Evaluation of Aurantiochytrium mangrovei Biomass Grown on Digestate as a Sustainable Feed Ingredient of Sea Bass, Dicentrarchus labrax, Juveniles and Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot-Scale Cultivation and Harvesting of Aurantiochytrium Mangrovei on Liquid Effluent from Anaerobic Digestion
2.1.1. Preparation of A. mangrovei Inoculum
2.1.2. Preparation of the Digestate for Integration in Culture Medium at Pilot Scale
2.1.3. Cultivation of A. mangrovei at Pilot Scale
2.1.4. Harvesting of A. mangrovei Culture at Pilot Scale
2.1.5. Monitoring of Culture Concentration and Cellular Parameters
2.2. Enzymatic Hydrolysis of A. mangrovei Biomass
2.3. Feed Formulation for Seabass Juveniles and Larvae
2.3.1. Seabass Juveniles Feed
2.3.2. Seabass Larvae Feed
2.4. Experimental Design
2.4.1. Sea Bass Juvenile Rearing
2.4.2. Sea Bass Larvae Rearing
2.5. Sampling and Biometric Measurements
2.5.1. Juvenile Sampling
2.5.2. Larvae Sampling
2.6. Biochemical Analysis
2.6.1. Lipid Extraction
2.6.2. Lipid Class Analysis
2.6.3. Separation of Polar Lipids (PL) and Neutral Lipids (NL)
2.6.4. Fatty Acid Analysis in Total Lipids (TL), Polar Lipids (PL) and Neutral Lipids (NL)
2.6.5. Analysis of Lipid Peroxidation
2.6.6. Statistical Analysis
3. Results
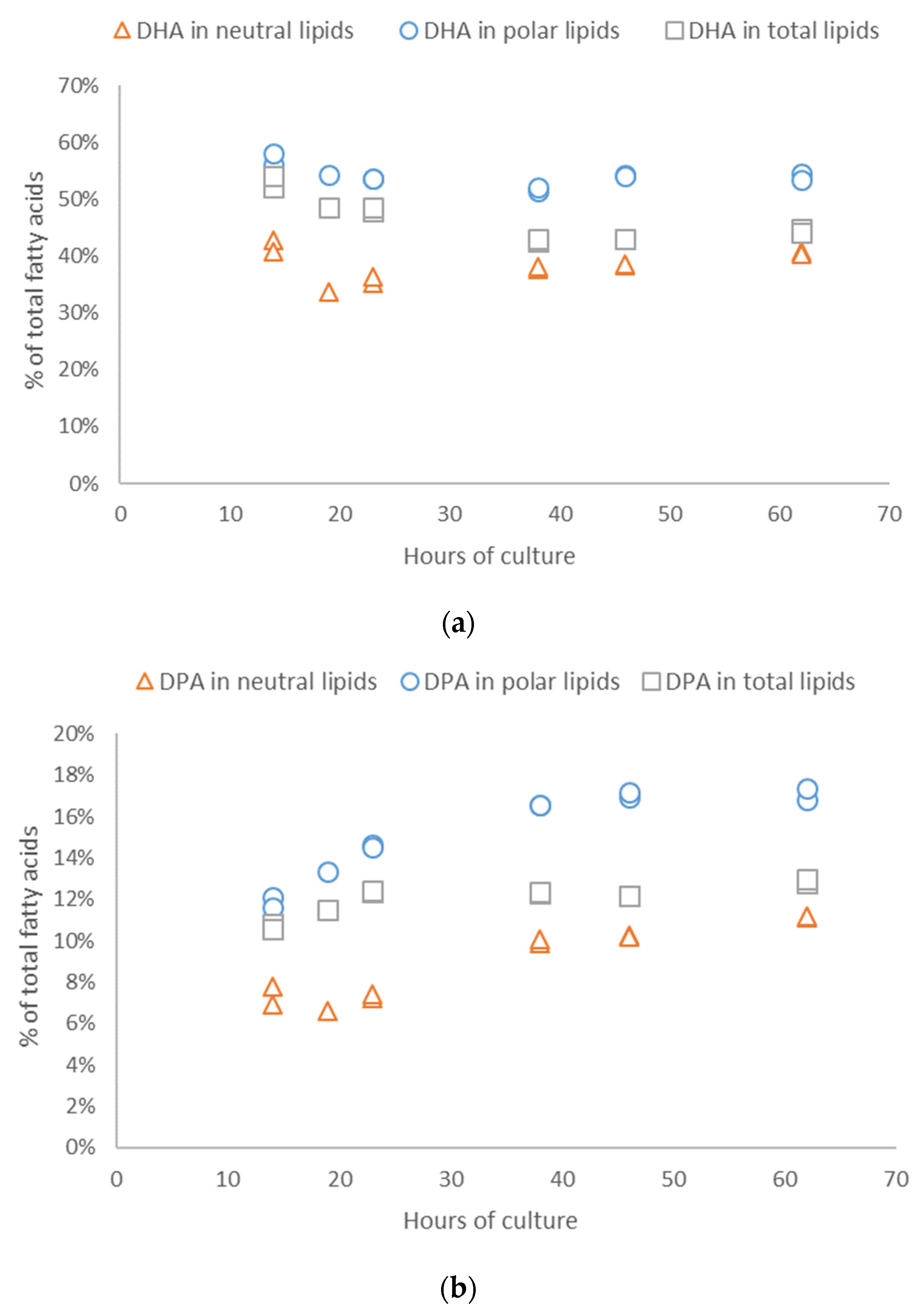
3.1. Cellular Parameters of Microalgae Culture
3.2. Biochemical Quality of Microalgae Culture and Concentrate
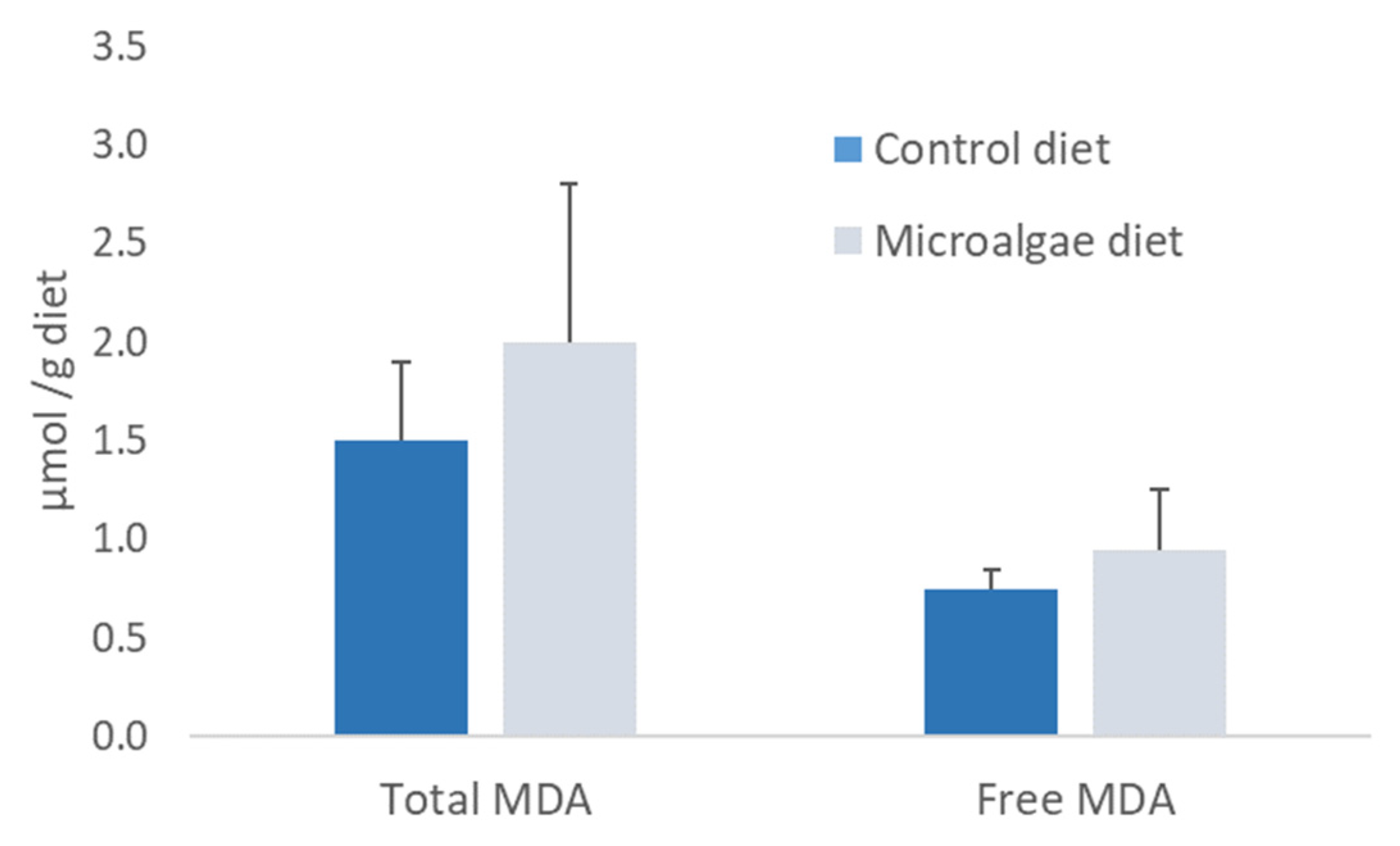
