Abstract
Pomegranate produces fruit with high nutritional value. Of particular interest is the woody part of the seed, which influences consumer acceptability and is rich in bioactive components. The aim of this study was to morphoanatomically and biochemically characterize the tegmen of local and commercial pomegranates grown in Italy. SEM and a texturometer were used for morphoanatomical and hardness analysis, respectively, and gravimetric and Soxhlet extraction, as well as a GS-MS instrument, were used for chemical analysis. In addition to the classic morphometric parameters, we described, for the first time, the bundles on pomegranate tegmen, identifying four forms (FAN, TREE, COMB and RAMIFIED); local cultivars showed a higher variability compared to the commercial cultivars. Lignin content showed no correlation with seed strength. We developed a new hierarchical model in which geometric parameters and the resistant volume of the lignified tissue can explain the variability in hardness observed in the tests. Quantitative differences were observed in total lipids and unsaponifiable fractions; in particular, all oils were rich in unsaturated fatty acids. We propose that bundles form as a novel trait for characterization and a new hierarchical model to evaluate the hardness of seeds. The content of fatty acids and other biocompounds in pomegranate seeds can promote their valorization as a resource rather than a waste product.
1. Introduction
Punica granatum L., known as pomegranate, is a species that has recently attracted considerable attention, owing to its nutritional properties and considerable health benefits of its fruit and its derivatives. Pomegranate is usually consumed directly as fresh seeds, fresh juice and processed products [1]. Moreover, it has considerable market potential as an ingredient in food supplements, functional food production, pharmacological and herbal products [2]. Modern consumers demand high-quality and typical products that are locally produced; therefore, a complete characterization of autochthonous pomegranate genotypes is essential, especially in countries such as Italy, where pomegranate cultivation is expanding and the biodiversity of the local germplasm remains poorly understood [3].
Traditional approaches for pomegranate characterization include molecular [4], morphological [5] and biochemical analyses [6,7]; particular attention has been paid to qualitative characteristics of the main edible part of the fruit (the fleshy, membranous and pulpy part of the seed from which the juice is obtained), whereas fewer studies have been conducted on the hard part of the seeds, often erroneously called the “woody portion” or “seed” [8]. In this regard, it is worth noting that the botanical terms used to define the parts of the pomegranate fruit and seed are often used improperly in various scientific articles. Several types of terminological errors are common in the literature, although often accepted in the scientific community, such as the use of the terms “aril” to define the whole seed and “seed” to define only the inner hard portion. Malgarejo et al. [5] attempted to clarify the terminology and defined the structure of the pomegranate “seed” as the whole grain, composed of the sarcotesta (fleshy outer testa; single layer of translucid and pulpy cells), mesotesta (internal sclerotized testa; hard and thick-walled) and tegmen (inner integument formed by two yellowish-brown membranous epidermal layers), with nucella and embryo within its structures. Other authors use the term “aril” for the fleshy external layer, which is described as a structure originating, unlike testa (actually sarcotesta), from the funicle, as a distinct outgrowth [9].
In this paper, the term “seed” is used to refer to the whole seed (also consisting of the pulpy part, sarcotesta), whereas the term “tegmen”, although botanically inaccurate, is used to refer to the woody hard part of the seed containing the inner layer of seed coat, the embryo and cotyledons, as defined by Tozzi et al. [10] and as used in our previous paper [3].
The morphoanatomical and phytochemical characterization of the tegmen is important for several reasons. As reported in the literature for other species, the traits of the seed (structure, size and bundles), in particular its integuments, are the least affected by environmental variations, so we can assume that the latter have stable and discriminating characteristics [11].
Therefore, the pomegranate tegmen size and the bundles present on its external surface could also be used for the characterization of genotypes. Bundles that run along the tegmen leave footprints (such as grooves), which have not been investigated by researchers to date. The woody part of the seed is of particular commercial importance. The presence of cellulose and lignin in the tegmen determines the “hardness” of the seed, making the fruit more or less appreciated by the consumer. Dalimov et al. [12] reported that pomegranate seeds contain high levels of lignin (21.44%), whereas Xia et al. [13] indicated a lignin content of 10.6% and 14.98% in Tunisia and Sanbai cvs, respectively. Fruits are generally classified according to the hardness of the seed as soft, semi-soft, semi-hard or hard [14]. The hardness can be determined by a sensory analysis [15] or by an instrumental analysis and expressed as Newton/mm2 or as kg/cm2. Patil et al. [14] reported that soft seeds have a hardness < 3.67 kg/cm2, semi-soft seeds have a hardness ranging from 3.67 to 4.2 kg/cm2, and hard seeds have a hardness > 4.2 kg/cm2. Zarai et al. [16,17] divided Iranian cvs into the following groups: soft-seeded (80–150 N), semi-soft-seeded (200–220 N), semi-hard-seeded (300–420 N) and hard-seeded (450–630 N). The woody index, with values ranging from 4.77 to 15.3%, indicates the quantity of the woody portion relative to the total weight of the seed [18]. Hardness, woody index and other qualitative parameters determine the potential use of cultivars. Genotypes with a soft tegmen are used for fresh consumption, whereas those with a hard tegmen are used for the production of industrial juices; particularly in the latter case, the tegmen constitutes processing waste [10,18,19].
The tegmen contains reserve tissue that is relatively rich in lipids, fiber and minerals [20,21]. In particular, pomegranate seed oil extracted from the reserve tissue, usually defined as “pomegranate seed oil (PSO)”, representing 10–20% of the seed’s dry weight, has recently attracted considerable economic attention [22]. PSO contains a high level of unsaturated fatty acids (up to 90%), namely punicic (conjugated linolenic acid isomer, 44–88%), linoleic (3–14%) and oleic (3–13%) acids, with minor amounts of saturated fatty acids, mostly palmitic (3–12%) and stearic acid (2–15%). Minor amounts of gadoleic, lignoceric, arachidic and myristic acids can also be found in PSO [18,20,23,24].
Furthermore, a high percentage (3–4%) of non-triglyceride substances (unsaponifiable fraction) has been detected in pomegranate tegmen of various origin, revealing a promising content of vitamin E isomers, squalene, phytosterols, aliphatic and triterpene alcohols [25]. The tegmen is, therefore, a valuable source of pharmaceutical and nutraceutical compounds and seed oil can be considered a byproduct from agro-industrial waste to create low-cost food supplements, believed to be effective in the prevention of oxidative damage, cancer or cardiovascular diseases [22]. In this context, the aim of this research (part of the multidisciplinary project aimed at valorization of minor varieties and their local products) was to characterize and describe, for the first time from a morphoanatomical and biochemical point of view, the woody seed part of the pomegranate Italian genotypes selected in the national territory, providing useful information for their possible introduction in agriculture, herbal and pharmaceutical sectors, also in an integrated and sustainable way.
2. Materials and Methods
2.1. Plant Material
The study was conducted on 13 local genotypes of P. granatum sampled in different Italian regions compared with 2 cultivars of commercial interest. The genotypes were located in Emilia Romagna (ER1; ER2; ER3; ER4; ER5; ER6; and ER7), Tuscany (TU1; TU2; and TU3), Apulia (AP1; AP2), and Basilicata (BA1), while the two commercial cultivars, Dente di Cavallo and Wonderful, were in the Sicily region. Accessions and codification are reported in Table 1.

Table 1.
Punica granatum L. genotypes studied, code (ID), accession name, and location.
The fruits were harvested at maturity from September to November in the different regions and transported to laboratory. Sample preparation was conducted following the protocol described by Beghè et al. 2019 [3]. Briefly, for each accession, 5 fruits of uniform size were harvested around the canopy. All seeds were extracted manually from each fruit. Sixty seeds were randomly selected for the morphological analysis that was conducted before and after the removal of sarcotesta by hand. The remaining seeds were separated by the sarcotesta and cleaned, dried in the oven for 24 h at 60 °C, aliquoted in tubes and stored at −80 °C until used for analysis.
2.2. Morphoanatomical Analysis
The tegmen of the different genotypes was subjected to morphological measurements following the UPOV [26] including length (mm), width (mm), shape (length/width ratio), perimeter (mm) and weight (g). All measurements were conducted with image analyzer NIS Elements D 4.00. The weight of whole seed and tegmen, determined using a precision balance accurate to 0.001 g., was used to calculate the woody index (WI) according to Hernández et al. [27], dividing the weight of the tegmen (g) by the weight of the seed (g), multiplying by 100, and expressing it as a percentage.
A GAIA3 TESCAN scanning electron microscope (SEM) was used to characterize the structure of bundles on the surface and the thickness of the sclerotized tegmen tissue. The samples were mounted on aluminum sample-holder stubs, spattered with silver (Emitech K575X, Emitech Ltd., Ashford, UK) and examined at 20 KV. The thickness was measured on micrographs of equatorially cut samples, using an image analysis system iTems. The thickness was calculated as the average value of eight spots in each tegmen cross section according to Calin Sanchez et al. [28].
2.3. Hardness Analysis
2.3.1. Texturometer Analysis
Tegmen hardness was determined using a Fource Gage PCE-FM200 texturometer. Results are expressed in kg/cm2 as force applied to the lateral side of tegmen until it breaks. Ten samples of each genotype were measured in three replications.
