Body Condition and Allometry of Free-Ranging Short-Finned Pilot Whales in the North Atlantic
Abstract
1. Introduction
2. Materials and Methods
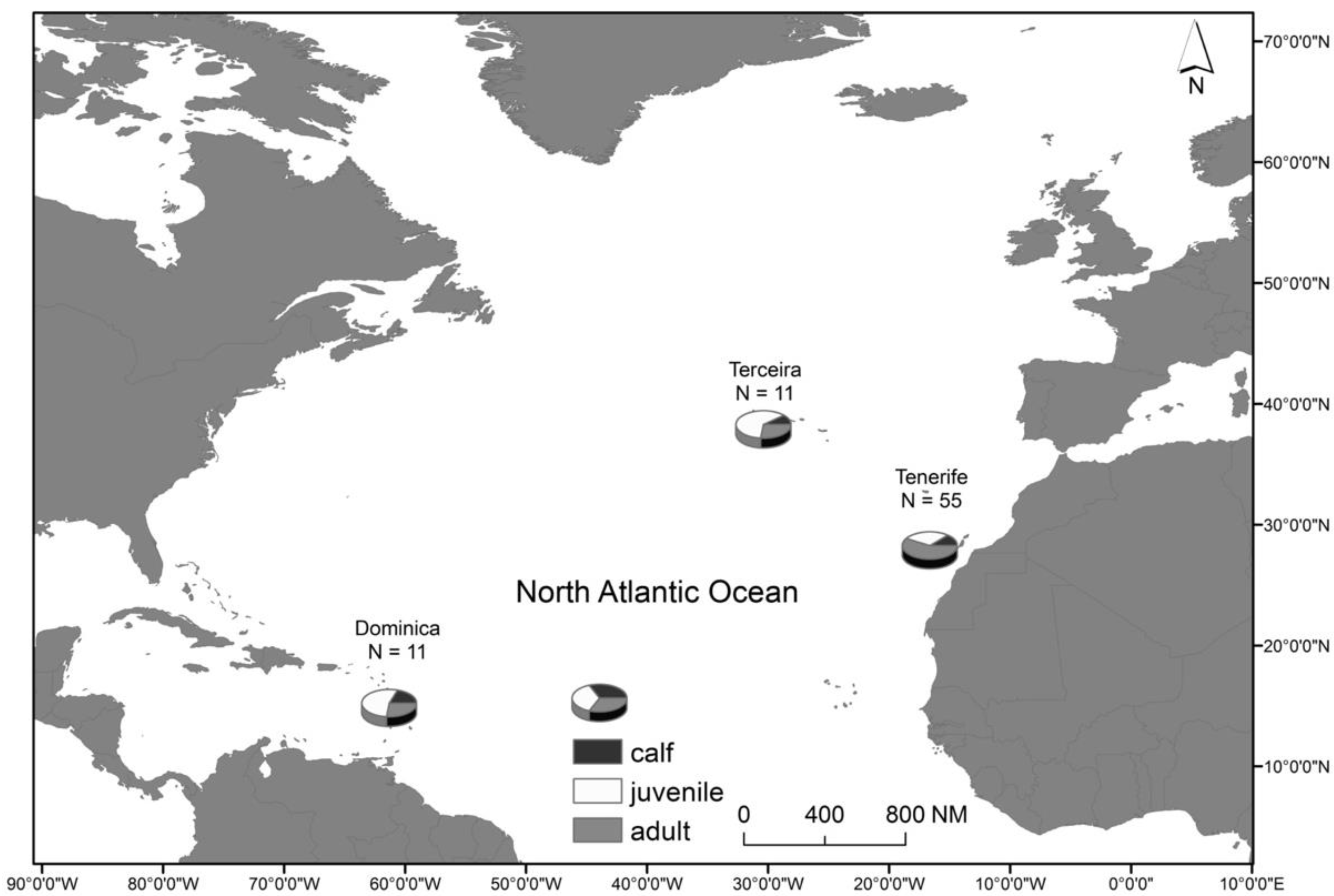
2.1. Study Area and Species
2.2. Data Collection: Measuring Pilot Whale Morphometrics
2.3. Data Processing of UAV Photographs to Extract Morphometric Values
2.4. Classification of Reproductive Classes
2.5. Body Shape, Allometrics, and Body Condition Analyses
2.6. Effects of Reproductive Class and Location on Body Condition
3. Results
3.1. Summary Statistics
3.2. Body Shape and Allometrics of Short-Finned Pilot Whales
3.3. Body Condition of Short-Finned Pilot Whales
4. Discussion
4.1. Body Shape and Allometrics of Pilot Whales
4.2. Body Condition of Pilot Whales
4.3. Future Conservation Efforts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilder, S.M.; Raubenheimer, D.; Simpson, S.J. Moving beyond body condition indices as an estimate of fitness in ecological and evolutionary studies. Funct. Ecol. 2016, 30, 108–115. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating Fitness: A Comparison of Body Condition Indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Festa-Bianchet, M.; Delorme, D.; Jorgenson, J. Body mass and individual fitness in female ungulates: Bigger is not always better. Proc. R. Soc. B Boil. Sci. 2000, 267, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Toïgo, C.; Gaillard, J.-M.; Van Laere, G.; Hewison, M.; Morellet, N. How does environmental variation influence body mass, body size, and body condition? Roe deer as a case study. Ecography 2006, 29, 301–308. [Google Scholar] [CrossRef]
- Tartu, S.; Bourgeon, S.; Aars, J.; Andersen, M.; Polder, A.; Thiemann, G.W.; Welker, J.M.; Routti, H. Sea ice-associated decline in body condition leads to increased concentrations of lipophilic pollutants in polar bears (Ursus maritimus) from Svalbard, Norway. Sci. Total Environ. 2017, 576, 409–419. [Google Scholar] [CrossRef]
- Pettis, H.; Rolland, R.; Hamilton, P.; Knowlton, A.; Burgess, E.; Kraus, S. Body condition changes from natural factors and fishing gear entanglements in North Atlantic right whales Eubalaena glacialis. Endanger. Species Res. 2016, 32, 237–249. [Google Scholar] [CrossRef]
- Ijsseldijk, L.L.; Hessing, S.; Mairo, A.; Doeschate, M.T.I.T.; Treep, J.; Broek, J.V.D.; Keijl, G.O.; Siebert, U.; Heesterbeek, H.; Gröne, A.; et al. Nutritional status and prey energy density govern reproductive success in a small cetacean. Sci. Rep. 2021, 11, 19201. [Google Scholar] [CrossRef]
- Lockyer, C.; Waters, T. Weights and anatomical measurements of Northeastern Atlantic Fin (Balaenoptera physalus, linnaeus) and Sei (B. borealis, lesson) whales. Mar. Mammal Sci. 1986, 2, 169–185. [Google Scholar] [CrossRef]