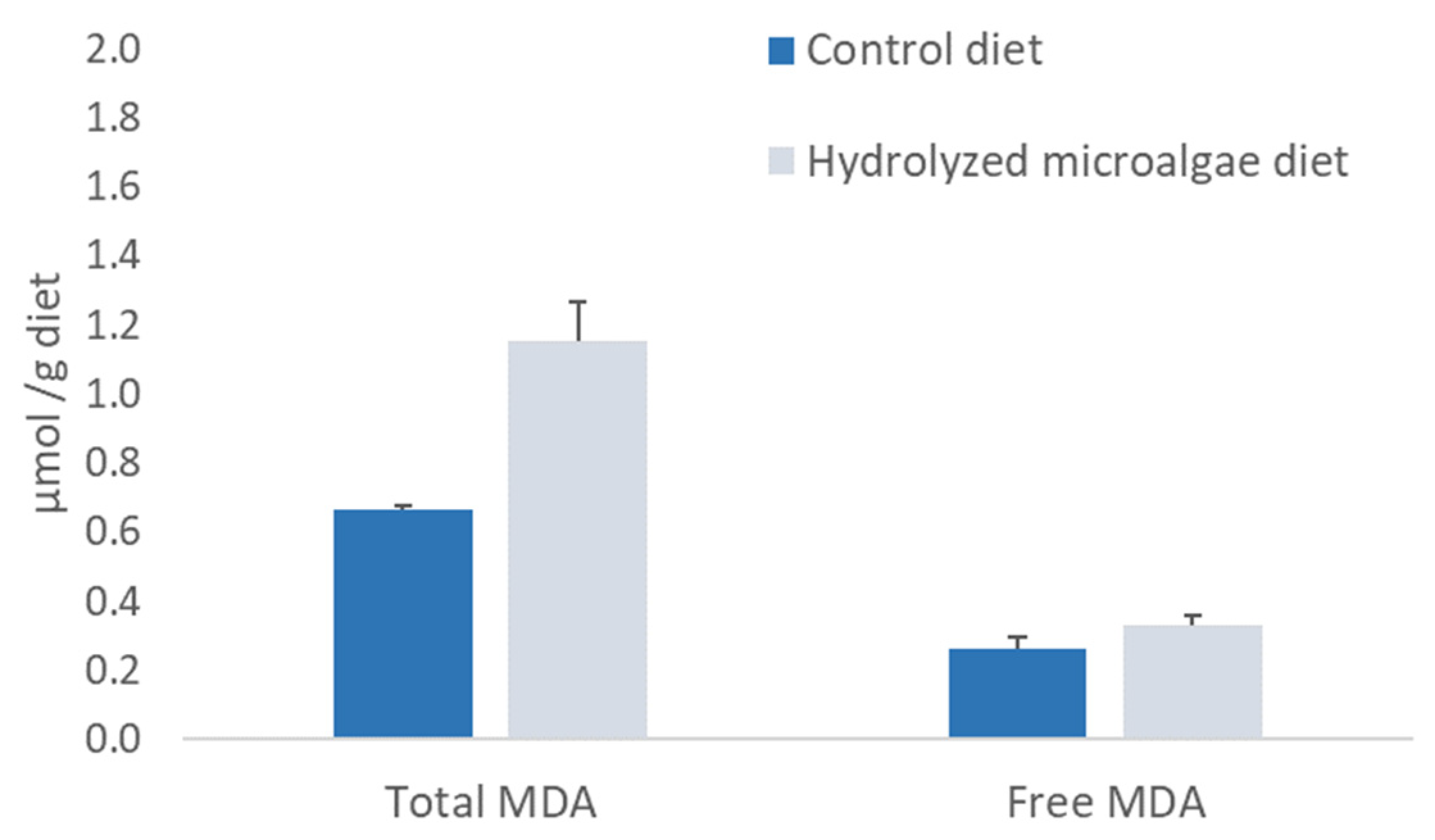
3.3. Biochemical Quality of Experimental Juvenile Diets and Larvae Micro-Diets
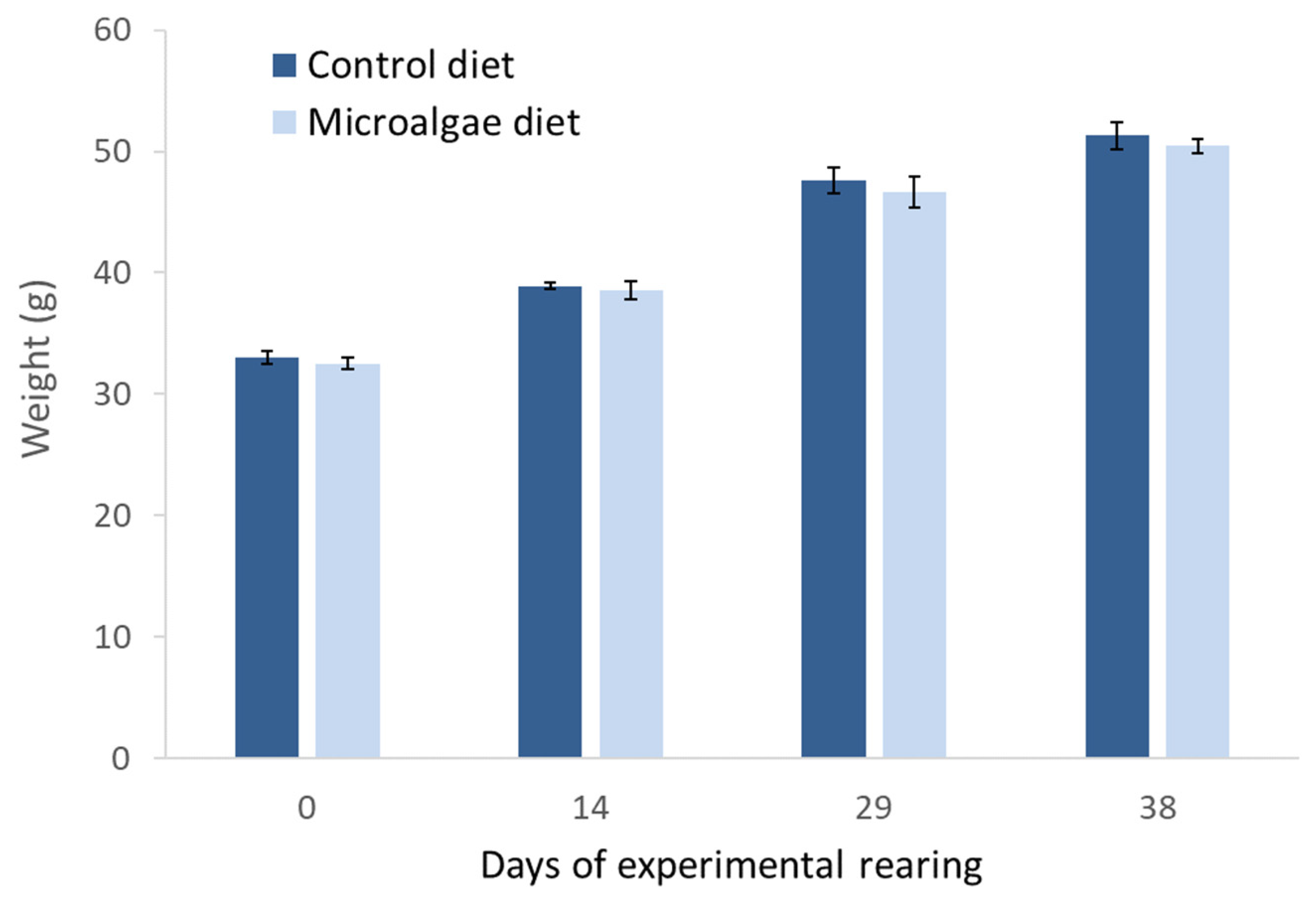
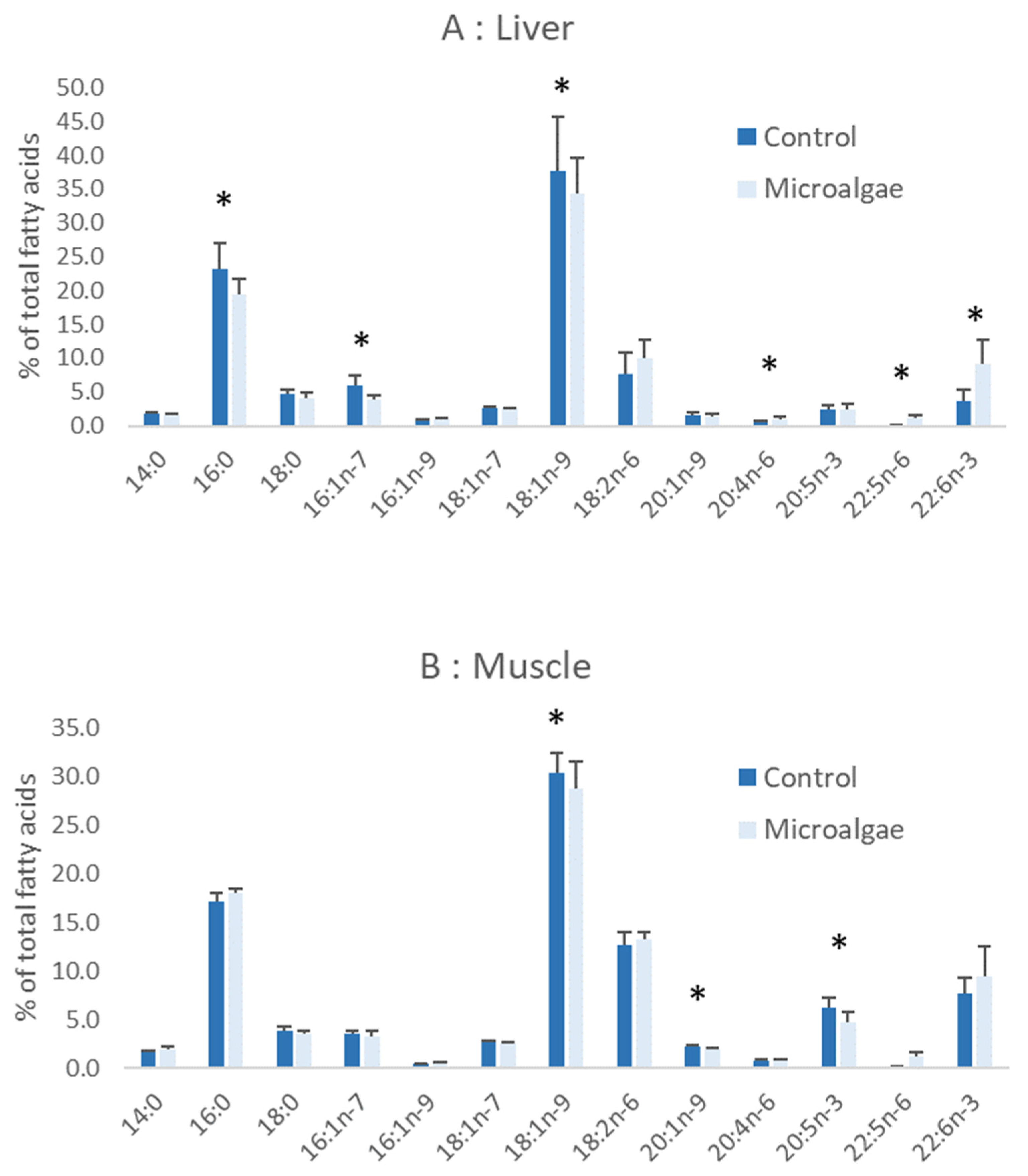
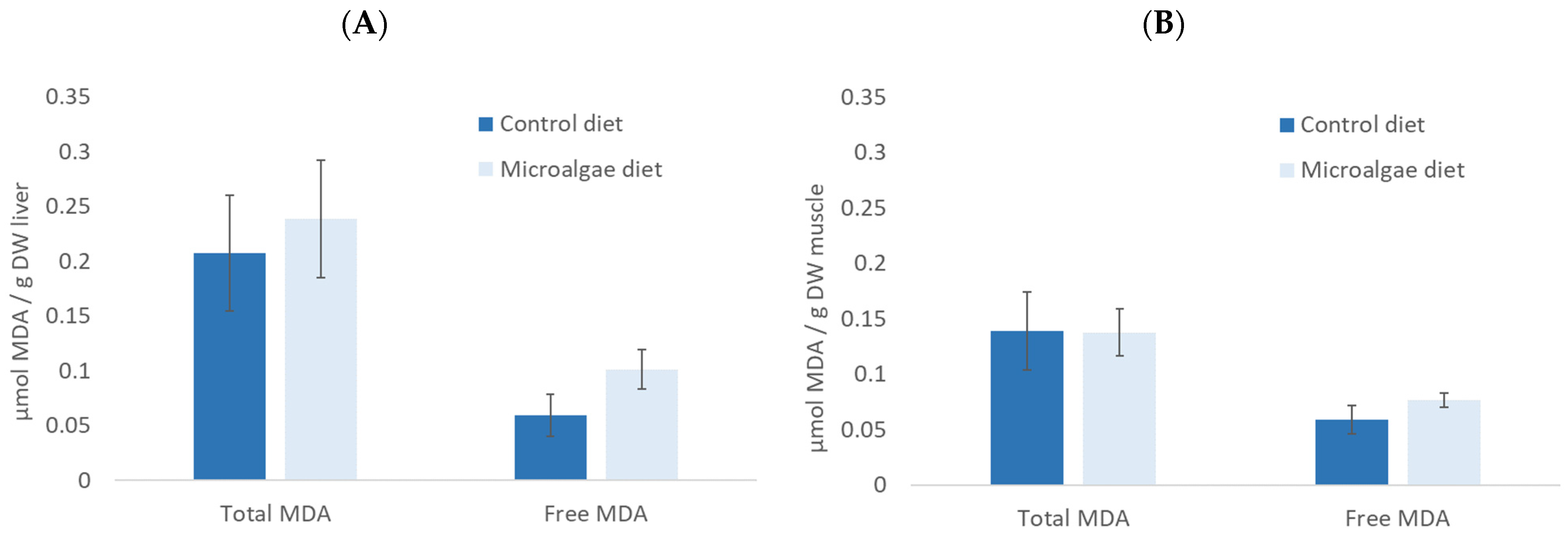
3.4. Sea Bass Juveniles Feeding Trial
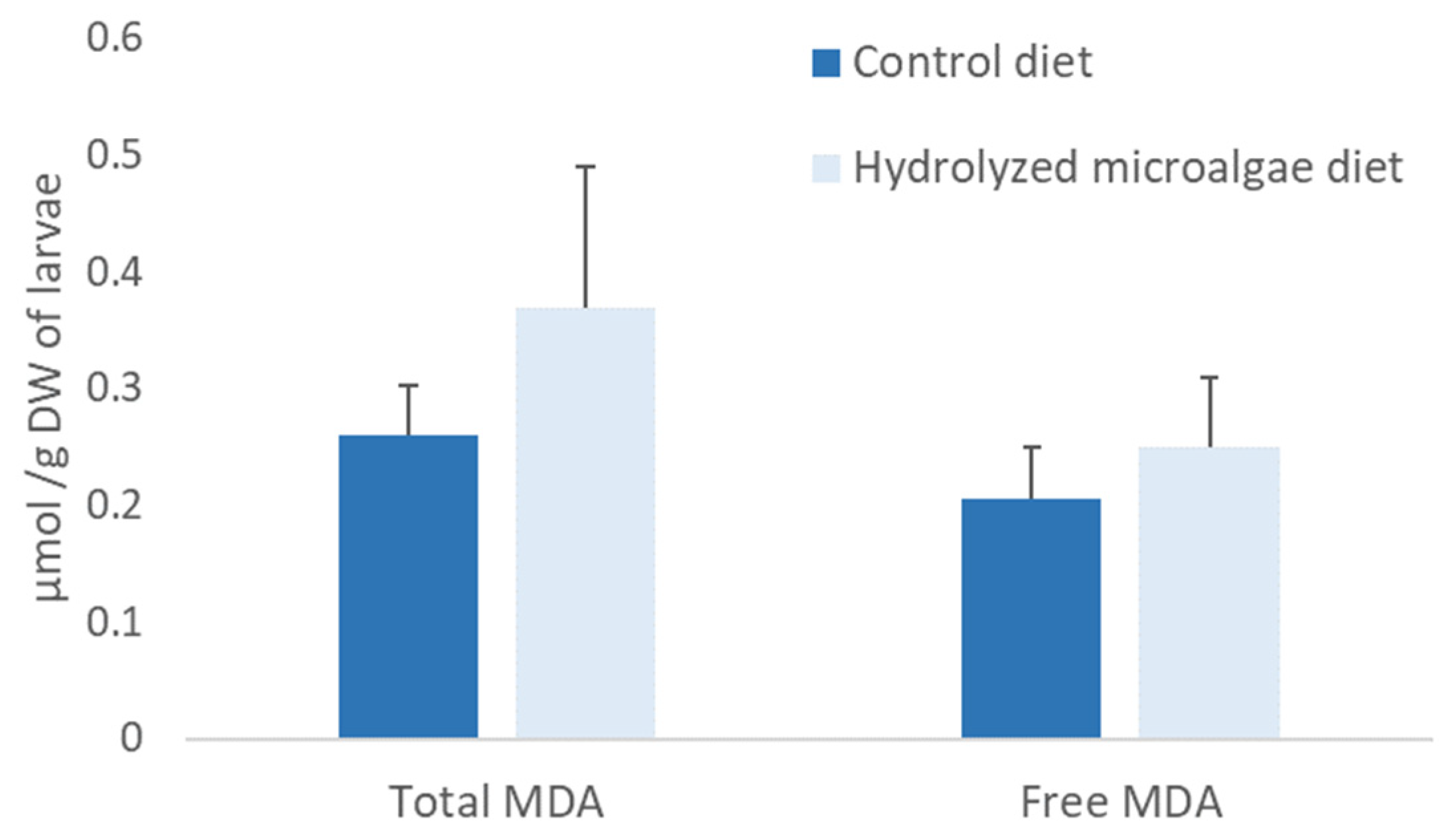
3.5. Sea Bass Larvae Feeding Trial
4. Discussion
4.1. From Microalgae Biomass Production to Fish Feed Formulation
4.1.1. Cellular and Biochemical Changes during Batch Cultivation
4.1.2. Biomass Downstream Processing
4.1.3. Fish Feed Formulation and Composition
4.2. Nutritional Value of Microalgae for Sea Bass Juveniles
4.3. Nutritional Value of Microalgae for Sea Bass Larvae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Global Overview on the Use of Fish Meal and Fish Oil in Industrially Compounded Aquafeeds: Trends and Future Prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding Aquaculture in an Era of Finite Resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Feed Matters: Satisfying the Feed Demand of Aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Jones, A.C.; Mead, A.; Kaiser, M.J.; Austen, M.C.V.; Adrian, A.W.; Auchterlonie, N.A.; Black, K.D.; Blow, L.R.; Bury, C.; Brown, J.H.; et al. Prioritization of Knowledge Needs for Sustainable Aquaculture: A National and Global Perspective. Fish Fish. 2015, 16, 668–683. