing Media Improve Tolerance and Nutrient Uptake in Ornamental Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Growing Media Characterisation
2.3. Plant Growth and Physiology
2.4. Statistical Analysis
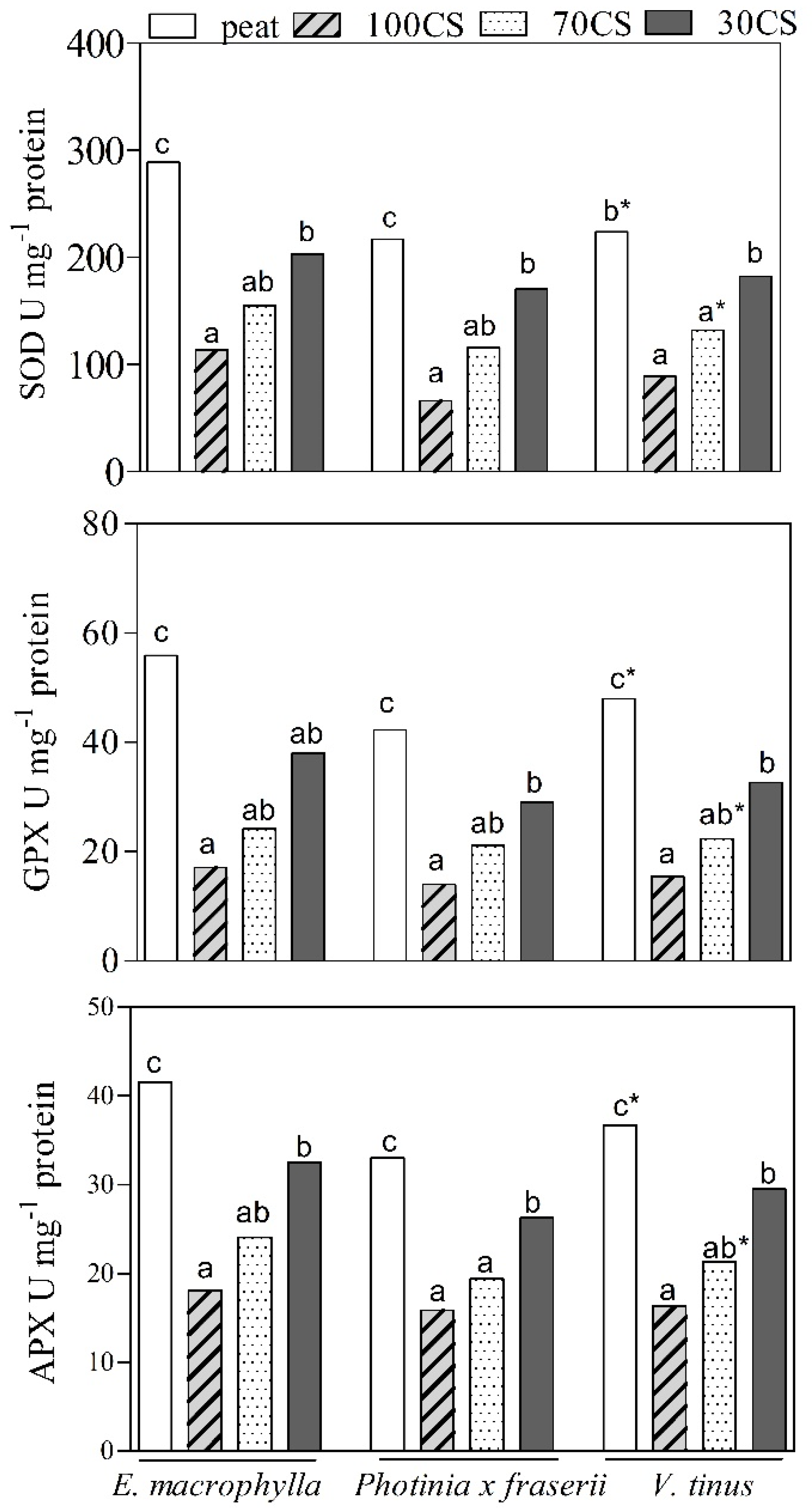
3. Results
3.1. Growing Media Properties
3.2. Plant Growth and Physiological Responses
3.3. Plant-Growing Media Relationship
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asaduzzaman, M. Soilless Culture: Use of Substrates for the Production of Quality Horticultural Crops; BoD—Books on Demand: Norderstedt, Germany, 2015. [Google Scholar]
- Raviv, M. Composts in growing media: What’s new and what’s next? Acta Hortic. 2013, 982, 39–47. [Google Scholar] [CrossRef]
- Cocozza, C.; Parente, A.; Zaccone, C.; Mininni, C.; Santamaria, P.; Miano, T. Comparative management of offshore posidonia residues: Composting vs. energy recovery. Waste Manag. 2011, 31, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mininni, C.; Santamaria, P.; Abdelrahman, H.M.; Cocozza, C.; Miano, T.; Montesano, F.; Parente, A. Posidonia-based compost as a peat substitute for lettuce transplant production. HortScience 2012, 47, 1438–1444. [Google Scholar] [CrossRef]
- Montesano, F.F.; Gattullo, C.E.; Parente, A.; Terzano, R.; Renna, M. Cultivation of potted sea fennel, an emerging mediterranean halophyte, using a renewable seaweed-based material as a peat substitute. Agriculture 2018, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Peruzzi, E.; Macci, C.; Doni, S.; Zelari, L.; Masciandaro, G. Co-composting as a management strategy for Posidonia oceanica residues and dredged sediments. Waste Biomass Valorization 2020, 11, 4907–4919. [Google Scholar] [CrossRef]
- Sednet. Available online: http://sednet.org (accessed on 25 July 2022).