- Braithwaite, J.E.; Meeuwig, J.J.; Letessier, T.B.; Jenner, K.C.S.; Brierley, A.S. From sea ice to blubber: Linking whale condition to krill abundance using historical whaling records. Polar Biol. 2015, 38, 1195–1202. [Google Scholar] [CrossRef]
- Christiansen, F.; Rodríguez-González, F.; Martínez-Aguilar, S.; Urbán, J.; Swartz, S.; Warick, H.; Vivier, F.; Bejder, L. Poor body condition associated with an unusual mortality event in gray whales. Mar. Ecol. Prog. Ser. 2021, 658, 237–252. [Google Scholar] [CrossRef]
- Christiansen, F.; Dawson, S.; Durban, J.; Fearnbach, H.; Miller, C.; Bejder, L.; Uhart, M.; Sironi, M.; Corkeron, P.; Rayment, W.; et al. Population comparison of right whale body condition reveals poor state of the North Atlantic right whale. Mar. Ecol. Prog. Ser. 2020, 640, 1–16. [Google Scholar] [CrossRef]
- Currie, J.J.; van Aswegen, M.; Stack, S.H.; West, K.L.; Vivier, F.; Bejder, L. Rapid weight loss in free ranging pygmy killer whales (Feresa attenuata) and the implications for anthropogenic disturbance of odontocetes. Sci. Rep. 2021, 11, 8181. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.L.; Ramp, C.A.; Sears, R.; Plourde, S.; Brosset, P.; Miller, P.J.; Hall, A.J. Declining reproductive success in the Gulf of St. Lawrence’s humpback whales (Megaptera novaeangliae) reflects ecosystem shifts on their feeding grounds. Glob. Chang. Biol. 2021, 27, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Víkingsson, G.; Gislason, A.; Lockyer, C.; New, L.; Thomas, L.; Hammond, P.S. Evidence for density-dependent changes in body condition and pregnancy rate of North Atlantic fin whales over four decades of varying environmental conditions. ICES J. Mar. Sci. 2013, 70, 1273–1280. [Google Scholar] [CrossRef]
- Lockyer, C. All creatures great and smaller: A study in cetacean life history energetics. J. Mar. Biol. Assoc. UK 2007, 87, 1035–1045. [Google Scholar] [CrossRef]
- Christiansen, F.; Vikingsson, G.A.; Rasmussen, M.H.; Lusseau, D. Female body condition affects foetal growth in a capital breeding mysticete. Funct. Ecol. 2014, 28, 579–588. [Google Scholar] [CrossRef]
- Christiansen, F.; Vivier, F.; Charlton, C.; Ward, R.; Amerson, A.; Burnell, S.; Bejder, L. Maternal body size and condition determine calf growth rates in southern right whales. Mar. Ecol. Prog. Ser. 2018, 592, 267–281. [Google Scholar] [CrossRef]
- Castrillon, J.; Bengtson Nash, S. Evaluating cetacean body condition; a review of traditional approaches and new developments. Ecol. Evol. 2020, 10, 6144–6162. [Google Scholar] [CrossRef]
- Durban, J.W.; Moore, M.J.; Chiang, G.; Hickmott, L.S.; Bocconcelli, A.; Howes, G.; Bahamonde, P.A.; Perryman, W.L.; Leroi, D.J. Photogrammetry of blue whales with an unmanned hexacopter. Mar. Mammal Sci. 2016, 32, 1510–1515. [Google Scholar] [CrossRef]