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Metian, M. Demand and Supply of Feed Ingredients for Farmed Fish and Crustaceans: Trends and Prospect—FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2011. [Google Scholar]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as Feed: Using Economic Allocation to Quantify the Fish In: Fish Out Ratio of Major Fed Aquaculture Species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and Genetically Engineered Oils as Replacements for Fish Oil in Aquaculture Feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef]
- Marchan, L.F.; Lee Chang, K.J.; Nichols, P.D.; Polglase, J.L.; Mitchell, W.J.; Gutierrez, T. Screening of New British Thraustochytrids Isolates for Docosahexaenoic Acid (DHA) Production. J. Appl. Phycol. 2017, 29, 2831–2843. [Google Scholar] [CrossRef] [PubMed]
- Winwood, R.J. Recent Developments in the Commercial Production of DHA and EPA Rich Oils from Micro-Algae. OCL 2013, 20, D604. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Replacement of Fish Oil with Thraustochytrid Schizochytrium Sp. L Oil in Atlantic Salmon Parr (Salmo Salar L) Diets. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 382–392. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Gerd Marit, B.; Mørkøre, T.; Krasnov, A.; Baeverfjord, G.; Ytrestøyl, T.; Carlehög, M.; Sweetman, J.; Ruyter, B. Microalgal Schizochytrium Limacinum Biomass Improves Growth and Filet Quality When Used Long-Term as a Replacement for Fish Oil, in Modern Salmon Diets. Front. Mar. