Analysis of Post-Exercise Acute Hemodynamic Sustainability in Different Training Methods in Paralympic Powerlifting Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Approach to the Problem
2.2. Sample
2.3. Instruments
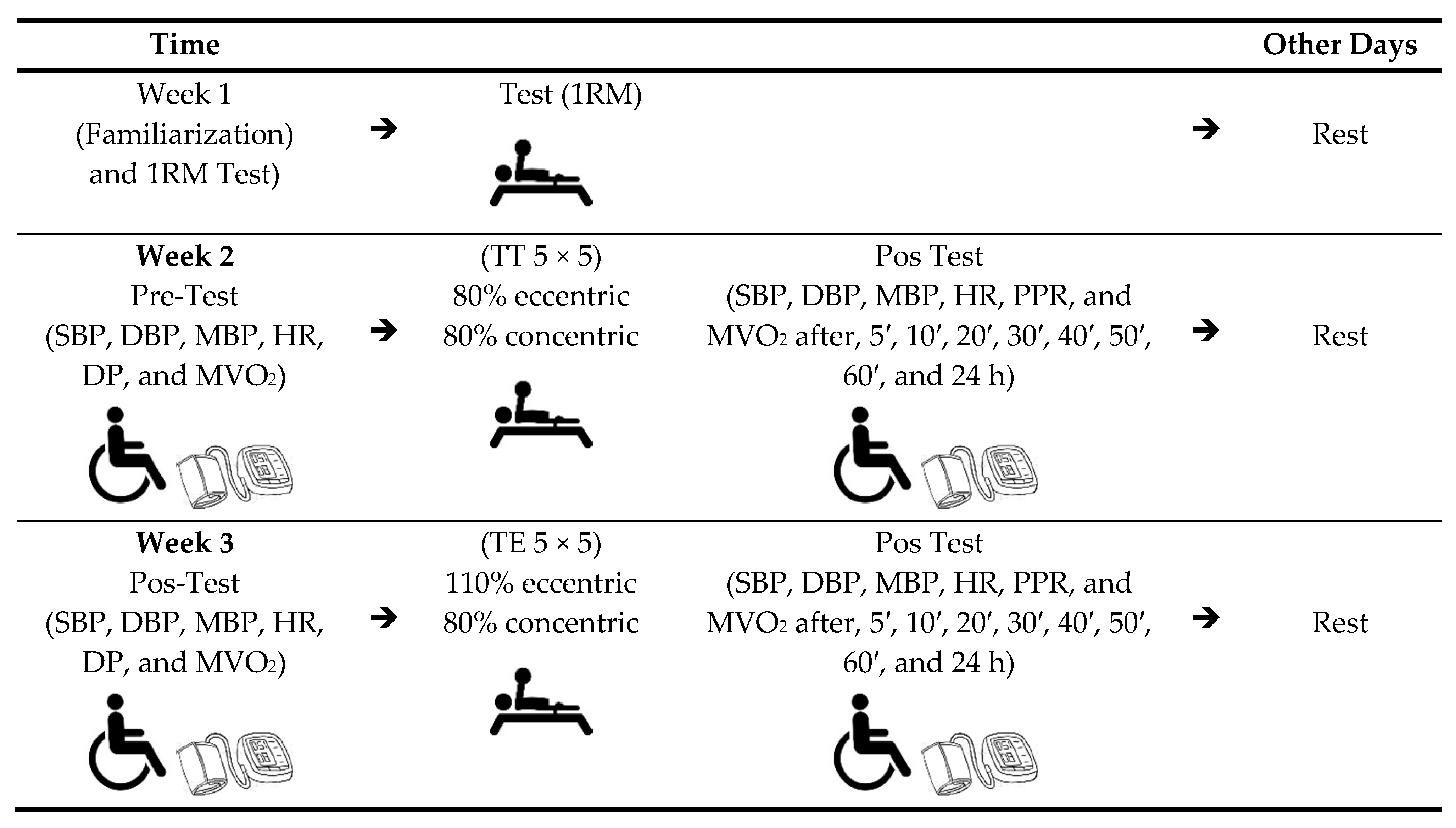
2.4. Procedures
2.4.1. Measurement of Blood Pressure and Heart Rate
2.4.2. Load Determination
2.4.3. Warm-Up
2.4.4. Training Procedures: Resistance, Traditional, and Eccentric
2.5. Statistics
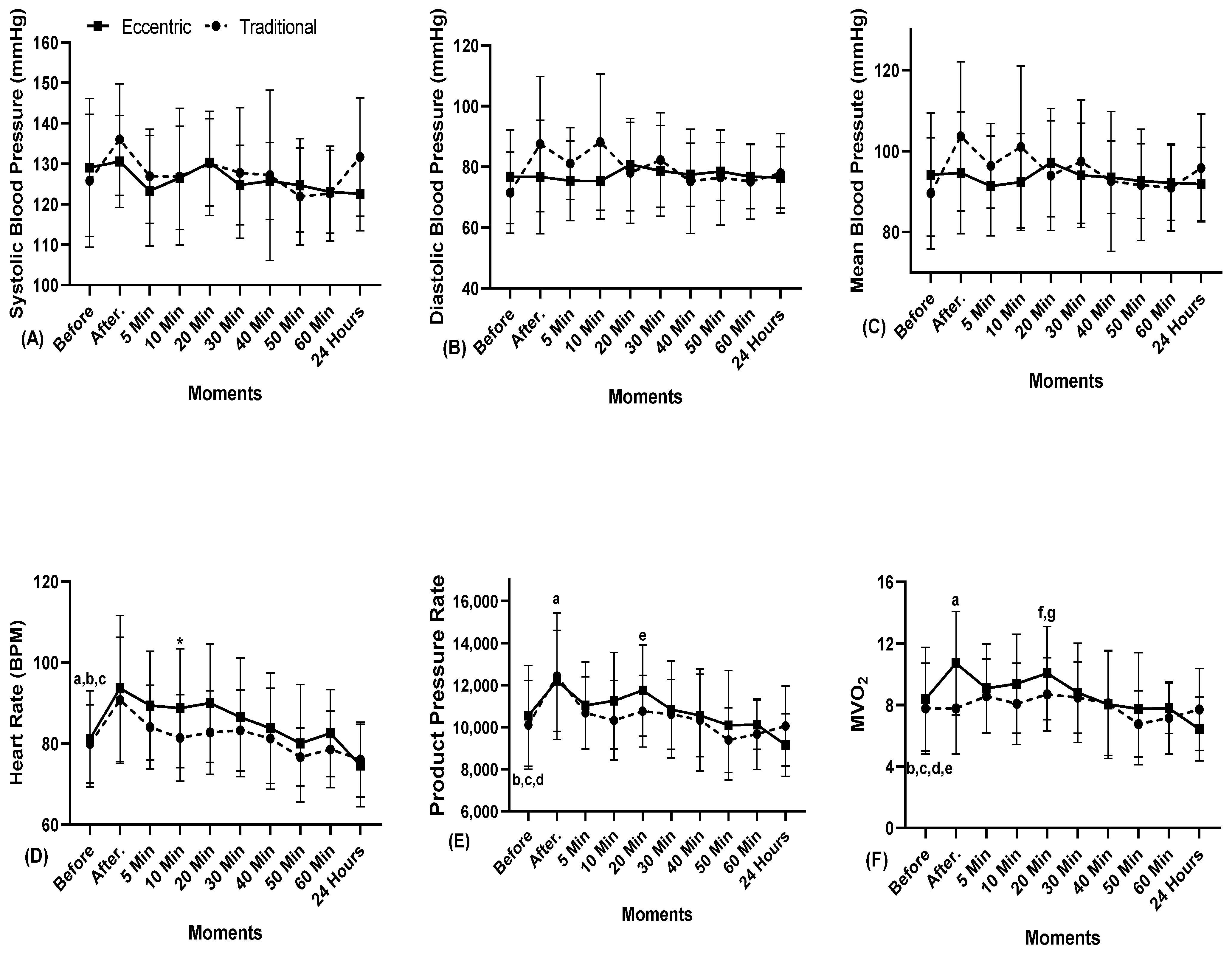
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Triani, F.D.S.; Lima, V.P.; Neto, V.G.C.; Monteiro, E.R. Correlation Between Body Mass Index, Muscle Power and Oxygen Consumption of Physical Education Students. J. Health Sci. 2018, 20, 29–33. [Google Scholar] [CrossRef]
- MacDougall, J.D.; McKelvie, R.S.; Moroz, D.E.; Sale, D.G.; McCartney, N.; Buick, F. Factors affecting blood pressure during heavy weight lifting and static contractions. J. Appl. Physiol. 1992, 73, 1590–1597. [Google Scholar] [CrossRef]
- Feriani, D.J.; Coelho-Júnior, H.J.; Scapini, K.B.; de Moraes, O.A.; Mostarda, C.; Ruberti, O.M.; Uchida, M.C.; Caperuto, C.; Irigoyen, M.C.; Rodrigues, B. Effects of inspiratory muscle exercise in the pulmonary function, autonomic modulation, and hemodynamic variables in older women with metabolic syndrome. J. Exerc. Rehabil. 2017, 13, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Murrant, C.L.; Fletcher, N.M.; Fitzpatrick, E.J.H.; Gee, K.S. Do skeletal muscle motor units and microvascular units align to help match blood flow to metabolic demand? Eur. J. Appl. Physiol. 