- Christiansen, F.; Dujon, A.M.; Sprogis, K.R.; Arnould, J.P.Y.; Bejder, L. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 2016, 7, e01468. [Google Scholar] [CrossRef]
- Fearnbach, H.; Durban, J.; Ellifrit, D.; Balcomb, K. Using aerial photogrammetry to detect changes in body condition of endangered southern resident killer whales. Endanger. Species Res. 2018, 35, 175–180. [Google Scholar] [CrossRef]
- Buckland, S.T.; Garner, G.W.; Amstrup, S.C.; Laake, J.L.; Manly, B.F.J.; McDonald, L.L.; Robertson, D.G. Marine Mammal Survey and Assessment Methods; Balkema, A.A., Ed.; CRC Press: Rotterdam, The Netherlands; Brookfield, WI, USA, 1999; p. 177. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L. Distance Sampling; Hall, C.A., Ed.; John Wiley & Sons, Ltd.: London, UK, 1993. [Google Scholar]
- Christie, A.I.; Colefax, A.P.; Cagnazzi, D. Feasibility of Using Small UAVs to Derive Morphometric Measurements of Australian Snubfin (Orcaella heinsohni) and Humpback (Sousa sahulensis) Dolphins. Remote Sens. 2022, 14, 21. [Google Scholar] [CrossRef]
- Christiansen, F.; Sironi, M.; Moore, M.J.; Di Martino, M.; Ricciardi, M.; Warick, H.A.; Irschick, D.J.; Gutierrez, R.; Uhart, M.M. Estimating body mass of free-living whales using aerial photogrammetry and 3D volumetrics. Methods Ecol. Evol. 2019, 10, 2034–2044. [Google Scholar] [CrossRef]
- Christiansen, F.; Sprogis, K.R.; Groß, J.; Castrillon, J.; Warick, H.A.; Leunissen, E.; Nash, S.B. Variation in outer blubber lipid concentration does not reflect morphological body condition in humpback whales. J. Exp. Biol. 2020, 223, jeb213769. [Google Scholar] [CrossRef] [PubMed]
- Glarou, M.; Gero, S.; Frantzis, A.; Brotons, J.M.; Vivier, F.; Alexiadou, P.; Cerdà, M.; Pirotta, E.; Christiansen, F. Estimating body mass of sperm whales from aerial photographs. Mar. Mammal Sci. 2022, 1–23. [Google Scholar] [CrossRef]
- Adamczak, S.K.; Pabst, A.; McLellan, W.A.; Thorne, L.H. Using 3D Models to Improve Estimates of Marine Mammal Size and External Morphology. Front. Mar. Sci. 2019, 6, 334. [Google Scholar] [CrossRef]
- Cheney, B.J.; Dale, J.; Thompson, P.M.; Quick, N.J. Spy in the sky: A method to identify pregnant small cetaceans. Remote Sens. Ecol. Conserv. 2022, 8, 492–505. [Google Scholar] [CrossRef]
- Stewart, J.D.; Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G.; Casler, P.K.; Ward, E.J.; Dapp, D.R. Survival of the fattest: Linking body condition to prey availability and survivorship of killer whales. Ecosphere 2021, 12, e03660. [Google Scholar] [CrossRef]
- Kasuya, T.; Matsui, S. Age determination and growth of the short finned pilot whale off the pacific coast of Japan. Sci. Rep. Whales Res. Inst. 1984, 35, 57–91. [Google Scholar]