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Parrish, C.C.; Simon, C.J.; Revill, A.T.; Nichols, P.D. Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo Salar) Fingerlings: Culture Performance and Fatty Acid Incorporation. JMSE 2020, 8, 207. [Google Scholar] [CrossRef]
- Hart, B.; Schurr, R.; Narendranath, N.; Kuehnle, A.; Colombo, S.M. Digestibility of Schizochytrium Sp. Whole Cell Biomass by Atlantic Salmon (Salmo Salar). Aquaculture 2021, 533, 736156. [Google Scholar] [CrossRef]
- Ganuza, E.; Benítez-Santana, T.; Atalah, E.; Vega-Orellana, O.; Ganga, R.; Izquierdo, M.S. Crypthecodinium Cohnii and Schizochytrium Sp. as Potential Substitutes to Fisheries-Derived Oils from Seabream (Sparus Aurata) Microdiets. Aquaculture 2008, 277, 109–116. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards Sustainable Aquafeeds: Complete Substitution of Fish Oil with Marine Microalga Schizochytrium Sp. Improves Growth and Fatty Acid Deposition in Juvenile Nile Tilapia (Oreochromis Niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Robinson, E.H.; Tucker, C.S.; Manning, B.B.; Khoo, L. Effects of Dried Algae Schizochytrium Sp., a Rich Source of Docosahexaenoic Acid, on Growth, Fatty Acid Composition, and Sensory Quality of Channel Catfish Ictalurus Punctatus. Aquaculture 2009, 292, 232–236. [Google Scholar] [CrossRef]