2.3.2. Gravimetric Chemical Analysis
Before analyses, the material was sieved and the 40- to 60-mesh fraction (corresponding to 250–420 µm) was used. Two grams of sieved material was used for each sample. Laboratory operations were carried out by a stepwise approach: (i) extraction of substances soluble in organic solvents; (ii) extraction of water-soluble compounds; (iii) assessment of acid lignin. Ash content was also measured on a separate sub-sample, following TAPPI T211-om02 [29], which provides weighing the residue after the meal had been calcinated at 550 °C in air for 2 h. The substances soluble in organic solvents were assessed by extracting wood meal in a 1:2 ethanol and toluene (6 h, 5 cycles per hour), using a Soxhlet extractor. After extraction with organic solvents, the water-soluble compounds were extracted from the wood flour (8 h, 2 cycles per hour). The lignin content was then assessed following the procedure described in the TAPPI T222-om02 [30] standard method for acid (Klason) lignin. The procedure involves a treatment with concentrated sulphuric acid at room temperature, followed by a treatment with diluted sulphuric acid at the relevant boiling temperature. All quantitative results are referred to the anhydrous initial weight of the flour. On the powder-reduced samples, the content of total organic carbon (C) and nitrogen (N) were determined through dry combustion, using an elemental analyzer (Carlo Erba NA 1500 CHNS Analyzer, Milan, Italy) according to Santi et al. [31]. From the nitrogen values, the protein content was assessed multiplying the determined nitrogen content by a nitrogen-to-protein conversion factor, 6.25 [32]. Given these values, the remaining parts, constituting the other substances (mainly polysaccharides, pectins, gums, etc.), were calculated by differences, as the complement to 100% of the sum of the results obtained from the other analyses.
2.4. Oil Analysis
2.4.1. Extraction of Pomegranate Oil and Chemical Characterization by GC-MS
Nine genotypes were subjected to oil analysis. Five grams of dried and cleaned tegmen was finely ground in a mortar with a pestle, then oil was extracted by using an automatic Soxhlet apparatus (SER 148/3 VELP SCIENTIFICA, Usmate Velate, Italy) with ethyl ether as solvent (60 min of immersion and 30 min of washing). The Soxhlet ether extract was evaporated under vacuum at 40 °C and pomegranate seed oil samples were kept at −20 °C and in the dark until analysis. The chemical characterization of pomegranate oils was made in stages. Each analysis was repeated three times and data were expressed as percent of dry matter.
2.4.2. Fatty Acids Profile
The fatty acid methyl esters were prepared according to ISO [33] and slightly adapted. Briefly, 100 mg of oil was dissolved in hexane (5 mL) containing 0.2 mg of the internal standard methyl pentacosanoate (C25:0), added to 1 mL of KOH 10% in methanol and mixed vigorously at room temperature for 5 min. The upper layer was neutralized with sodium bisulfate and centrifuged, and 100 µL of the hexane phase was withdrawn and diluted to match the linearity range of the GC–MS instrument. Then, samples were split-injected (1 μL) on a Thermo Scientific Trace 1300 gas-chromatograph (Thermo Scientific, Waltham, MA, USA) carrying a SUPELCOWAX® 10 capillary column (30 m × 0.25 mm × 0.25 µm, Supelco, Bellafonte, PA, USA) coupled to a Thermo Scientific Trace ISQ mass spectrometer (Thermo Scientific, Waltham, MA, USA). Carrier gas was helium (1 mL/min), injector and detector temperatures were kept at 250 °C, while oven temperature was programmed from 80 to 240 °C at 20 °C/min. Acquisition mode: scan (m/z 40–411).
Identification and concentration of each detected fatty acid was determined in relation to the concentration of the internal standard after calculating the response factors using the Supelco 37 Component FAME Mix (Sigma Aldrich, Saint Louis, MO, USA). Finally, results were expressed as relative percentage of total FAMEs.
2.4.3. Unsaponifiable Fraction Analysis
The unsaponifiable fraction was extracted and silylated according to ISO [34], with slight modifications. Briefly, 5 g of pomegranate oil and 100 mL of a 2.2 N potassium hydroxide solution in ethanol–water (8:2 v/v) were put into a 250 mL flask and saponification reaction was carried out by boiling and stirring the sample for 1 h. After cooling, 100 mL of distilled water was added, and the sample transferred to a separating funnel and extracted 4 times with 50 mL of ethyl ether. The ether extracts were pooled into a separating funnel and washed with distilled water (50 mL each time), until the wash gave a neutral reaction. The wash water was removed, and the organic sample was dried with anhydrous sodium sulphate, filtered and taken to dryness. Then, the residue was dissolved in 5 mL of hexane and added to 1 mL of 5-alfa-cholestan-3-beta-ol solution (internal standard, at 0.3 mg/mL in hexane). The unsaponifiable matter was isolated by silica gel (60 Å, 230–400 mesh particle size, 40–63 µm) column chromatography and eluted with a solution of hexane-ethyl acetate (8:2 v/v). Finally, after the solution was filtered and taken to dryness, the residue was silylated with 600 µL of hexamethyldisilazane (HMDS) and 300 µL of trichloromethylsilane (TCMS) at 60 °C for 1 h. Composition analysis was determined by split-injecting 1 µL of the silylated solutions in the GC-MS system.
GC–MS analysis was performed on a 7820A gas chromatograph coupled to an 5977B mass selective detector (Agilent technologies, Santa Clara, CA, USA) with a DB5 (J&W) capillary column (temperature: 80 °C for 2 min, 15 °C/min until 280 °C, 280 °C for 20 min). Acquisition mode: scan (m/z 40–550). Unsaponifiable components were identified and quantified, by means of the internal standard (5-alfa-cholestan-3-beta-ol), as previously reported by Caligiani et al. [22].
2.5. Statistical Analysis
All results are expressed as the mean ± standard error. Linear regression was performed to assess the existent correlation between hardness and tegmen geometrical parameters. Analysis of Variance (ANOVA) and Tukey’s HSD post hoc test (p ≤ 0.05) were carried out for comparing groups of interest. Statistical significance was fixed using a conventional p value ≤ 0.05. Additionally, Principal component analysis (PCA) was performed for the data oil analysis, and PCA score plot was used to discriminate among the genotypes according to their oil composition. The analysis was performed using XLSTAT 2009 software (AddinsoftTM1995-2009).
3. Results and Discussions
3.1. Morphoanatomical Analysis
Mean values of the tegmen morphometric characters are reported in Table 2.

Table 2.
Morphological parameters of tegmen of Punica granatum L. genotypes.
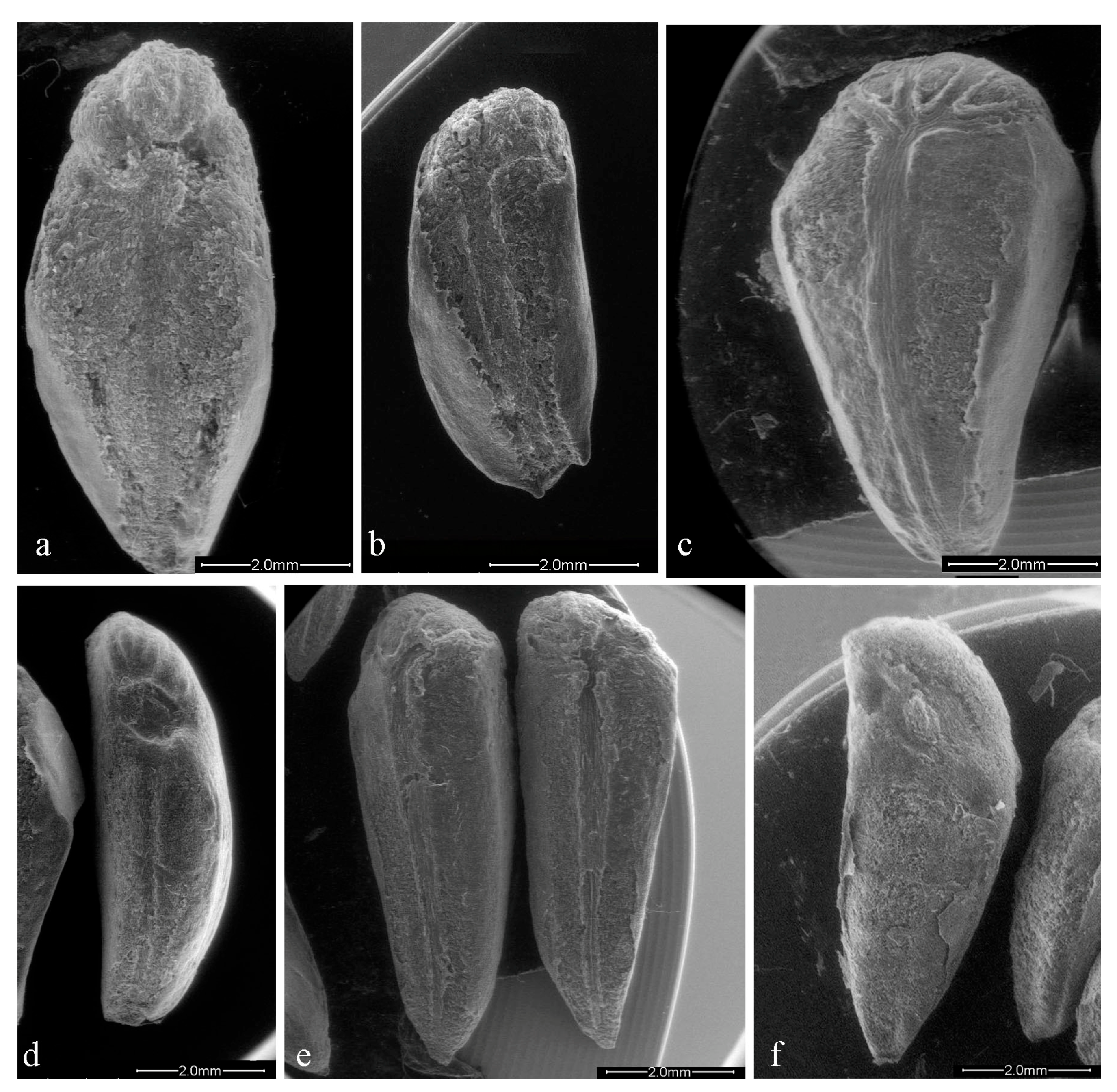
The genotypes of the Emilia Romagna region simultaneously recorded the major tegmen length, 7.20 mm in ER2 (Figure 1a), and the minor one, 5.33 mm in ER7 (Figure 1b), and the minor width, 2.22 mm in genotype ER5 (Figure 1d), while the major width is reported in “Wonderful” with 3.57 mm (Figure 1c; Table 2). The length/width ratio, which gives information about the more or less elongated shape, was very low in ER2 and “Wonderful”, (2.06 and 1.99, respectively) which resulted in a more rounded shape, while it was higher in BA1 (2.93, Figure 1e), ER5 (2.82) and API1 (2.64, Figure 1f) with a more elongated shape (Table 2). The average tegmen weight was 0.025 g; 10 genotypes were heavier and 6 were lighter. Among the local genotypes, the weights ranged between 0.029 g (ER3) and 0.015 g (TU2). Among all genotypes analyzed, the commercial cultivars (“Dente di Cavallo” and “Wonderful”) had a heavier tegmen than the local ecotypes, 0.038 and 0.032 g, respectively.