- Heimlich-Boran, J.R. Social Organisation of the Short-Finned Pilot Whale (Globicephala macrorhynchus) with Special Reference to the Comparative Social Ecology of Delphinids; University of Cambridge: Cambridge, UK, 1993. [Google Scholar]
- Kasuya, T.; Tai, S. Life history of short finned pilot whale stocks off Japan and a description of the fishery. In Biology of Northern Hemisphere Pilot Whales; Donova, G., Lockyer, C., MArtin, A., Eds.; IWC: Cambridge, UK, 1993; p. 479. [Google Scholar]
- Alves, F.; Dinis, A.; Nicolau, C.; Ribeiro, C.; Kaufmann, M.; Fortuna, C.; Freitas, L. Survival and abundance of short-finned pilot whales in the archipelago of Madeira, NE Atlantic. Mar. Mammal Sci. 2015, in press. [CrossRef]
- Würsig, B.; Thewissen, J.G.M.; Kovacs, K. Encyclopedia of Marine Mammals, 3rd ed.; Academic Press: London, UK, 2018. [Google Scholar]
- Téllez, R.; Mignucci-Giannoni, A.A.; Caballero, S. Initial description of short-finned pilot whale (Globicephala macrorhynchus) genetic diversity from the Caribbean. Biochem. Syst. Ecol. 2014, 56, 196–201. [Google Scholar] [CrossRef]
- Caldwell, D.K.; Erdman, D.S. The Pilot Whale in the West Indies. J. Mammal. 1963, 44, 113–115. [Google Scholar] [CrossRef]
- Van Cise, A.M.; Baird, R.W.; Baker, C.S.; Cerchio, S.; Claridge, D.; Fielding, R.; Hancock-Hanser, B.; Marrero, J.; Martien, K.K.; Mignucci-Giannoni, A.A.; et al. Oceanographic barriers, divergence, and admixture: Phylogeography and taxonomy of two putative subspecies of short-finned pilot whale. Mol. Ecol. 2019, 28, 2886–2902. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, T.; Mayashita, T.; Kasamatsu, F. Segregation of two forms of short finned pilot whales off the Pacific coast of Japan. Sci. Rep. Whales Res. Inst. 1988, 39, 77–90. [Google Scholar]
- Oremus, M.; Gales, R.; Dalebout, M.L.; Funahashi, N.; Endo, T.; Kage, T.; Steel, D.; Baker, S.C. Worldwide mitochondrial DNA diversity and phylogeography of pilot whales (Globicephala spp.). Biol. J. Linn. Soc. 2009, 98, 729–744. [Google Scholar] [CrossRef]
- Irvine, A.B.; Scott, M.D.; Wells, R.S.; Mead, J.G. Stranding of the pilot whale, Globicephala macrorhynchus, in Florida and South Carolina. Fish. Bull. 1979, 77, 511–513. [Google Scholar]
- Hohn, A.A.; Rotstein, D.S.; Harms, C.A.; Southall, B.L. Report on Marine Mammal Unusual Mortality Event UMESE0501Sp: Multispecies Mass Stranding of Pilot Whales (Globicephala macrorhynchus), Minke Whale (Balaenoptera acutorostrata) and Dwarf Sperm Whales (Kogia sima) in North Carolina on 15–16 January 2005. Noaa Technical Memorandum Nmfs-Sefsc-537. 2006. Available online: https://aquadocs.org/handle/1834/19915 (accessed on 23 June 2022).