- Terova, G.; Moroni, F.; Antonini, M.; Bertacchi, S.; Pesciaroli, C.; Branduardi, P.; Labra, M.; Porro, D.; Ceccotti, C.; Rimoldi, S. Using Glycerol to Produce European Sea Bass Feed With Oleaginous Microbial Biomass: Effects on Growth Performance, Filet Fatty Acid Profile, and FADS2 Gene Expression. Front. Mar. Sci. 2021, 8, 715078. [Google Scholar] [CrossRef]
- Kissinger, K.R.; García-Ortega, A.; Trushenski, J.T. Partial Fish Meal Replacement by Soy Protein Concentrate, Squid and Algal Meals in Low Fish-Oil Diets Containing Schizochytrium Limacinum for Longfin Yellowtail Seriola Rivoliana. Aquaculture 2016, 452, 37–44. [Google Scholar] [CrossRef]
- García-Ortega, A.; Kissinger, K.R.; Trushenski, J.T. Evaluation of Fish Meal and Fish Oil Replacement by Soybean Protein and Algal Meal from Schizochytrium Limacinum in Diets for Giant Grouper Epinephelus Lanceolatus. Aquaculture 2016, 452, 1–8. [Google Scholar] [CrossRef]
- Cahu, C.; Zambonino Infante, J. Substitution of Live Food by Formulated Diets in Marine Fish Larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- Cahu, C.L.; Zambonino Infante, J.L.; Quazuguel, P.; Le Gall, M.M. Protein Hydrolysate vs. Fish Meal in Compound Diets for 10-Day Old Sea Bass Dicentrarchus Labrax Larvae. Aquaculture 1999, 171, 109–119. [Google Scholar] [CrossRef]
- Zambonino Infante, J.L.; Cahu, C.L.; Peres, A. Partial Substitution of Di- and Tripeptides for Native Proteins in Sea Bass Diet Improves Dicentrarchus Labrax Larval Development. J. Nutr. 1997, 127, 608–614. [Google Scholar] [CrossRef]