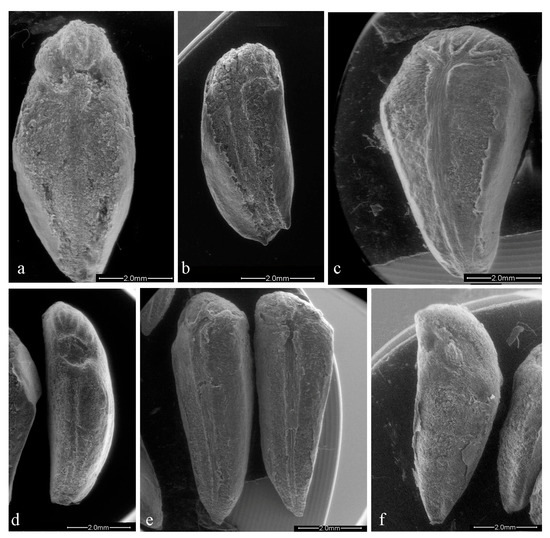
Figure 1.
Tegmen surface of different genotypes: (a) ER2; (b) ER7; (c) Wonderful; (d) ER5; (e) BA1; (f) AP1.
Differences between the samples were also observed for the Wood index (Table 2). ER4 showed the highest WI with 11.9 % while Apulia samples recorded the lowest value (AP1 4.25 % and AP2 4.80 %). Wood Index is a parameter that evaluates the sensory attribute of the wood perception and defines the fruit quality. Consumers appreciate more fruits with a low woody perception [28]. Many of the morphological characters reported in the present research showed values comparable to those presented by other authors [35,36].
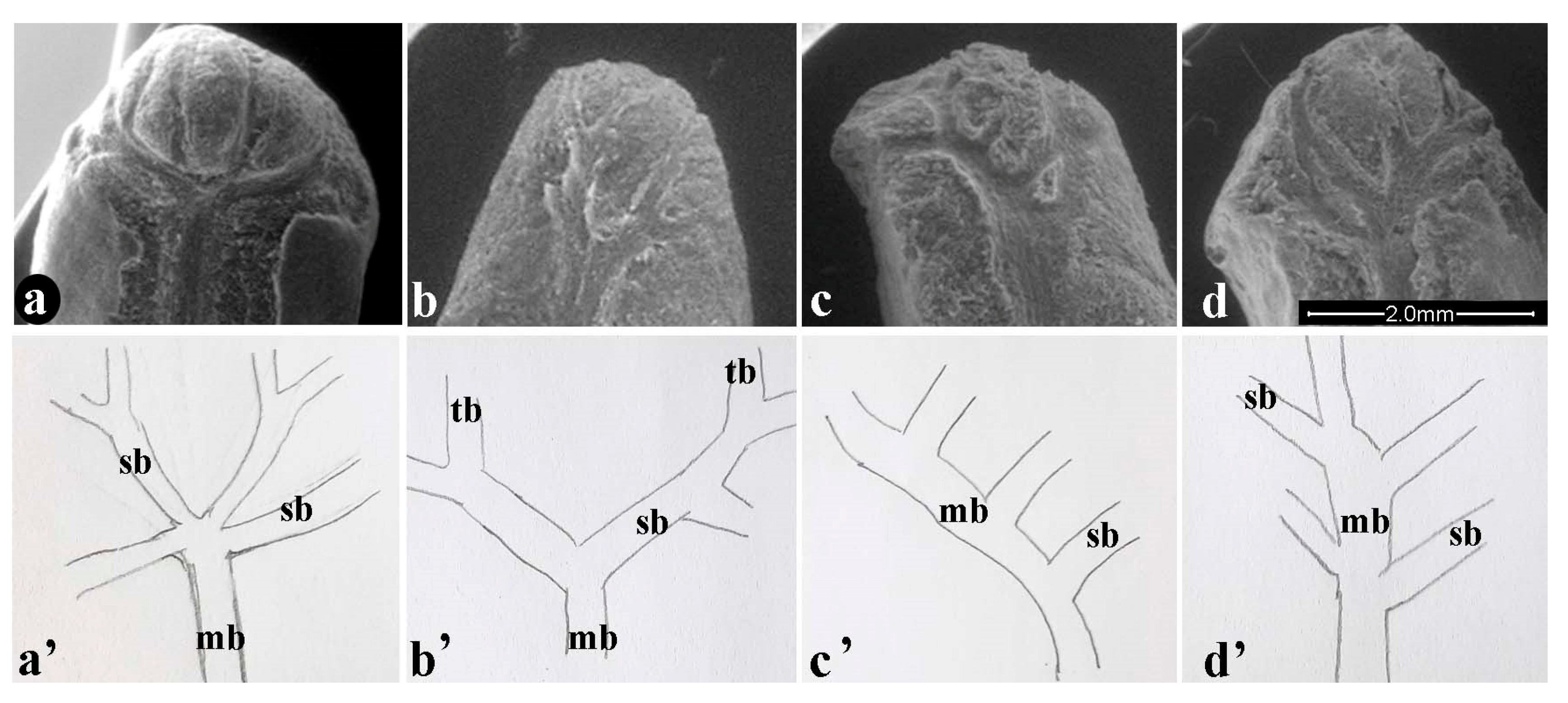
The pomegranate tegmen is traversed by a main bundle that splits at the distal part, giving rise to several shapes. The characteristics of the bundles of the tegmen are shown in Figure 2. The results identified four forms of bundle structure, which we defined as: FAN: the main vascular bundle develops in an enlargement (more or less extended) from which three to six secondary bundles radially branch out (Figure 2a,a’); TREE: the main vascular bundle divides into two secondary bundles that in turn branch off into tertiary bundles, in variable numbers from zero to seven (Figure 2b,b’); COMB: the secondary bundles, on average from two to seven, are generated from only one part of the main bundle, as in a comb (Figure 2c,c’); RAMIFIED: the main vascular bundle divides to the right and left in secondary bundles from three to nine (Figure 2d,d’).
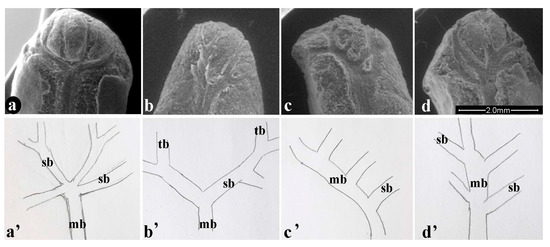
Figure 2.
Bundles on tegmen (a–d); identified structures (a’–d’): (a’) Fan; (b’) Tree; (c’) Comb; (d’) Ramified; mb: main bundle; sb: secondary bundle; tb: tertiary bundle.