- Kasuya, T.; Marsh, H. Life history and reproductive biology of the short-finned pilot whale, Globicephala macrorhynchus, off the Pacific coast of Japan. Rep. Int. Whal. Commn. Spec. Issue 1984, 6, 259–310. [Google Scholar]
- Ellis, S.; Franks, D.W.; Nattrass, S.; Currie, T.E.; Cant, M.A.; Giles, D.; Balcomb, K.C.; Croft, D.P. Analyses of ovarian activity reveal repeated evolution of post-reproductive lifespans in toothed whales. Sci. Rep. 2018, 8, 12833. [Google Scholar] [CrossRef]
- Soto, N.A.; Johnson, M.P.; Madsen, P.T.; Díaz, F.; Domínguez, I.; Brito, A.; Tyack, P. Cheetahs of the deep sea: Deep foraging sprints in short finned pilot whales off Tenerife (Canary Islands). J. Anim. Ecol. 2008, 77, 936–947. [Google Scholar] [CrossRef]
- Jensen, F.H.; Bejder, L.; Wahlberg, M.; de Soto, N.A.; Johnson, M.; Madsen, P. Vessel noise effects on delphinid communication. Mar. Ecol. Prog. Ser. 2009, 395, 161–175. [Google Scholar] [CrossRef]
- Arranz, P.; Glarou, M.; Sprogis, K.R. Decreased resting and nursing in short-finned pilot whales when exposed to louder petrol engine noise of a hybrid whale-watch vessel. Sci. Rep. 2021, 11, 21195. [Google Scholar] [CrossRef] [PubMed]
- Servidio, A.; Pérez-Gil, E.; Pérez-Gil, M.; Cañadas, A.; Hammond, P.S.; Martín, V. Site fidelity and movement patterns of short-finned pilot whales within the Canary Islands: Evidence for resident and transient populations. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29 (Suppl. S1), 227–241. [Google Scholar] [CrossRef]
- Silva, M.A.; Prieto, R.; Cascão, I.; Seabra, M.; Machete, M.; Baumgartner, M.F.; Santos, R.S. Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores. Mar. Biol. Res. 2014, 10, 123–137. [Google Scholar] [CrossRef]
- Boisseau, O.; Leaper, R.; Moscrop, A. Observations of Small Cetaceans in the Eastern Caribbean; International Whaling Commission Scientific Committee Paper SC/58/SM24; International Whaling Commission Scientific Committee: Munich, Germany, 2006. [Google Scholar]
- Jefferson, T.; Webber, M.; Pitman, R.L. Marine Mammals of the World: A Comprehensive Guide to Their Identification. Marine Mammals of the World: A Comprehensive Guide to Their Identification; Elsevier: Amsterdam, The Netherland, 2008. [Google Scholar]
- Servidio, A. Distribution, Social Structure and Habitat Use of Short-Finned Pilot Whale, Globicephala macrorhynchus, in the Canary Islands. Ph.D. Thesis, University of St Andrews, St Andrews, UK, 2014. [Google Scholar]
- Puig-Lozano, R.; Fernández, A.; Saavedra, P.; Tejedor, M.; Sierra, E.; De La Fuente, J.; Xuriach, A.; Díaz-Delgado, J.; Rivero, M.A.; Andrada, M.; et al. Retrospective Study of Traumatic Intra-Interspecific Interactions in Stranded Cetaceans, Canary Islands. Front. Vet. Sci. 2020, 7, 107. [Google Scholar] [CrossRef]
- Prieto, R.; Fernandes, M. Revision of the occurrence of the long-finned pilot whale Globicephala melas (Traill, 1809), in the Azores. Arquipel.-Life Mar. Sci. 2007, 24, 65–69. [Google Scholar]
- Arkowitz, R.; Rommel, S. Force and bending moment of the caudal muscles in the shortfin pilot whale. Mar. Mammal Sci. 1985, 1, 203–209. [Google Scholar] [CrossRef]
- Norris, K.S. Standardized methods for measuring and recording data on smaller cetaceans. J. Mammal. 1961, 42, 471–476. [Google Scholar] [CrossRef]
- Cheney, B.; Wells, R.S.; Barton, T.R.; Thompson, P.M. Laser photogrammetry reveals variation in growth and early survival in free-ranging bottlenose dolphins. Anim. Conserv. 2018, 21, 252–261. [Google Scholar] [CrossRef]
- Dawson, S.M.; Bowma, M.H.; Leunissen, E.; Sirguey, P. Inexpensive Aerial Photogrammetry for Studies of Whales and Large Marine Animals. Front. Mar. Sci. 2017, 4, 366. [Google Scholar] [CrossRef]
- Gonzalez, A.; Lopez, A. First recorded mass stranding of short finned pilot whales (Globicephala macrorhynchus) in the northeastern Atlantic. Mar. Mammal Sci. 2000, 16, 640–646. [Google Scholar] [CrossRef]