- Gisbert, E.; Skalli, A.; Fernández, I.; Kotzamanis, Y.; Zambonino-Infante, J.L.; Fabregat, R. Protein Hydrolysates from Yeast and Pig Blood as Alternative Raw Materials in Microdiets for Gilthead Sea Bream (Sparus Aurata) Larvae. Aquaculture 2012, 338–341, 96–104. [Google Scholar] [CrossRef]
- Delcroix, J.; Gatesoupe, F.-J.; Desbruyères, E.; Huelvan, C.; Le Delliou, H.; Le Gall, M.-M.; Quazuguel, P.; Mazurais, D.; Zambonino-Infante, J.L. The Effects of Dietary Marine Protein Hydrolysates on the Development of Sea Bass Larvae, Dicentrarchus Labrax, and Associated Microbiota. Aquac. Nutr. 2015, 21, 98–104. [Google Scholar] [CrossRef]
- Yamasaki, T.; Aki, T.; Shinozaki, M.; Taguchi, M.; Kawamoto, S.; Ono, K. Utilization of Shochu Distillery Wastewater for Production of Polyunsaturated Fatty Acids and Xanthophylls Using Thraustochytrid. J. Biosci. Bioeng. 2006, 102, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Silkina, A.; Fernandes, F.; Fuentes, G.C.; Ndovela, V.; Gayo, P.J.I.; De la Broise, D.; Soudant, P.; Chauchat, L.; Seelam, J.S.; Fernandes de Souza, M.; et al. Best Practices for Microalgal Production Using Nutrient Rich Digestate as a Waste-Based Medium; Public Output Report of the ALG-AD Project. 2021. Available online: https://www.nweurope.eu/projects/project-search/alg-ad-creating-value-from-waste-nutrients-by-integrating-algal-and-anaerobic-digestion-technology/publications/the-alg-ad-project-reports-and-deliverables/ (accessed on 27 September 2022).