- Ugolini, F.; Calzolari, C.; Lanini, G.M.; Massetti, L.; Pollaki, S.; Raschi, A.; Sabatini, F.; Tagliaferri, G.; Ungaro, F.; Massa, D.; et al. Testing decontaminated sediments as a substrate for ornamentals in field nursery plantations. J. Environ. Manag. 2017, 197, 681–693. [Google Scholar] [CrossRef]
- Ugolini, F.; Mariotti, B.; Maltoni, A.; Tani, A.; Salbitano, F.; Izquierdo, C.G.; Macci, C.; Masciandaro, G.; Tognetti, R. A tree from waste: Decontaminated dredged sediments for growing forest tree seedlings. J. Environ. Manag. 2018, 211, 269–277. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M.; Urbaniak, M. An assessment of bottom sediment as a source of plant nutrients and an agent for improving soil properties. Environ. Eng. Manag. J. 2019, 18, 1647–1656. [Google Scholar] [CrossRef]
- Tozzi, F.; Pecchioli, S.; Renella, G.; Melgarejo, P.; Legua, P.; Macci, C.; Doni, S.; Masciandaro, G.; Giordani, E.; Lenzi, A. Remediated marine sediment as growing medium for lettuce production: Assessment of agronomic performance and food safety in a pilot experiment. J. Sci. Food Agric. 2019, 99, 5624–5630. [Google Scholar] [CrossRef]
- Macci, C.; Vannucchi, F.; Doni, S.; Peruzzi, E.; Lucchetti, S.; Castellani, M.; Masciandaro, G. Recovery and environmental recycling of sediments: The experience of CNR-IRET Pisa. J. Soils Sediment. 2022, 22, 2865–2872. [Google Scholar] [CrossRef]
- Koniarz, T.; Baran, A.; Tarnawski, M. Agronomic and environmental quality assessment of growing media based on bottom sediment. J. Soils Sediment. 2022, 22, 1355–1367. [Google Scholar] [CrossRef]
- Braga, B.B.; de Carvalho, T.R.A.; Brosinsky, A.; Foerster, S.; Medeiros, P.H.A. From waste to resource: Cost-benefit analysis of reservoir sediment reuse for soil fertilization in a semiarid catchment. Sci. Total Environ. 2019, 670, 158–169. [Google Scholar] [CrossRef]
- Kiani, M.; Raave, H.; Simojoki, A.; Tammeorg, O.; Tammeorg, P. Recycling Lake sediment to agriculture: Effects on plant growth, nutrient availability, and leaching. Sci. Total Environ. 2021, 753, 141984. [Google Scholar] [CrossRef]
- Regulation 2019:1009. Regulation (EU) 2019/1009 of the European parliament and of the council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Gazz. Uff. 2019, 170, 1–114. [Google Scholar]
- D.lgs. 75/2010. Decreto Legislativo 29 aprile 2010, n. 75. Riordino e revisione della disciplina in materia di fertilizzanti, a norma dell’articolo 13 della legge 7 luglio 2009, n. 88. Gazz. Uffi Ciale 2010, 106, 1–146. [Google Scholar]
- Renella, G. Recycling and reuse of sediments in agriculture: Where Is the Problem? Sustainability 2021, 13, 1648. [Google Scholar] [CrossRef]
- Peruzzi, E.; Macci, C.; Doni, S.; Longo, V.; Souid, A.; Ugolini, F.; Zelari, L.; Masciandaro, G. Posidonia oceanica based-compost and dredged sediments as a growth substrate for ornamental plants. Acta Hortic. 2021, 1305, 317–324. [Google Scholar] [CrossRef]
- Doni, S.; Macci, C.; Peruzzi, E.; Iannelli, R.; Ceccanti, B.; Masciandaro, G. Decontamination and functional reclamation of dredged brackish sediments. Biodegradation 2013, 24, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Welsh, D.F. Effect of Irrigation Regimes on Plant Performance and Root Characteristics of Container-Grown Photinia x Fraseri. Ph.D. Thesis, Texas A&M University, Texas, TX, USA, 1989. [Google Scholar]