- Alves, M.; Gaillard, F.; Sparrow, M.; Knoll, M.; Giraud, S. Circulation patterns and transport of the Azores Front-Current system. Deep.-Sea Res. II 2002, 19, 3983–4002. [Google Scholar] [CrossRef]
- Minobe, S.; Kuwano-Yoshida, A.; Komori, N.; Xie, S.-P.; Small, R.J. Influence of the Gulf Stream on the troposphere. Nature 2008, 452, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Noren, S.; Schwarz, L.; Chase, K.; Aldrich, K.; Oss, K.M.-V.; Leger, J.S. Validation of the photogrammetric method to assess body condition of an odontocete, the shortfinned pilot whale Globicephala macrorhynchus. Mar. Ecol. Prog. Ser. 2019, 620, 185–200. [Google Scholar] [CrossRef]
- Fish, F.E.; Howle, L.E.; Murray, M.M. Hydrodynamic flow control in marine mammals. Integr. Comp. Biol. 2008, 48, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Pabst, D.A. To bend a dolphin: Convergence of force transmission designs in cetaceans and scombrid fishes. Am. Zool. 2000, 40, 146–155. [Google Scholar] [CrossRef]
- Mclellan, W.A.; Koopman, H.N.; Rommel, S.A.; Read, A.J.; Potter, C.W.; Nicolas, J.R.; Westgate, A.J.; Pabst, D.A. Ontogenetic allometry and body composition of harbour porpoises (Phocoena phocoena, L.) from the western North Atlantic. J. Zool. 2002, 257, 457–471. [Google Scholar] [CrossRef]
- Tønnesen, P.; Gero, S.; Ladegaard, M.; Johnson, M.; Madsen, P.T. First-year sperm whale calves echolocate and perform long, deep dives. Behav. Ecol. Sociobiol. 2018, 72, 165. [Google Scholar] [CrossRef]
- Marón, C.F.; Lábaque, M.C.; Beltramio, L.; Di Martino, M.; Alzugaray, L.; Ricciardi, M.; Ajó, A.A.F.; Adler, F.R.; Seger, J.; Sironi, M.; et al. Patterns of blubber fat deposition and evaluation of body condition in growing southern right whale calves (Eubalaena australis). Mar. Mammal Sci. 2021, 37, 1309–1329. [Google Scholar] [CrossRef]
- Adamczak, S.K.; Holser, R.R.; Costa, D.P.; McCabe, E.J.B.; Wells, R.S. Body Composition of Common Bottlenose Dolphins in Sarasota Bay, Florida. Front. Mar. Sci. 2021, 8, 581. [Google Scholar] [CrossRef]
- Bierlich, K.C.; Hewitt, J.; Bird, C.N.; Schick, R.S.; Friedlaender, A.; Torres, L.G.; Dale, J.; Goldbogen, J.; Read, A.J.; Calambokidis, J.; et al. Comparing Uncertainty Associated with 1-, 2-, and 3D Aerial Photogrammetry-Based Body Condition Measurements of Baleen Whales. Front. Mar. Sci. 2021, 8, 1729. [Google Scholar] [CrossRef]
- Abouheif, E.; Fairbairn, D.J. A comparative analysis of allometry for sexual size dimorphism: Assessing Rensch’s rule. Am. Nat. 1997, 149, 540–562. [Google Scholar] [CrossRef]
- Clark, S.; Odell, D. Allometric Relationships and Sexual Dimorphism in Captive Killer Whales (Orcinus orca). J. Mammal. 1999, 80, 777. [Google Scholar] [CrossRef]
- Dawson, S.M.; Chessum, C.J.; Hunt, P.J.; Slooten, E. An Inexpensive, Stereophotographic Technique to Measure Sperm Whales from Small Boats. Rep. Int. Whal. Comm. 1995, 45, 431–436. [Google Scholar]
- Murphy, S.; Rogan, E. External morphology of the short-beaked common dolphin, Delphinus delphis: Growth, allometric relationships and sexual dimorphism. Acta Zool. 2006, 87, 315–329. [Google Scholar] [CrossRef]
- van Aswegen, M.; Christiansen, F.; Symons, J.; Mann, J.; Nicholson, K.; Sprogis, K.; Bejder, L. Morphological differences between coastal bottlenose dolphin (Tursiops aduncus) populations identified using non-invasive stereo-laser photogrammetry. Sci. Rep. 2019, 9, 12235. [Google Scholar] [CrossRef]
- Noren, S.R.; Schwarz, L.; Robeck, T.R. Topographic Variations in Mobilization of Blubber in Relation to Changes in Body Mass in Short-Finned Pilot Whales (Globicephala macrorhynchus). Physiol. Biochem. Zool. 2021, 94, 228–240. [Google Scholar] [CrossRef]