- De la Broise, D.; Ventura, M.; Chauchat, L.; Guerreiro, M.; Michez, T.; Vinet, T.; Gautron, N.; Le Grand, F.; Bideau, A.; Goïc, N.L.; et al. Scale-Up to Pilot of a Non-Axenic Culture of Thraustochytrids Using Digestate from Methanization as Nitrogen Source. Marine Drugs 2022, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Lelong, A.; Hégaret, H.; Soudant, P. Cell-Based Measurements to Assess Physiological Status of Pseudo-Nitzschia Multiseries, a Toxic Diatom. Res. Microbiol. 2011, 162, 969–981. [Google Scholar] [CrossRef]
- Hidalgo, F.; Alliot, E.; Thebault, H. Influence of Water Temperature on Food Intake, Food Efficiency and Gross Composition of Juvenile Sea Bass, Dicentrarchus Labrax. Aquaculture 1987, 64, 199–207. [Google Scholar] [CrossRef]
- Sardenne, F.; Bodin, N.; Metral, L.; Crottier, A.; Le Grand, F.; Bideau, A.; Brisset, B.; Bourjea, J.; Saraux, C.; Bonhommeau, S.; et al. Effects of Extraction Method and Storage of Dry Tissue on Marine Lipids and Fatty Acids. Anal. Chim. Acta 2019, 1051, 82–93. [Google Scholar] [CrossRef]
- Marty, Y.; Soudant, P.; Perrotte, S.; Moal, J.; Dussauze, J.; Samain, J.F. Identification and Occurrence of a Novel Cis-4,7,10,Trans-13-Docosatetraenoic Fatty Acid in the Scallop Pecten Maximus (L.). J. Chromatogr. A 1999, 839, 119–127. [Google Scholar] [CrossRef]
- Morita, E.; Kumon, Y.; Nakahara, T.; Kagiwada, S.; Noguchi, T. Docosahexaenoic Acid Production and Lipid-Body Formation in Schizochytrium Limacinum SR21. Mar. Biotechnol. 2006, 8, 319–327. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Mbifile, M.D.; Li, C.; Yang, H.; Wang, W. Improvement of Docosahexaenoic Acid Fermentation from Schizochytrium Sp. AB-610 by Staged PH Control Based on Cell Morphological Changes. Eng. Life Sci. 2017, 17, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and Characterization of Polyunsaturated Fatty Acid Producing Thraustochytrium Species: Screening of Strains and Optimization of Omega-3 Production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Nham Tran, T.L.; Miranda, A.F.; Gupta, A.; Puri, M.; Ball, A.S.; Adhikari, B.; Mouradov, A. The Nutritional and Pharmacological Potential of New Australian Thraustochytrids Isolated from Mangrove Sediments. Mar. Drugs 2020, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, J.; Publishing, P.; Kaushik, S.; Bergot, P.; Metailler, R. Nutrition and Feeding of Fish and Crustaceans; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; ISBN 978-1-85233-241-9. [Google Scholar]
- Remize, M.; Planchon, F.; Loh, A.N.; Le Grand, F.; Bideau, A.; Le Goic, N.; Fleury, E.; Miner, P.; Corvaisier, R.; Volety, A.; et al. Study of Synthesis Pathways of the Essential Polyunsaturated Fatty Acid 20:5n-3 in the Diatom Chaetoceros Muelleri Using 13C-Isotope Labeling. Biomolecules 2020, 10, 797. [Google Scholar] [CrossRef]
- Puccinelli, E.; Sardenne, F.; Pecquerie, L.; Fawcett, S.E.; Machu, E.; Soudant, P. Omega-3 Pathways in Upwelling Systems: The Link to Nitrogen Supply. Front. Mar. Sci. 2021, 8, 664601. [Google Scholar] [CrossRef]
- Magalhães, R.; Guerreiro, I.; Santos, R.A.; Coutinho, F.; Couto, A.; Serra, C.R.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Oxidative Status and Intestinal Health of Gilthead Sea Bream (Sparus Aurata) Juveniles Fed Diets with Different ARA/EPA/DHA Ratios. Sci. Rep. 2020, 10, 13824. [Google Scholar] [CrossRef]
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater Microalgae (Schizochytrium Sp.) as a Substitute to Fish Oil for Shrimp Feed. Sci. Rep. 2019, 9, 6178. [Google Scholar] [CrossRef]
- Lyons, P.P.; Turnbull, J.F.; Dawson, K.A.; Crumlish, M. Effects of Low-Level Dietary Microalgae Supplementation on the Distal Intestinal Microbiome of Farmed Rainbow Trout Oncorhynchus Mykiss (Walbaum). Aquac. Res. 2017, 48, 2438–2452. [Google Scholar] [CrossRef]
- De Souza, F.P.; de Lima, E.C.S.; Urrea-Rojas, A.M.; Suphoronski, S.A.; Facimoto, C.T.; Júnior, J.d.S.B.; de Oliveira, T.E.S.; Pereira, U.d.P.; Santis, G.W.D.; de Oliveira, C.A.L.; et al. Effects of Dietary Supplementation with a Microalga (Schizochytrium Sp.) on the Hemato-Immunological, and Intestinal Histological Parameters and Gut Microbiota of Nile Tilapia in Net Cages. PLoS ONE 2020, 15, e0226977. [Google Scholar] [CrossRef]
- Izquierdo, M. Essential Fatty Acid Requirements in Mediterranean Fish Species. Besoins Acides Aminés Indispens. Chez Especes Méditerranéennes Poisson. 2005, 63, 91–102. [Google Scholar]
- Mourente, G. Accumulation of DHA (Docosahexaenoic Acid; 22:6n-3) in Larval and Juvenile Fish Brain. In The big fish Bang; Institute of Marine Research: Bergen, Norway, 2003; pp. 239–258. [Google Scholar]