- Guérin, V.; Lemaire, F.; Marfà, O.; Caceres, R.; Giuffrida, F. Growth of Viburnum tinus in peat-based and peat-substitute growing media. Sci. Hortic. 2001, 89, 129–142. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Jia, B.; Wu, Q.; Zang, D.; Yu, X. Analysis of the genetic diversity of the coastal and island endangered plant species Elaeagnus macrophylla via conserved DNA-derived polymorphism marker. PeerJ 2020, 8, e8498. [Google Scholar] [CrossRef] [Green Version]
- EN 13037:2011; Soil Improvers and Growing Media—Determination of pH. European Committee for Standardization (CEN): Bruxelles, Belgium, 2011.
- EN 13038:2011; Soil Improvers and Growing Media—Determination of Electrical Conductivity. European Committee for Standardization (CEN): Bruxelles, Belgium, 2011.
- EN 13041:2011; Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. European Committee for Standardization (CEN): Bruxelles, Belgium, 2011.
- Rodelo-Torrente, S.; Espinosa, A.C.T.; Pallares, M.M.; Osorio, D.P.; Echeverría-González, A. Soil fertility in agricultural production units of tropical areas. Glob. J. Environ. Sci. Manag. 2022, 8, 403–418. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Souid, A.; Bellani, L.; Gabriele, M.; Pucci, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B.; Longo, V. Phytochemical and biological activities in Limonium species collected in different biotopes of Tunisia. Chem. Biodivers. 2019, 16, e1900216. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalase and Peroxidase. Method Enzymol. 1955, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Montesano, F.F.; Parente, A.; Grassi, F.; Santamaria, P. Posidonia-based compost as a growing medium for the soilless cultivation of tomato. Int. Symp. Grow. Media Soil. Cultiv. 2013, 1034, 277–282. [Google Scholar] [CrossRef]
- Mininni, C.; Grassi, F.; Traversa, A.; Cocozza, C.; Parente, A.; Miano, T.; Santamaria, P. Posidonia oceanica (L.) based compost as substrate for potted basil production. J. Sci. Food Agric. 2015, 95, 2041–2046. [Google Scholar] [CrossRef]
- Kitir, N.; Yildirim, E.; Şahin, Ü.; Turan, M.; Ekinci, M.; Ors, S.; Kul, R.; Ünlü, H.; Ünlü, H. Peat use in horticulture. In Peat; Topcuoglu, B., Turan, M., Eds.; IntechOpen: London, UK, 2018; pp. 75–90. [Google Scholar]
- Chang, W.; Sui, X.; Fan, X.; Jia, T.; Song, F. Arbuscular Mycorrhizal Symbiosis Modulates Antioxidant Response and Ion Distribution in Salt-Stressed Elaeagnus angustifolia Seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.W.; Donahue, R.L. Soils: An Introduction to Soils and Plant Growth, 6th ed.; Prentice-Hall International, Inc.: Hoboken, NJ, USA, 1990. [Google Scholar]