- Irschick, D.J.; Martin, J.; Siebert, U.; Kristensen, J.H.; Madsen, P.T.; Christiansen, F. Creation of accurate 3D models of harbor porpoises (Phocoena phocoena) using 3D photogrammetry. Mar. Mammal Sci. 2021, 37, 482–491. [Google Scholar] [CrossRef]
- Biuw, M.; McConnell, B.; Bradshaw, C.; Burton, H.; Fedak, M. Blubber and buoyancy: Monitoring the body condition of free-ranging seals using simple dive characteristics. J. Exp. Biol. 2003, 206, 3405–3423. [Google Scholar] [CrossRef]
- Narazaki, T.; Isojunno, S.; Nowacek, D.P.; Swift, R.; Friedlaender, A.S.; Ramp, C.; Smout, S.; Aoki, K.; Deecke, V.B.; Sato, K.; et al. Body density of humpback whales (Megaptera novaengliae) in feeding aggregations estimated from hydrodynamic gliding performance. PLoS ONE 2018, 13, e0200287. [Google Scholar] [CrossRef]
- Thorne, L.H.; Foley, H.J.; Baird, R.W.; Webster, D.L.; Swaim, Z.T.; Read, A.J. Movement and foraging behavior of short-finned pilot whales in the Mid-Atlantic Bight: Importance of bathymetric features and implications for management. Mar. Ecol. Prog. Ser. 2017, 584, 245–257. [Google Scholar] [CrossRef]
- Abecassis, M.; Polovina, J.; Baird, R.; Copeland, A.; Drazen, J.; Domokos, R.; Oleson, E.; Jia, Y.; Schorr, G.S.; Webster, D.L.; et al. Characterizing a Foraging Hotspot for Short-Finned Pilot Whales and Blainville’s Beaked Whales Located off the West Side of Hawai‘i Island by Using Tagging and Oceanographic Data. PLoS ONE 2015, 10, e0142628. [Google Scholar] [CrossRef]
- Fernandez, M.; Alves, F.; Ferreira, R.; Fischer, J.-C.; Thake, P.; Nunes, N.; Caldeira, R.; Dinis, A. Modeling Fine-Scale Cetaceans’ Distributions in Oceanic Islands: Madeira Archipelago as a Case Study. Front. Mar. Sci. 2021, 8, 870. [Google Scholar] [CrossRef]
- Víkingsson, G.A. Body condition of fin whales during summer off Iceland. In Developments in Marine Biology; Blix, A.S., Walløe, L., Ulltang, Ø., Eds.; Elsevier Science: New York, NY, USA, 1995; pp. 361–369. [Google Scholar]
- Lemos, L.S.; Burnett, J.D.; Chandler, T.E.; Sumich, J.L.; Torres, L.G. Intra- and inter-annual variation in gray whale body condition on a foraging ground. Ecosphere 2020, 11, e03094. [Google Scholar]
- Lockyer, C.H. Seasonal changes in body fat condition of northeast Atlantic pilot whales, and their biological significance. Rep. Int. Whal. Comm. Spec. Issue 1993, 14, 324–350. [Google Scholar]
- Vélez-Belchí, P.; González-Carballo, M.; Pérez-Hernández, M.D.; Hernández-Guerra, A. Open Ocean Temperature and Salinity Trends in the Canary Current Large Marine Ecosystem. Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem; Valdés, L., Déniz-Gonzalez, I., Eds.; IOC Technical Series; IOC-UNESCO: Paris, France, 2015; Volume 115. [Google Scholar]
- Yonekura, M.; Matsui, S.; Kasuya, T. On the external characters of Globicephala macrorhynchus off Taiji, Pacific coast of Japan. Sci. Rep. Whales Res. Inst. 1980, 32, 67–95. [Google Scholar]
- Cantor, M.; Gero, S.; Whitehead, H.; Rendell, L. Sperm Whale: The Largest Toothed Creature on Earth, in Ethology and Behavioral Ecology of Odontocetes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 261–280. [Google Scholar]
- Mahaffy, S.D.; Baird, R.W.; McSweeney, D.J.; Webster, D.L.; Schorr, G.S. High site fidelity, strong associations, and long-term bonds: Short-finned pilot whales off the island of Hawai‘i. Mar. Mammal Sci. 2015, 31, 1427–1451. [Google Scholar] [CrossRef]
- Augusto, J.F.; Frasier, T.R.; Whitehead, H. Using photography to determine sex in pilot whales (Globicephala melas) is not possible: Males and females have similar dorsal fins. Mar. Mammal Sci. 2013, 29, 213–220. [Google Scholar] [CrossRef]
- Arranz, P.; de Soto, N.A.; Madsen, P.T.; Sprogis, K.R. Whale-watch vessel noise levels with applications to whale-watching guidelines and conservation. Mar. Policy 2021, 134, 104776. [Google Scholar] [CrossRef]