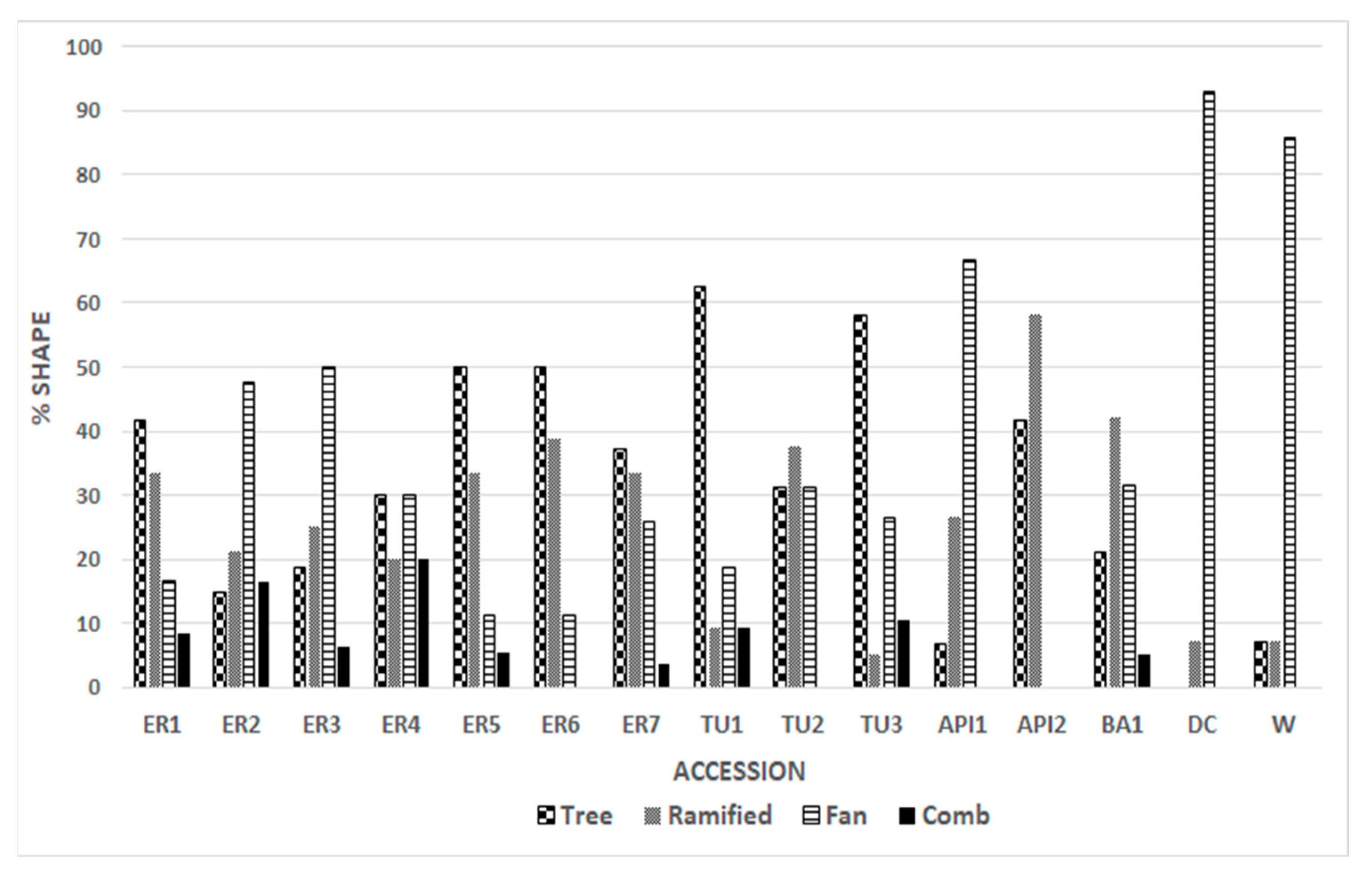
The genotypes analyzed showed variability in the distribution of the four bundle forms (Figure 3). Most of the Emilia Romagna genotypes recorded the predominant TREE form (ER1; ER4; ER5; ER6; ER7), while the ER2 and ER3 accessions showed that the most common form was FAN. In the Tuscan TU1 and TU3 genotypes, a percentage of about 60% of TREE forms was recorded. AP1 and AP2 showed a difference in distribution, whereby 66.6% of the tegmen of AP1 had the FAN form, while 58.3% of AP2 had the RAMIFIED form; interestingly, AP2 had no tegmen with the FAN and COMB forms (Figure 3). “Dente di Cavallo” and “Wonderful” presented almost exclusively the FAN form. In the local genotypes, we observed the presence of all described forms, and thus a wider variability than in the commercial cultivars, where about 90 percent of the tegmina belong to a single structural form. The results lead us to hypothesize that most local genotypes have maintained a wider variability, probably due to less selective pressure by humans than commercial cultivars. “Wonderful” was selected from a mixture of varieties in 1896 in California, USA [37] and “Dente di Cavallo” was selected in Sicily in 1960 [38] from landraces of local germplasm. Human selection and subsequent vegetative propagation led to the loss of genetic diversity.
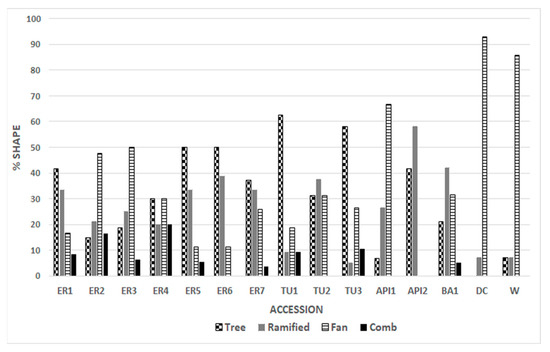
Figure 3.
Percentages of the 4 structures for each genotype. In almost all the genotypes, there is the prevalence of one form.
3.2. Hardness and Chemical Analysis
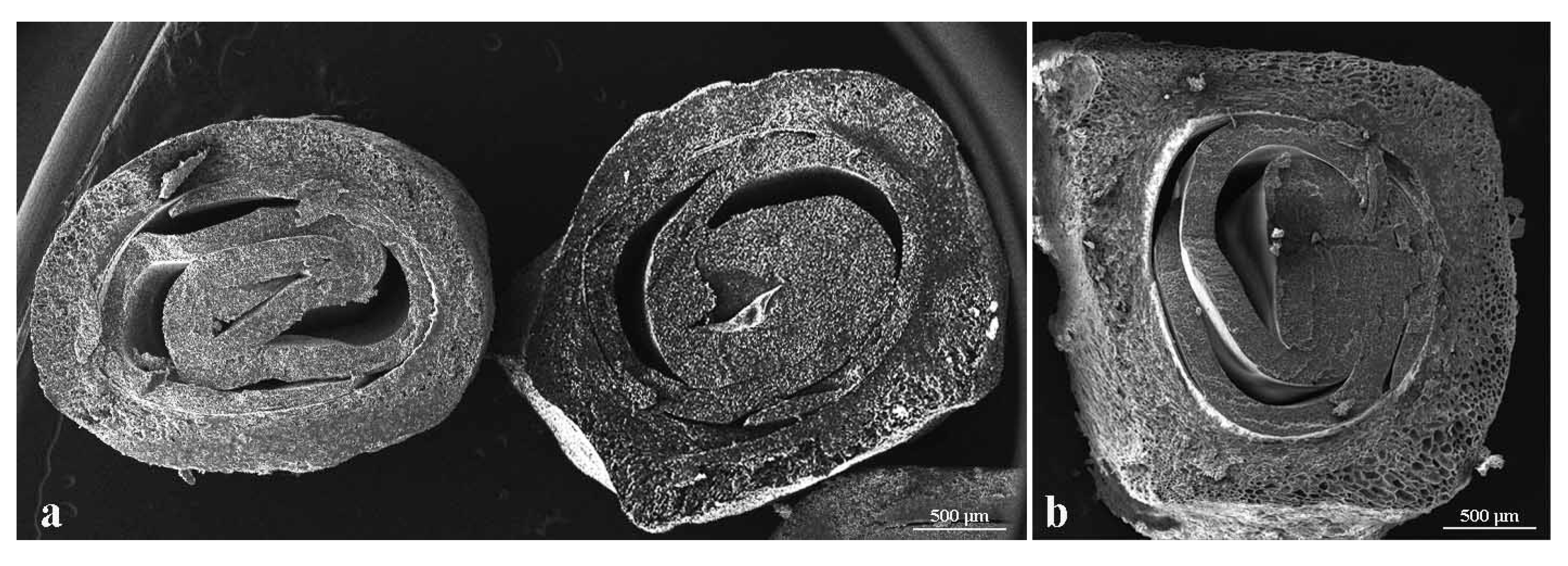
The results obtained in mechanical tests and in chemical analyses showed differences among the analyzed genotypes (Table 3). The highest values of tegmen hardness were found in TU3 (10.41 kg/cm2) and “Wonderful” (10.37 kg/cm2), while the lowest were observed in ER3 (2.68 kg/cm2) and ER1 (2.88 kg/cm2). The results placed our accessions in the soft (seed hardness <3.67 kg/cm2); semi-soft (from 3.67 to 4.2 kg/cm2) and hard (>4.2 kg/cm2) categories. according to Patil et al. [14]. Thickness of the sclerotized seed coat recorded variations between 362.5 µm in TU2 (Figure 4a) and 450.3 µm in “Dente di Cavallo” (Figure 4b).

Table 3.
Mean value of chemomechanical and relevant geometrical parameters of tegmen of Punica granatum L. genotypes. OE = organic extractives; AE = aqueous extractives; L = lignin; A = ash; P = protein; OS = other substances; T = thickness sclerotized seed coat; Geometrical Parameter. Mean values, standard error.
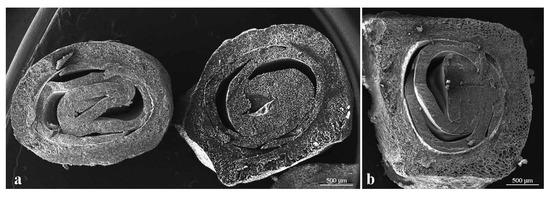
Figure 4.
Equatorial section of tegmen used to measure the thickness of the sclerotized seed coat; (a) TU2; (b) “Dente di Cavallo”.
Chemical analysis showed that, in the tegmen, the lignin amount varied from 16.5 % in “Wonderful” to 31.1 % in TU3 whereas the ‘other substances’ were the most abundant, with their values ranging from 24.1 % in AP1 to 50.6 % in ER1 (Table 3). Similar results were reported in previous studies [39]. High values of organic extractives (OE) were generally observed (from 7.1 % in TU3 to 25.6 % in “Wonderful”). OE are composed of apolar or slightly polar compounds, such as oils or fatty acids, and can be also associated with tannins, phenols and polyphenols [40]. The values obtained for the parameters analyzed are in agreement with those reported by authors for other cultivars [41].
3.3. Mechanical Data
Many of studies associate tegmen hardness with lignin and cellulose content [12,41,42]. In our study, the lignin amount was very similar for the analyzed material, which was contrasting with the high variability observed in hardness tests (Table 3). In fact, correlation tests did not show any relationship between lignin content and seed strength (data not shown). Thus, to explain this behavior, the related data were analyzed more in depth.
Pomegranate seeds have an approximately ovoidal shape and can be considered as empty shells, since the tissues of the embryo are very soft compared to the tegmen. Several models can be found in the literature for the compressive strength of spherical shells [43,44]. Assuming the shell deformation is elastic (that is, the seeds have a predominantly brittle behavior), the load, P, can be expressed according to Young and Budynas [45]
where T is the specimen thickness, t is the ring thickness at the center, E is the elastic modulus, D is the specimen diameter, and is the specimen deflection.