- Lu, Z.; Lu, J.; Pan, Y.; Lu, P.; Li, X.; Cong, R.; Ren, T. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ. 2016, 39, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, Q.; Zheng, C.; Yang, J. The interaction between particulate organic matter and copper, zinc in paddy soil. Environ. Pollut. 2018, 243 Pt B, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-T.; Wang, Y.-J.; Li, C.-B.; He, J.-Z.; Gao, J.; Zhou, G.-M.; Friedman, S.P.; Sparks, D.L. Effect of Organic Matter on Sorption of Zn on Soil: Elucidation by Wien Effect Measurements and EXAFS Spectroscopy. Environ. Sci. Technol. 2016, 50, 2931–2937. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, F.; Scartazza, A.; Scatena, M.; Rosellini, I.; Tassi, E.; Cinelli, F.; Bretzel, F. De-inked paper sludge and mature compost as high-value components of soilless substrate to support tree growth. J. Clean. Prod. 2021, 290, 125176. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H. The physiological basis of containerised tree seedling ‘transplant shock’: A review. Aust. For. 2005, 68, 112–120. [Google Scholar] [CrossRef]
- Marschner, H. (Ed.) Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- De Micco, V.; Arena, C.; Amitrano, C.; Rouphael, Y.; De Pascale, S.; Cirillo, C. Changes in Morpho-Anatomical and Eco-Physiological Responses of Viburnum tinus L. var lucidum as Modulated by Sodium Chloride and Calcium Chloride Salinization. Horticulturae 2022, 8, 119. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Liao, L.L.; Liu, S.; Nie, M.M.; Li, J.; Zhang, L.D.; Ma, J.F.; Chen, Z.C. Magnesium deficiency triggers SGR—Mediated chlorophyll degradation for magnesium remobilization. Plant Physiol. 2019, 181, 262–275. [Google Scholar] [CrossRef] [Green Version]
- Tewari, R.K.; Kumar, P.; Tewari, N.; Srivastava, S.; Sharma, P.N. Macronutrient deficiencies and differential antioxidant responses—Influence on the activity and expression of superoxide dismutase in maize. Plant Sci. 2004, 166, 687–694. [Google Scholar] [CrossRef]



| Indicator | Italian Regulation 75/2010 1 | EU Fertilisers Regulation 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Base Growing Media | Mixed Growing Media | Growing Medium | S ** | P ** | 100 CS ** | 30 CS | 70 CS ** | |
| Zn (mg kg−1) | <500 * | <500 | 237 | 15.2 | 35.2 | 182 | 115 | |
| Cd (mg kg−1) | <1.5 * | <1.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | |
| Ni (mg kg−1) | <100 * | <50 | 44.3 | 9.65 | 28.6 | 35.3 | 29.1 | |
| Pb (mg kg−1) | <140 * | <120 | 46.1 | 15.8 | 12.8 | 39.1 | 35.8 | |
| Cu (mg kg−1) | <230 * | <200 | 51.7 | 12.5 | 12.3 | 41.2 | 30.1 | |
| Hg (mg kg−1) | <1.5 * | <1 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | |
| Total organic carbon(%) | >8 | >4 | 1.81 | 27.9 | 22.2 | 7.65 | 3.33 | |
| Total Nitrogen (%) | 1.031 | 0.579 | 0.334 | 0.158 | ||||
| pH | 3.5–7.5 | 4.5–8.5 | 8.6 | 6.7 | 6.7 | 7.6 | 7.4 | |
| Electrical conductivity (dS m−1) | <0.7 | <1 | 0.28 | 0.30 | 0.70 | 0.19 | 0.19 | |
| Dry Bulk density (g cm−3) | <0.45 | <0.95 | 1.36 | 0.36 | 0.29 | 0.66 | 0.86 | |
| P | 100 CS | 70 CS | 30 CS | Reference Values * | ||
|---|---|---|---|---|---|---|
| Zn | 15a ** | 35b ** | 115c | 182d ** | ||
| Cu | 12a ** | 12a ** | 30b | 41c ** | ||
| K | 2500b | 2190a | 2902c | 3414d | ||
| Mg | 5000a | 4460a | 5605b | 6471b | ||
| Ca | 8000a | 17,400b | 23,960c | 28,544c | ||
| Ca/Mg | 1.6a | 3.9b | 4.2b | 4.4b | <1 | Calcium deficiency |
| 1–2 | Low level of Ca to Mg | |||||
| 2–5 | ideal | |||||
| >5 | Mg deficiency | |||||
| Mg/K | 2.0a | 2.0a | 1.9a | 1.9a | <1 | Mg deficiency |
| 1–3 | Acceptable | |||||
| 3 | ideal | |||||
| 3–18 | Acceptable | |||||
| >18 | K deficiency | |||||