- Carrillo, M.; Ritter, F. Increasing numbers of ship strikes in the Canary Islands: Proposals for immediate action to reduce risk of vessel-whale collisions. J. Cetacacean. Res. Manag. 2010, 11, 131–138. [Google Scholar]
- De stephanis, R.; Urkiola, E. Collisions between Ships and Cetaceans in Spain; International Whaling Commission Scientific Committee SC/58/BC5; International Whaling Commission Scientific Committee: Munich, Germany, 2006; p. 6. [Google Scholar]
- Sprogis, K.R.; Videsen, S.; Madsen, P.T. Vessel noise levels drive behavioural responses of humpback whales with implications for whale-watching. eLife 2020, 9, e56760. [Google Scholar] [CrossRef]
- Booth, C.G.; Sinclair, R.R.; Harwood, J. Methods for Monitoring for the Population Consequences of Disturbance in Marine Mammals: A Review. Front. Mar. Sci. 2020, 7, 115. [Google Scholar] [CrossRef]
- Bejder, L.; Samuels, A.; Whitehead, H.; Gales, N.; Mann, J.; Connor, R.; Heithaus, M.; Watson-Capps, J.; Flaherty, C.; Krützen, M. Decline in Relative Abundance of Bottlenose Dolphins Exposed to Long-Term Disturbance. Conserv. Biol. 2006, 20, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Lusseau, D.; Slooten, E.; Currey, R. Unsustainable Dolphin-watching Tourism in Fiordland, New Zealand. Tour. Mar. Environ. 2006, 3, 173–178. [Google Scholar] [CrossRef]





| Life History Parameters | North Pacific ‘Naisa’ Morphotype |
|---|---|
| Max. length (female–male) | 4.05–5.80 m |
| Max. weight | 800–2300 kg |
| Weight at birth | 37 kg |
| Length at birth | 1.4 m |
| Length at weaning | 2.5 m |
| Length at functional sexual maturity (female–male) | 3.2–4.2 m |
| Reproductive Class | n | Body Length (m) Mean [min,max] | Body Volume (m3) Mean [min,max] | Body Condition (%) Mean [min,max] |
|---|---|---|---|---|
| Calves | 9 | 2.37 [2.09–2.48] | 0.23 [0.15–0.32] | 1.48 [−14.11–29.28] |
| Juveniles | 31 | 2.90 [2.55–3.20] | 0.39 [0.25–0.64] | 0.93 [−34.75–52.43] |
| Adults | 37 | 3.72 [3.20–4.57] | 0.72 [0.46–1.13] | 1.40 [−17.61–30.46] |
| Total | 77 | 2.99 [2.09–4.57] | 0.44 [0.15–1.13] | 0.87 [−34.75–52.43] |
| North Atlantic (This Study) | Florida and South Carolina [41] * | North Carolina [42] ** | Galicia (Eastern North Atlantic) [59] * | Captivity [62] | |
|---|---|---|---|---|---|
| Calves | 2.37 (0.11) N = 9 | 2.15 (0.12) N = 9 | 1.88 (0.27) N = 4 | 1.35 N = 1 | NA |
| Juveniles | 2.90 (0.18) N = 31 | 2.91 (0.24) N = 92 | 2.81 (0.28) N = 6 | 2.73 (0.42) N= 5 | NA |
| Adults | 3.72 (0.44) N = 37 | 3.80 (0.48) N = 35 | 3.60 (0.16) N = 17 | 3.91 (0.33) N = 6 | 3.85 (0.38) N = 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arranz, P.; Christiansen, F.; Glarou, M.; Gero, S.; Visser, F.; Oudejans, M.G.; Aguilar de Soto, N.; Sprogis, K. Body Condition and Allometry of Free-Ranging Short-Finned Pilot Whales in the North Atlantic. Sustainability 2022, 14, 14787. https://doi.org/10.3390/su142214787
Arranz P, Christiansen F, Glarou M, Gero S, Visser F, Oudejans MG, Aguilar de Soto N, Sprogis K. Body Condition and Allometry of Free-Ranging Short-Finned Pilot Whales in the North Atlantic. Sustainability. 2022; 14(22):14787. https://doi.org/10.3390/su142214787
Chicago/Turabian StyleArranz, Patricia, Fredrik Christiansen, Maria Glarou, Shane Gero, Fleur Visser, Machiel G. Oudejans, Natacha Aguilar de Soto, and Kate Sprogis. 2022. "Body Condition and Allometry of Free-Ranging Short-Finned Pilot Whales in the North Atlantic" Sustainability 14, no. 22: 14787. https://doi.org/10.3390/su142214787
APA StyleArranz, P., Christiansen, F., Glarou, M., Gero, S., Visser, F., Oudejans, M. G., Aguilar de Soto, N., & Sprogis, K. (2022). Body Condition and Allometry of Free-Ranging Short-Finned Pilot Whales in the North Atlantic. Sustainability, 14(22), 14787. https://doi.org/10.3390/su142214787