In our case, we considered the pomegranate seeds as spherical; that is obviously an approximation, because their aspect ratio is generally higher than 2 (Table 2). Moreover, the transversal section of seeds is not perfectly round, and the lignified part thickness is quite irregular (Figure 4). Thus, the following approximations can be considered:
where W is the tegmen width, and Equation (1) can be simplified as follows:
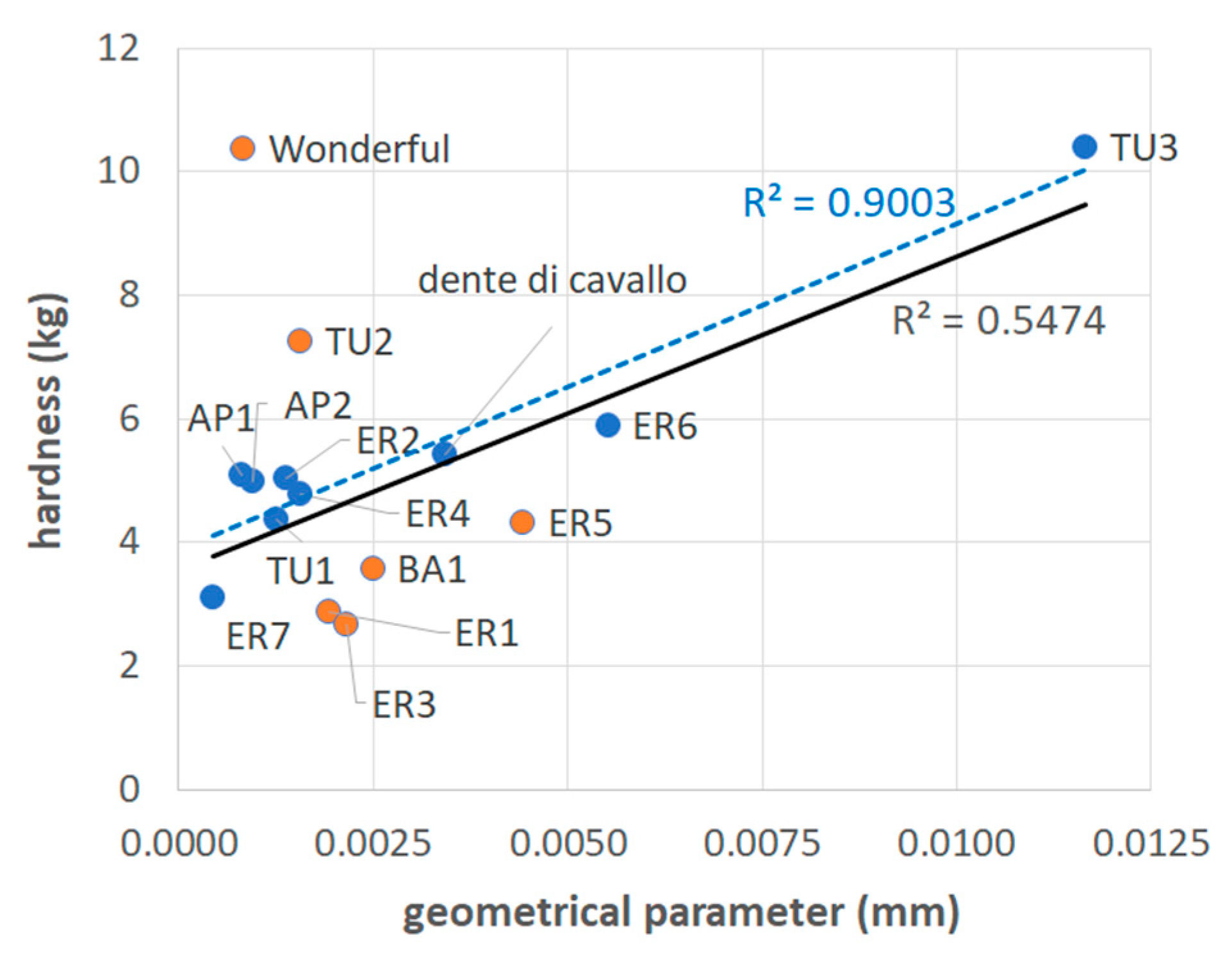
The calculated values of this geometrical parameter are reported in Table 3. The obtained graph between the values of hardness and those of the geometrical parameter is shown in Figure 5. It can be observed how, despite the mentioned approximation, the proposed mechanical model can explain quite well the observed differences in hardness values of several samples (blue spots in Figure 5). However, not all mechanical values are simply related to the geometrical parameter. In fact, if the orange spots in Figure 5 are taken out from the set used to calculate the coefficient of determination of the linear regression line, R2, this value passes from 0.55 to 0.90 (Figure 5).
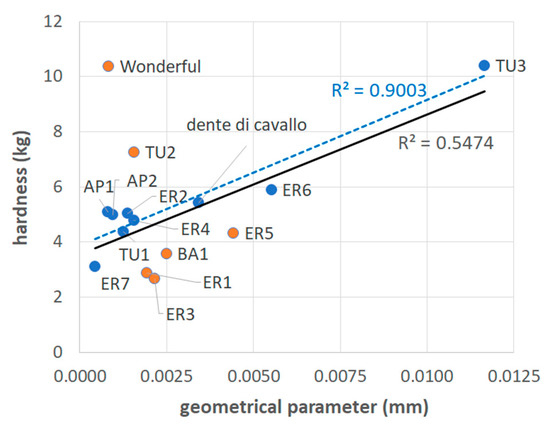
Figure 5.
Mean values of hardness vs. geometrical parameters for the various considered samples. The continuous, black-colored regression line is related to the whole set of samples excluding the Wonderful cultivar. The associated R2 is also in black. The dotted blue-colored regression line is related to a set where the samples in orange were excluded. The associated R2 is in blue.
It can be noted that the samples represented by the orange spots in Figure 5 are characterized by very similar values of the geometrical parameter, included in the range 1.6 · 10−3 for TU2 to 2.5 · 10−3 for BA1. To these values, “Wonderful” (0.8 · 10−3) and ER5 (4.4 · 10−3) were added, as they are also appreciably far from the regression line.
For these samples, it was hypothesized that a chemical difference should exist, which overlaps to the geometrical-type one. Thus, for these samples, the geometrical volume of the equivalent spherical cell, corresponding to the sclerotized seed coat, was calculated as:
and this value was multiplied by the lignin amount:
Vres = V ⋯ L
Vres represents the resistant volume of sclerotized seed coat, that is the percentage of the seed volume occupied by lignin (the structural chemical component of the cell wall able to oppose compressive stress).
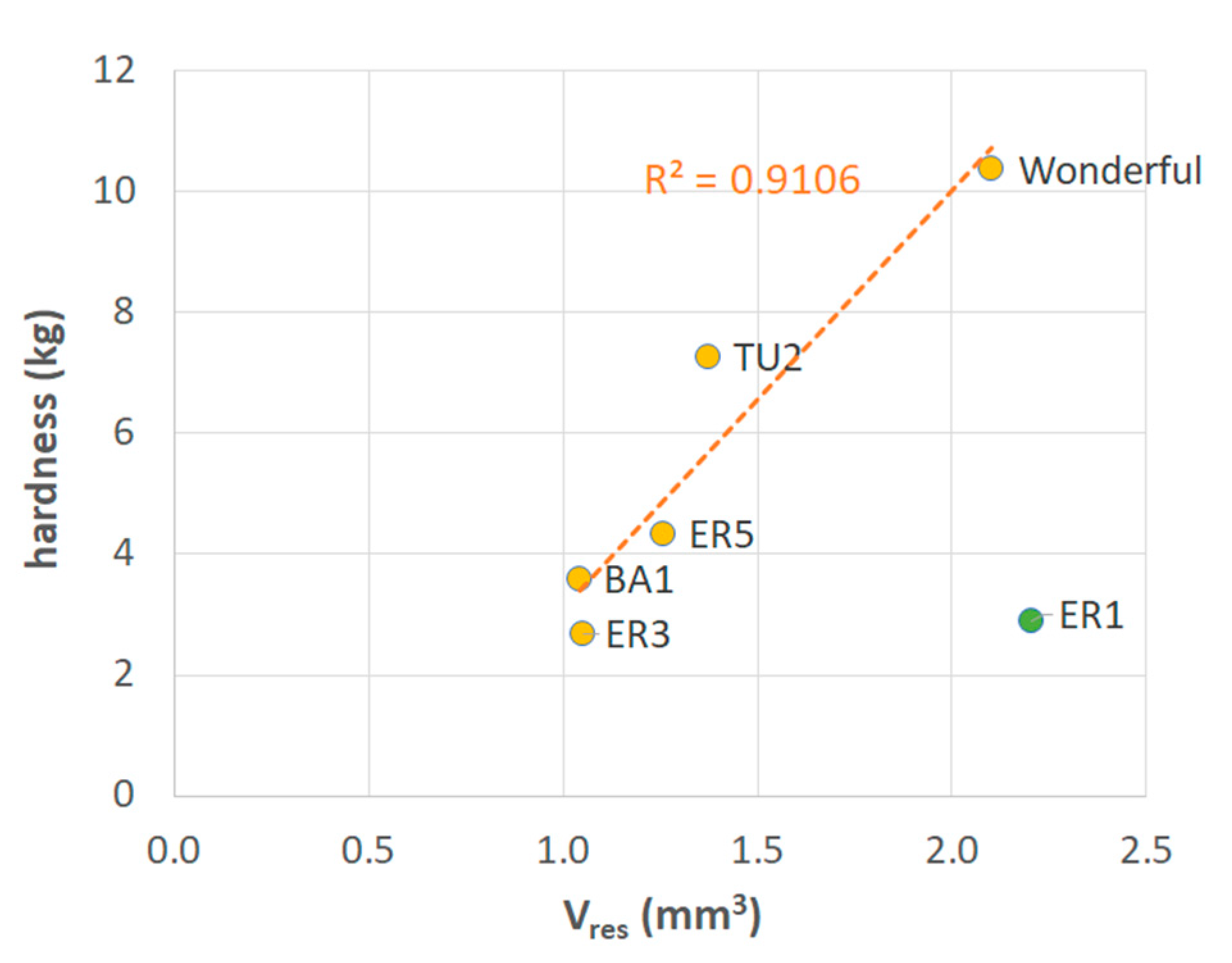
Figure 6 shows that five out of the six samples selected on the basis of Figure 5 were very well aligned on a straight line (R2 = 0.91). This implies that the hypothesized mechanism that chemical factors, in addition to geometric ones, may play a role in determining seed strength, is significant. Accordingly, if the geometric characteristics of the tegmen (in particular, the thickness and diameter of the sclerotized layer) are not sufficient to explain the observed differences in hardness, then the chemical composition of the tissue should be considered, since for a similar tegmen geometry, the higher or lower amount of lignin in the walls may result in higher or lower strength, respectively.