- Cahu, C.; Zambonino Infante, J.; Escaffre, A.-M.; Bergot, P.; Kaushik, S. Preliminary Results on Sea Bass (Dicentrarchus Labrax) Larvae Rearing with Compound Diet from First Feeding. Comparison with Carp (Cyprinus Carpio) Larvae. Aquaculture 1998, 169, 1–7. [Google Scholar] [CrossRef]
- Süzer, C.; Kamacı, H.O.; Çoban, D.; Saka, Ş.; Fırat, K.; Karacaoğlan, A. Early Weaning of Sea Bass (D. Labrax) Larvae: Effects on Growth Performance and Digestive Enzyme Activities. TrJFAS 2011, 11, 491–497. [Google Scholar]

| Experimental Juvenile Diets | ||
|---|---|---|
| Ingredients (% of Dry Weight Diet) | Control | Microalgae |
| Fish meal | 64 | 52 |
| Microalgae biomass | 0 | 15 |
| CPSP 90 (pre-digest fishmeal) | 10 | 10 |
| fish oil | 1 | 0 |
| rapeseed oil | 1 | 1 |
| rapeseed lecithin | 14 | 13 |
| Starch | 5 | 5 |
| Vitamin mix 1 | 3 | 3 |
| Mineral mix 2 | 1 | 1 |
| Cellulose | 1 | 0 |
| Total | 100 | 100 |
| Calculated proximal composition (% dry weight) | ||
| Proteins % DW | 52.1 | 48.2 |
| Lipids % DW | 18.5 | 17.8 |
| EPA+DHA % DW | 1.1 | 1.1 |
| EPA+DHA % lipids | 6.1 | 6.0 |
| Experimental Larvae Micro-Diets | ||
|---|---|---|
| Ingredients (% of Dry Weight Diet) | Control | Microalgae |
| Fish meal | 62 | 68 |
| Hydrolyzed microalgae biomass | 0 | 14 |
| CPSP 90 (pre-digest fishmeal) | 10 | 0 |
| Tuna oil | 1 | 0 |
| Soya lecithin | 13 | 13 |
| Vitamin mix 1 | 3 | 3 |
| Mineral mix 2 | 3 | 2 |
| Cellulose | 8 | 0 |
| Total | 100 | 100 |
| Calculated proximal composition (% dry weight) | ||
| Proteins % DW | 50.7 | 50.1 |
| Lipids % DW | 16.5 | 17.6 |
| EPA+DHA % DW | 1.1 | 1.2 |
| % of Total FA | Control Diet | Microalgae Diet | ||
|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |
| 14:0 | 1.6 | 0.1 | 1.6 | 0.1 |
| 15:0 | 0.2 | 0.0 | 0.8 | 0.0 |
| 16:0 | 12.1 | 0.1 | 17.4 | 0.2 |
| 17:0 | 0.2 | 0.0 | 0.3 | 0.0 |
| 18:0 | 1.8 | 0.0 | 1.7 | 0.0 |
| 20:0 | 0.2 | 0.0 | 0.2 | 0.0 |
| 16:1n-7 | 2.2 | 0.1 | 1.4 | 0.0 |
| 18:1n-7 | 2.6 | 0.1 | 2.3 | 0.1 |
| 18:1n-9 | 39.2 | 0.2 | 35.5 | 0.2 |
| 20:1n-11 | 0.5 | 0.0 | 0.4 | 0.0 |
| 20:1n-7 | 0.1 | 0.0 | 0.1 | 0.0 |
| 20:1n-9 | 1.9 | 0.1 | 1.5 | 0.1 |
| 22:1n-11 | 1.8 | 0.1 | 1.6 | 0.1 |
| 22:1n-9 | 0.3 | 0.0 | 0.2 | 0.0 |
| 24:1n-9 | 0.7 | 0.0 | 0.6 | 0.0 |
| 16:2n-4 | 0.2 | 0.0 | 0.1 | 0.0 |
| 16:3n-4 | 0.1 | 0.0 | 0.0 | 0.0 |
| 16:4n-1 | 0.2 | 0.0 | 0.1 | 0.0 |
| 18:2n-4 | 0.1 | 0.0 | 0.0 | 0.0 |
| 18:2n-6 | 19.4 | 0.4 | 17.3 | 0.2 |
| 18:3n-3 | 3.2 | 0.0 | 2.8 | 0.0 |
| 18:4n-3 | 0.6 | 0.0 | 0.4 | 0.0 |
| 20:4n-3 | 0.2 | 0.0 | 0.2 | 0.0 |
| 20:4n-6 | 0.4 | 0.0 | 0.4 | 0.0 |
| 20:5n-3 | 3.3 | 0.0 | 1.7 | 0.1 |
| 22:5n-3 | 0.5 | 0.0 | 0.3 | 0.0 |
| 22:5n-6 | 0.4 | 0.1 | 1.8 | 0.1 |
| 22:6n-3 | 4.1 | 0.2 | 7.8 | 0.3 |
| Total Branched | 0.3 | 0.0 | 0.2 | 0.0 |
| Total SFA | 16.2 | 0.1 | 22.2 | 0.3 |
| Total MUFA | 49.9 | 0.4 | 44.0 | 0.3 |
| Total PUFA | 33.6 | 0.4 | 33.5 | 0.6 |
| DHA/EPA | 1.2 | 0.0 | 4.5 | 0.1 |
| Micro-Diet Conditioning | Tank Number | Survival % |
|---|---|---|
| 1 | 1.4 | |
| Control | 2 | 2.7 |
| 3 | 26.4 | |
| 1 | 0.9 | |
| Hydrolyzed microalgae | 2 | 1.7 |
| 3 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soudant, P.; Ventura, M.; Chauchat, L.; Guerreiro, M.; Mathieu-Resuge, M.; Le Grand, F.; Simon, V.; Collet, S.; Zambonino-Infante, J.-L.; Le Goïc, N.; et al. Evaluation of Aurantiochytrium mangrovei Biomass Grown on Digestate as a Sustainable Feed Ingredient of Sea Bass, Dicentrarchus labrax, Juveniles and Larvae. Sustainability 2022, 14, 14573. https://doi.org/10.3390/su142114573
Soudant P, Ventura M, Chauchat L, Guerreiro M, Mathieu-Resuge M, Le Grand F, Simon V, Collet S, Zambonino-Infante J-L, Le Goïc N, et al. Evaluation of Aurantiochytrium mangrovei Biomass Grown on Digestate as a Sustainable Feed Ingredient of Sea Bass, Dicentrarchus labrax, Juveniles and Larvae. Sustainability. 2022; 14(21):14573. https://doi.org/10.3390/su142114573
Chicago/Turabian StyleSoudant, Philippe, Mariana Ventura, Luc Chauchat, Maurean Guerreiro, Margaux Mathieu-Resuge, Fabienne Le Grand, Victor Simon, Sophie Collet, José-Luis Zambonino-Infante, Nelly Le Goïc, and et al. 2022. "Evaluation of Aurantiochytrium mangrovei Biomass Grown on Digestate as a Sustainable Feed Ingredient of Sea Bass, Dicentrarchus labrax, Juveniles and Larvae" Sustainability 14, no. 21: 14573. https://doi.org/10.3390/su142114573
APA StyleSoudant, P., Ventura, M., Chauchat, L., Guerreiro, M., Mathieu-Resuge, M., Le Grand, F., Simon, V., Collet, S., Zambonino-Infante, J.-L., Le Goïc, N., Lambert, C., Fernandes, F., Silkina, A., de Souza, M. F., & de la Broise, D. (2022). Evaluation of Aurantiochytrium mangrovei Biomass Grown on Digestate as a Sustainable Feed Ingredient of Sea Bass, Dicentrarchus labrax, Juveniles and Larvae. Sustainability, 14(21), 14573. https://doi.org/10.3390/su142114573