| Ca/K | 3.2a | 7.9b | 8.3b | 8.4b | <30 | suitable |
| >30 | K deficiency | |||||
| (Ca + Mg)/K | 5.2a | 10.0b | 10.2b | 10.3b | <40 | K suitable |
| >40 | K deficiency | |||||
| E. macrophylla | Photinia × fraseri | V. tinus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | 100 CS | 70 CS | 30 CS | P | 100 CS | 70 CS | 30 CS | P | 100 CS | 70 CS | 30 CS | ||
| SRG | % | 261a | 256a | 253a | 204a | 215a | 179a | 180a | 170a | 170a * | 141a | 159a * | 118a |
| GS | mmol m−2 s−1 | 115a | 126a | 190a | 117a | 72a | 126a | 85a | 82a | 110a * | 98a | 93a * | 61a |
| TR | mmol H2O m−2 s−1 | 2.44a | 2.52a | 3.43b | 2.44a | 1.67a | 2.51a | 2.08a | 1.81a | 2.49a * | 2.20a | 2.07a * | 1.53a |
| PN | µmol CO2 m−2 s−1 | 8.00a | 8.35a | 13.73b | 7.80a | 4.52a | 7.60b | 5.70a | 3.83a | 8.10ab * | 9.37b | 6.70ab * | 3.60a |
| Chl a | mg g−1 dy weight | 967a | 1096a | 937a | 902a | 940a | 666a | 791a | 864a | 1291a | 1198a | 1266a | 1420a |
| E. macrophylla | Photinia × fraseri | V. tinus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | 100 CS | 70 CS | 30 CS | P | 100 CS | 70 CS | 30 CS | P | 100 CS | 70 CS | 30 CS | ||
| Zn | mg kg−1 | 3.91a | 8.59b | 4.02a | 9.02b | 2.64a | 3.85a | 1.88a | 6.38b | 4.90a | 10.7c | 10.4bc | 8.92b |
| Cu | mg kg−1 | 3.11a | 3.74a | 6.95b | 9.86c | 2.10a | 2.41ab | 1.87a | 2.66b | 4.67a | 5.28b | 5.63b | 6.64b |
| Mn | mg kg−1 | 173b | 127a | 203bc | 218c | 46a | 45a | 67b | 46a | 102a | 117a | 148b | 180c |
| K | g kg−1 | 5.98a | 5.36a | 4.54a | 4.87a | 8.92b | 7.87ab | 7.82ab | 6.78a | 9.23b | 7.43a | 8.14ab | 6.67a |
| Mg | g kg−1 | 1.01b | 1.03b | 0.86a | 1.07b | 1.87a | 2.12a | 1.72a | 1.92a | 1.39a | 1.76b | 1.49ab | 1.88b |
| Ca | g kg−1 | 5.64a | 6.32a | 6.39a | 8.08b | 7.57a | 11.9b | 8.25b | 11.9b | 2.91a | 5.22b | 4.43ab | 6.17b |
| PC1 | PC2 | |
|---|---|---|
| SOD | −0.917 * | 0.009 |
| GPX | −0.898 * | 0.079 |
| APX | −0.869 * | 0.018 |
| Cu l | −0.109 | −0.896 * |
| Zn l | 0.243 | −0.629 * |
| Mn l | −0.529 | −0.786 * |
| K l | 0.112 | 0.626 * |
| Mg l | 0.452 | 0.661 * |
| Ca l | 0.387 | 0.430 |
| PN | 0.051 | −0.546 |
| Mg/K | 0.771 * | −0.311 |
| Ca/K | 0.770 * | −0.328 |
| (Ca + Mg)/K | 0.815 * | −0.343 |
| Proportion of variance | 38.28% | 26.60% |
| Cumulative proportion of variance | 38.28% | 64.88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannucchi, F.; Macci, C.; Doni, S.; Longo, V.; Ugolini, F.; Masciandaro, G.; Peruzzi, E. Posidonia-Based Compost and Dredged Sediment in Growing Media Improve Tolerance and Nutrient Uptake in Ornamental Plants. Sustainability 2022, 14, 14419. https://doi.org/10.3390/su142114419
Vannucchi F, Macci C, Doni S, Longo V, Ugolini F, Masciandaro G, Peruzzi E. Posidonia-Based Compost and Dredged Sediment in Growing Media Improve Tolerance and Nutrient Uptake in Ornamental Plants. Sustainability. 2022; 14(21):14419. https://doi.org/10.3390/su142114419
Chicago/Turabian StyleVannucchi, Francesca, Cristina Macci, Serena Doni, Vincenzo Longo, Francesca Ugolini, Grazia Masciandaro, and Eleonora Peruzzi. 2022. "Posidonia-Based Compost and Dredged Sediment in Growing Media Improve Tolerance and Nutrient Uptake in Ornamental Plants" Sustainability 14, no. 21: 14419. https://doi.org/10.3390/su142114419