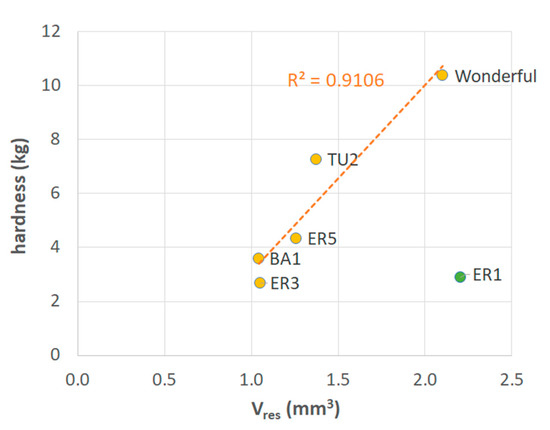
Figure 6.
Mean values of hardness vs. resistant volume of sclerotized seed coat for the samples selected on the basis of Figure 5. The regression line is related to the whole set of samples excluding ER1.
This circumstance also leads to two other more considerations:
- −
- The existence of the relationship shown in Figure 6 where only lignin is involved in the calculation of Veff implies that the other components, that is, proteins, extractives, ash and polysaccharides, do not appreciably contribute to mechanical strength in seeds, because none of them are structured enough to resist external stresses. The importance of lignin has been also highlighted by Dalimov et al. [12] and Xue et al. [46].
- −
- A hierarchical importance can be attributed to the factors considered in the present analysis, with the geometric characteristics (the thickness and diameter of the sclerotized layer) being the first parameters to be considered for seed hardness analysis. Therefore, a thicker and smaller seed (high value of the geometrical parameter) will be expectedly harder than a thin-walled large seed. In case unexpected values are obtained, a combination of lignin content and sclerotized integument volume should be also considered.
3.4. Oil Analysis
A subsample of the Italian pomegranate accessions was subjected to pomegranate seed oil (PSO) analysis. The subsample is formed by eight oils from accessions located in different areas of Northern, Central, and Southern Italy (ER5, ER6, ER7, TU1, TU2, TU3, AP1, and AP2), and one obtained from a commercial cultivar (Dente di Cavallo) with its origin in a typical Mediterranean cultivation area (Sicily, Southern Italy).
The samples showed great variability in total lipid content (in percentage of tegmen weight), ranging from 14% for TU1 to 25% for AP2 (Table 4). The results are consistent with other studies: for example, Kyralan et al. [47] reported for 15 Turkish pomegranate cultivars an oil content from 13.95% to 24.13% and Ferrara et al. [48] reported a wider range (4.9–26.8%) for the oil extract by local and commercial pomegranates grown in Italy. The differences in oil yield reported in the literature could be dependent on different factors as well as the varieties, the environmental conditions, or the methodology of extraction, such as Soxhlet, microwave irradiation, ultrasonic irradiation, normal stirring, etc. [22,49,50].

Table 4.
Lipid content (% on dry matter) and Fatty Acid profile (relative %) in the analyzed Punica granatum L. oils. Mean values and standard deviation. Data (in raw) marked different letters are significantly different from each other (Tukey’s HSD, p ≤ 0.05).
3.4.1. Fatty Acids Profile
Table 4 shows the percentages of fatty acids found in oil samples. The two major saturated fatty acids (SAF) identified were palmitic (C16:0) and stearic (C18:0) acids, which ranged from 3.1% (Dente di Cavallo) to 7.2% (ER5) and 2% (AP2) to 3.6% (Dente di Cavallo), respectively. The reported values for these fatty acids in the literature ranged from 2.2% to 14.9% and from 0.3% to 9.9%, respectively [23].
Regarding the monounsaturated fatty acids (MUFA), oleic acid was the predominant one in pomegranate seed oils and accounted for 2.9% to 10.7% of total fatty acids for the AP1 and ER6 accessions, respectively.
Considering the polyunsaturated fatty acid (PUFA) composition, the predominant fatty acids were the conjugated linoleic acid (CLnA) isomers, predominantly punicic acid which, in the literature, is generally reported as the sum of all conjugated C18:3. The percentage of CLnA in oil pomegranate is very peculiar of this species, being naturally rare and representing an important source of bioactive molecules with abundant health benefits [20]. Studies carried out on ten Spanish cultivars showed a total percentage of punicic acid ranging from 77.3% to 83.6% [23]. In Iranian samples [51], conjugated C18:3 was found in a percentage ranging from 79.4% to 82.4% in agreement with our results ranging from 68% for ER6 to 86% for AP1 and AP2.
As shown in Table 4, it has been confirmed that the unsaturated fatty acids were dominant fatty acids in all samples. The total unsaturated fatty acids (MUFA+PUFA) ranged from 89% (ER5) to 94% (TU3 and AP2) of the total fatty acids.
The ranges of SFA, MUFA and PUFA among the studied pomegranates’ accessions were: 6.0% for AP2 and TU2; 11% for ER5 and SFA; 3.5% for AP1; 11% for ER2 and MUFA; 80% for ER5 and ER6; and 94 % for AP2, TU2, and PUFA.
The saturated/unsaturated acid ratio varied between 0.06 (TU1 and AP2) and 0.12 (ER5), a range that was like that reported by Fadavi et al. [52] (0.06–0.32%). This parameter can be indicative of the quality of the oil; specifically, oil with lower saturated/unsaturated ratio can be more appreciated because of the higher content of polyunsaturated fatty acids.
3.4.2. Unsaponifiable Fraction Analysis
The unsaponifiable fraction, comprised of triterpene alcohols, carotenoids, tocopherols, policosanols, phytosterols, squalene, and other compounds, is less abundant in oil seed (about 0.5–1%); however, it represents another important source of different healthy compounds [22].
In this research, we have investigated the content of sterols, triterpene alcohols, octacosanol and squalene (Table 5). Regarding the total content of sterols, all samples presented the same detected compounds; however, the quantity of each of them was very variable. β sitosterol was found to be the most abundant sterol in all samples, according to other studies [23].

Table 5.
Sterols, triterpene alcohols (=4,4-dimethylsterols), octacosanol and squalene content in Punica granatum L. seed oils (mg/kg fat). Mean values and standard deviation.
4,4-Dimethylsterols (triterpene alcohols) are naturally occurring phytosterols that are suggested to exert beneficial effects in human health, such as reducing inflammation and atherogenic-related risks through the regulation of the endogenous cannabinoid system among the mechanisms of action [53]. These compounds were detected in all the tested samples in accordance with that reported by Caligiani et al. [22] and expressed as mg/kg fat of total 4,4-dimethylsterols (Table 5) as their identification was partially neglected because of co-elution or unclear fragmentation issues.
Squalene was detected in all samples and ranged from about 700 mg/kg in AP2 samples to 1200 mg/kg in the TU1 sample (Table 5) presenting values in line with other studies [22,54]. Squalene is beneficial to human health, and pomegranate oil can be considered a fairly good source of this compound if compared with other known squalene-rich plants such as olive, amaranthus, nut, grape, and poppy oil [21,22,54].
Finally, octacosanol, one of the most abundant policosanols detected in P. granatum species [22], was detected in all oil samples as the main alcohol (Table 5). This compound represents a good source of health benefits and, like sterols, is increasingly utilized in synergy in foods or supplements [55].
3.4.3. PCA Analysis
A PCA was performed to evaluate the relationship of the different genotypes and to enable a better interpretation of all oil data for each pomegranate.
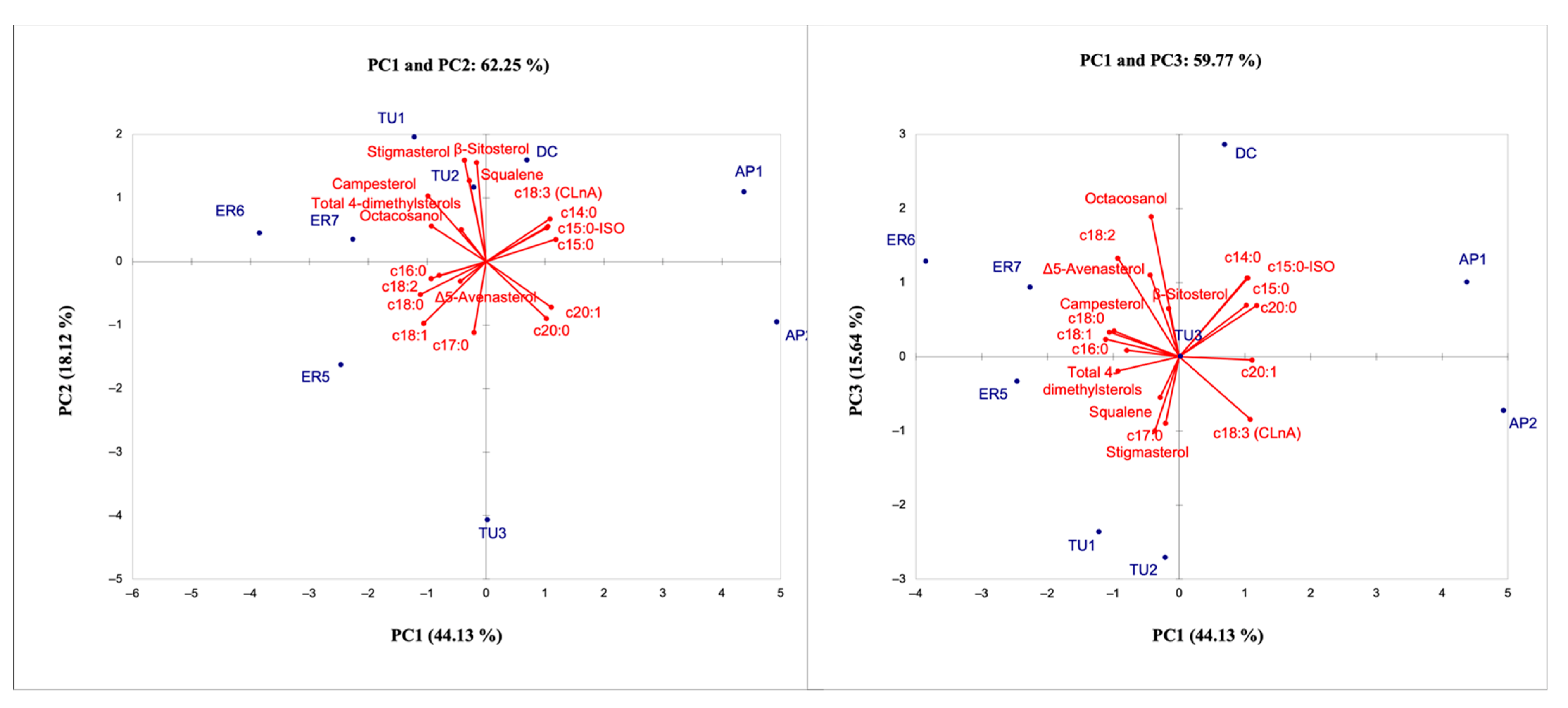
The results of PCA revealed the existence of large variability among accessions. The total variance explained by the first three principal components (PCs) in the model was 77.89%. The PC1 explained the 44.13% of the total variance and the traits with the greatest weight on this component were related to fatty acids (all fatty acids, except C17:0) and unsaponifiable lipid (total 4-dimethylsterols and campesterol). The PC2, explaining 18.12% of the variability, showed a positive load for stigmasterol, β-sitosterol and squalene, whereas the PC3, explaining 15.64% of the variability, showed a positive load with octacosanol. The score plot PCA showed a distribution of the pomegranate genotypes according to their main characteristics, suggesting the capability of the oil compound dataset to successfully differentiate the pomegranate genotypes (Figure 7).
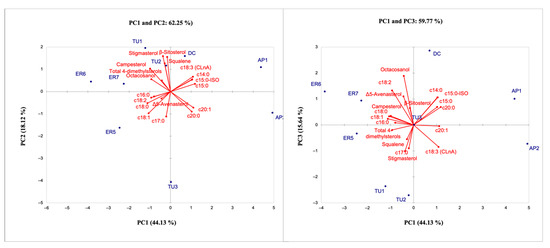
Figure 7.
Loading plots of the first, second and third Principal Component showing the lipid compounds and the position of accessions.
In particular, the greatest differences were identified between local accessions originating from Northern Italy, characterized for their higher content of fatty acids C18:0, C18:1, C18:2, and campesterol; and the accessions from Southern Italy, characterized for their content of SAF (C14, C15, and C20) and the high percentage of CLnA. From the results obtained for the samples analyzed, it appears evident that the lipidic profiles were similar in terms of composition among the different oils, but the abundance of specific compounds may be diverse depending on the different genotypes in the study; moreover, good separation between the geographical origins considered was achieved. In fact, it is known that the content of fatty acids and secondary metabolites is affected by many factors including genetic background and geographical position. Costa et al. [56] showed that fatty acids of pomegranate seeds are influenced by their position and that the bioactive compounds can be used to determine the geographical origin. The same hypothesis was proposed, concerning volatile components of the pomegranate juice from Italian and Montenegrin areas [2].
4. Conclusions
The results of this investigation displayed that the pomegranate accessions studied, grown in different areas of Italy, had a tegmen that presented differences in the morphoanatomical and chemical properties and that may be important for their future potential use.
To the best of our knowledge, for the first time, by analyzing the outer part of the tegmen using a scanning electron microscope, we highlighted the different structures that the bundles determine on the surface. The study identified four forms (FAN, TREE, COMB and RAMIFIED) that can be considered “cultivar specific” since the different accessions showed the prevalence of one shape over the others. The morphoanatomical and electron microscopy analyses have been effective tools for the characterization of cultivars of Punica granatum, that could potentially be used for seeds of other species.
Our results did not show a correlation between lignin content and tegmen hardness. Instead, our proposed model explained that the hardness of the tegmen is related to geometric parameters, such as seed coat thickness and diameter of the tegmen, and to the volume occupied by lignin in the sclerotized tissue. The model explained the observed variability in hardness by placing the analyzed accessions between soft and hard categories. The hierarchical model reported here can be applied to a wide range of woody seeds.
Concerning oil content, the results show that the pomegranate accessions studied are a good source of essential fatty acids (mainly punicic acid) and bioactive compounds that have interesting health properties, therefore confirming the importance of tegmen potential by-products for the food and pharmaceutical industry.
In conclusion, the accessions studied can be used for new production, cultivation in marginal environments, or, because of their characteristics, for breeding purposes.
Author Contributions
Conceptualization, D.B., R.P. and C.G.; Methodology, D.B., R.P., B.P., A.C. and C.G.; Validation, D.B., C.G., V.L., M.R. and R.P.; Technical support, M.A., L.F. and F.B.; Writing—Original Draft Preparation, D.B., R.P. and C.G.; Writing—Review and Editing, D.B., B.P., R.P., A.C., V.L., M.R., T.G. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are pleased to acknowledge all the persons and institutions that collaborated in location and conservation of the Italian pomegranate trees. We acknowledge Carolyn Keating and Andrea Fabbri for helpful comments and suggestions.
Conflicts of Interest
The Authors declare no conflict of interest in this work.
References
- Tarantino, A.; Difonzo, G.; Disciglio, G.; Frabboni, L.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Fresh pomegranate juices from cultivars and local ecotypes grown in southeastern Italy: Comparison of physicochemical properties, antioxidant activity and bioactive compounds. J. Sci. Food Agric. 2022, 102, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Beghè, D.; Cirlini, M.; Beneventi, E.; Miroslav, Č.; Tatjana, P.; Ganino, T.; Dall’Asta, C. Volatile profile of Italian and Montenegrine pomegranate juices for geographical origin classification. Eur. Food Res. Technol. 2021, 247, 211–220. [Google Scholar] [CrossRef]
- Beghè, D.; Fabbri, A.; Petruccelli, R.; Marieschi, M.; Torelli, A.; Ganino, T. Morphological and molecular characterization of ancient pomegranate (Punica granatum L.) accessions in Northern Italy. Adv. Hortic. Sci. 2019, 33, 581–592. [Google Scholar]
- Marieschi, M.; Torelli, A.; Beghè, D.; Bruni, R. Authentication of Punica granatum L.: Development of SCAR markers for the detection of 10 fruits potentially used in economically motivated adulteration. Food Chem. 2016, 202, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, P.; Núñez-Gómez, D.; Legua, P.; Martínez-Nicolás, J.J.; Almansa, M.S. Pomegranate (Punica granatum L.) a dry pericarp fruit with fleshy seeds. Trends Food Sci. Technol. 2020, 102, 232–236. [Google Scholar] [CrossRef]
- Calani, L.; Beghè, D.; Mena, P.; Del Rio, D.; Bruni, R.; Fabbri, A.; Dall’Asta, C.; Galaverna, G. Ultra-HPLC–MS n (poly) phenolic profiling and chemometric analysis of juices from ancient Punica granatum L. cultivars: A nontargeted approach. J. Agric. Food Chem. 2013, 61, 5600–5609. [Google Scholar] [CrossRef]
- El Moujahed, S.; Dinica, R.M.; Cudalbeanu, M.; Avramescu, S.M.; Msegued Ayam, I.; Ouazzani Chahdi, F.; Rodi, Y.K.; Errachidi, F. Characterizations of Six Pomegranate (Punica granatum L.) Varieties of Global Commercial Interest in Morocco: Pomological, Organoleptic, Chemical and Biochemical Studies. Molecules 2022, 27, 3847. [Google Scholar] [CrossRef]
- Pujari, K.H.; Rane, D.A. Concept of seed hardness in pomegranate-I) Anatomical studies in soft and hard seeds of ‘Muskat’ pomegranate. In Proceedings of the 3rd International Symposium on Pomegranate and Minor Mediterranean Fruits, Tai’an, China, 20 September 2013; pp. 97–104. [Google Scholar]
- Prakash, N. Sexual Reproduction in Seed Plants. An Embryological Approach; University of New England: Arminade, Australia, 1986; p. 84. [Google Scholar]
- Tozzi, F.; Núñez-Gómez, D.; Legua, P.; Del Bubba, M.; Giordani, E.; Melgarejo, P. Qualitative and varietal characterization of pomegranate peel: High-value co-product or waste of production? Sci. Hortic. 2022, 291, 110601. [Google Scholar] [CrossRef]
- Trujillo, I.; Ojeda, M.A.; Urdiroz, N.M.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C.M. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet. Genomes 2014, 10, 141–155. [Google Scholar] [CrossRef]
- Dalimov, D.N.; Dalimova, G.N.; Bhatt, M. Chemical composition and lignins of tomato and pomegranate seeds. Chem. Nat. Compd. 2003, 39, 37–40. [Google Scholar] [CrossRef]
- Xia, X.; Li, H.; Cao, D.; Luo, X.; Yang, X.; Chen, L.; Liu, B.; Wang, Q.; Jin, D.; Cao, S. Characterization of a NAC transcription factor involved in the regulation of pomegranate seed hardness (Punica granatum L.). Plant Physiol. Biochem. 2019, 139, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.G.; Singh, N.V.; Bohra, A.; Jamma, S.; Manjunatha, N.; Karuppannan, D.B.; Venkatesh, F.C.; Sharma, J.; Marathe, R.A. Novel miRNA-SSRs for improving seed hardness trait of pomegranate (Punica granatum L.). Front. Genet. 2022, 13, 866504. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.J.; Melgarejo, P.; Hernández, F.A.; Salazar, D.M.; Martinez, R. Seed characterisation of five new pomegranate (Punica granatum L.) varieties. Sci. Hortic. 2006, 110, 241–246. [Google Scholar] [CrossRef]
- Zarei, A.; Zamani, Z.; Fatahi, R.; Mousavi, A.; Salami, S.A. A mechanical method of determining seed-hardness in pomegranate. J. Crop Improv. 2013, 27, 444–459. [Google Scholar] [CrossRef]
- Zarei, A.; Zamani, Z.; Fatahi, R.; Mousavi, A.; Salami, S.A.; Avila, C.; Cánovas, F.M. Differential expression of cell wall related genes in the seeds of soft-and hard-seeded pomegranate genotypes. Sci. Hortic. 2016, 205, 7–16. [Google Scholar] [CrossRef]
- Alcaraz-Mármol, F.; Nuncio-Jáuregui, N.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez, J.J.; Hernández, F. Determination of fatty acid composition in arils of 20 pomegranates cultivars grown in Spain. Sci. Hortic. 2015, 197, 712–718. [Google Scholar] [CrossRef]
- Montefusco, A.; Durante, M.; Migoni, D.; de Caroli, M.; Ilahy, R.; Pék, Z.; Helyes, L.; Fanizzi, F.P.; Mita, G.; Piro, G.; et al. Analysis of the phytochemical composition of pomegranate fruit juices, peels and kernels: A comparative study on four cultivars grown in southern Italy. Plants 2021, 10, 2521. [Google Scholar] [CrossRef]
- Caligiani, A. Pomegranate seed oil conjugated linoleic acids: Characterization and health effects. In Pomegranate: Chemistry, Processing and Health Benefits; Caligiani, A., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 105–120. [Google Scholar]
- Liu, N.; Ren, G.; Faiza, M.; Li, D.; Cui, J.; Zhang, K.; Yao, X.; Zhao, M. Comparison of conventional and green extraction methods on oil yield, physicochemical properties, and lipid compositions of pomegranate seed oil. J. Food Compos. Anal. 2022, 114, 104747. [Google Scholar] [CrossRef]
- Caligiani, A.; Bonzanini, F.; Palla, G.; Cirlini, M.; Bruni, R. Characterization of a potential nutraceutical ingredient: Pomegranate (Punica granatum L.) seed oil unsaponifiable fraction. Plant Foods Hum. Nutr. 2010, 65, 277–283. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.M.; Ramalhosa, E.; Casal, S. Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compos. Anal. 2015, 39, 13–22. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Nunez-Gomez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- International Union for the Protection of New Varieties of Plants. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability: Pomegranate; International Union for the Protection of New Varieties of Plants: Geneva, Switzerland, 2013. [Google Scholar]
- Hernández, F.; Legua, P.; Martínez, R.; Melgarejo, P.; Martínez, J.J. Fruit quality characterization of seven pomegranate accessions (Punica granatum L.) grown in Southeast of Spain. Sci. Hortic. 2014, 175, 174–180. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Cano-Lamadrid, M.; Alcaraz-Mármol, F.; García-Sánchez, F.; Hernández, F.; Martínez-Nicolás, J.J. A new combined sensory-instrumental tool for pomegranate seed hardness determination. J. Sci. Food Agric. 2021, 101, 1355–1363. [Google Scholar] [CrossRef]
- TAPPI T 211 om-02; Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. Technical Association of the Pulp and Paper Industry (TAPPI):: Peachtree Corners, GA, USA, 2002.
- TAPPI T 222 om-02; Acid Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2002.
- Santi, C.A.; Cortes, S.; D’Acqui, L.P.; Sparvoli, E.; Pushparaj, B. Reduction of organic pollutants in olive mill wastewater by using different mineral substrates as adsorbents. Bioresour. Technol. 2008, 99, 1945–1951. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and OILS–Gas Chromatography of Fatty Acid Methyl Esters-Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 12228-1:2014; Determination of Individual and Total Sterols Contents–Gas Chromatographic Method–Part 1: Animal and Vegetable Fats and Oils. International Organization for Standardization: Geneva, Switzerland, 2014.
- Ferrara, G.; Cavoski, I.; Pacifico, A.; Tedone, L.; Mondelli, D. Morpho-pomological and chemical characterization of pomegranate (Punica granatum L.) genotypes in Apulia region, Southeastern Italy. Sci. Hortic. 2011, 130, 599–606. [Google Scholar] [CrossRef]
- Barone, E.; Sottile, F.; Caruso, T.; Marra, F.P. Preliminary Observations on Some Sicilian pomegranate (Punica granatum l.) Varieties. In Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology; Melgarejo, P., Martínez-Nicolás, J.J., Martínez-Tomé, J., Ciheam, Z., Eds.; Centre International de Hautes Etudes Agronomiques Méditerranéennes: Paris, France, 2000; pp. 137–141. [Google Scholar]
- Chandra, R.; Babu, K.D.; Jadhav, V.T.; Jaime, A.; Silva, T.D. Origin, history and domestication of pomegranate. Fruit Veg. Cereal Sci. Biotechnol. 2010, 2, 1–6. [Google Scholar]
- Damigella, P. La coltura del melograno nella Sicilia orientale. Tec. Agric. 1960, 6, 1–15. [Google Scholar]
- Talekar, S.; Patti, A.F.; Singh, R.; Vijayraghavan, R.; Arora, A. From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 2018, 112, 790–802. [Google Scholar] [CrossRef]
- Pizzo, B.; Pometti, C.L.; Charpentier, J.P.; Boizot, N.; Saidman, B.O. Relationships involving several types of extractives of five native argentine wood species of genera Prosopis and Acacia. Ind. Crops Prod. 2011, 34, 851–859. [Google Scholar] [CrossRef]
- Xue, H.; Cao, S.; Li, H.; Zhang, J.; Niu, J.; Chen, L.; Zhao, D. De novo transcriptome assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.). PLoS ONE 2017, 12, e0178809. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, P.; Sánchez, M.; Hernández, F.; Martínez, J.J.; Amorós, A. Parameters for determining the hardness and pleasantness of pomegranates (Punica granatum L.). Options Méditerranéennes Série A Séminaires Méditerranéens 2000, 42, 225–230. [Google Scholar]
- Wang, C.H.; Mai, Y.W. Deformation and fracture of Macadamia nuts. Part 2: Microstructure and fracture mechanics analysis of nutshell. Int. J. Fract. 1994, 69, 67–85. [Google Scholar] [CrossRef]
- Lu, Y.; Carroll, J.D.; Long, K.N.; Long, R. Failure of brittle micro-spherical shells embedded in elastomer matrix under indentation. Compos. Part B Eng. 2019, 173, 106870. [Google Scholar] [CrossRef]
- Young, W.C.; Budynas, R.C. Roark’s Formulas for Stress and Strain, 7th ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Xue, H.; Cao, S.Y.; Li, H.; Zhang, J.; Niu, J.; Chen, L.; Zhao, D. De novo assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.). In II Asian Horticultural Congress; ISHS Acta Horticulturae: Chengdu, China, 2016; Volume 1, pp. 131–146. [Google Scholar]
- Kýralan, M.; Gölükcü, M.; Tokgöz, H. Oil and conjugated linolenic acid contents of seeds from important pomegranate cultivars (Punica granatum L.) grown in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 985–990. [Google Scholar] [CrossRef]
- Ferrara, G.; Giancaspro, A.; Mazzeo, A.; Giove, S.L.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Trani, A.; Gambacorta, G.; Blanco, A.; et al. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci. Hortic. 2014, 178, 70–78. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Habibnia, M.; Ghavami, M.; Ansaripour, M.; Vosough, S. Chemical evaluation of oils extracted from five different varieties of Iranian pomegranate seeds. J. Food Sci. Technol. 2012, 35–40. [Google Scholar]
- Fadavi, A.; Barzegar, M.; Azizi, M.H. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J. Food Compos. Anal. 2006, 19, 676–680. [Google Scholar] [CrossRef]
- Zhang, T.; Xie, L.; Liu, R.; Chang, M.; Zhang, H.; Jin, Q.; Wang, X. Revisiting the 4, 4-dimethylsterols profile from different kinds of vegetable oils by using GC-MS. LWT 2020, 124, 109163. [Google Scholar] [CrossRef]
- Verardo, V.; Garcia-Salas, P.; Baldi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Pomegranate seeds as a source of nutraceutical oil naturally rich in bioactive lipids. Food Res. Int. 2014, 65, 445–452. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, F.; Luo, F.; Lin, Q. Octacosanol and health benefits: Biological functions and mechanisms of action. Food Biosci. 2022, 101632. [Google Scholar] [CrossRef]
- Costa, A.M.M.; Silva, L.O.; Torres, A.G. Chemical composition of commercial cold-pressed pomegranate (Punica granatum) seed oil from Turkey and Israel, and the use of bioactive compounds for samples’ origin preliminary discrimination. J. Food Compos. Anal. 2019, 75, 8–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).