2021, 121, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Poton, R.; Polito, M.D. Hemodynamic response to resistance exercise with and without blood flow restriction in healthy subjects. Clin. Physiol. Funct. Imaging 2014, 36, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Del Antonio, T.T.; de Assis, M.R. Rate-pressure product and variation of heart rate after isokinetic effort in adults and elderly. Rev. Bras. Med. Esporte 2017, 23, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.D.M.; Machado, C.A.; Poli-De-Figueiredo, C.E.; Amodeo, C.; Mion, D.; et al. Brazilian Guidelines on Blood Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef] [PubMed]
- Marçal, I.R.; Amaral, V.T.D.; Fernandes, B.; de Abreu, R.M.; Alvarez, C.; Guimarães, G.V.; Cornelissen, V.A.; Ciolac, E.G. Acute high-intensity interval exercise versus moderate-intensity continuous exercise in heated water-based on hemodynamic, cardiac autonomic, and vascular responses in older individuals with hypertension. Clin. Exp. Hypertens. 2022, 44, 427–435. [Google Scholar] [CrossRef]
- Ferrari, R.; Cadore, E.L.; Périco, B.; Kothe, G.B. Acute effects of body-weight resistance exercises on blood pressure and glycemia in middle-aged adults with hypertension. Clin. Exp. Hypertens. 2020, 43, 63–68. [Google Scholar] [CrossRef]
- Wegmann, M.; Hecksteden, A.; Poppendieck, W.; Steffen, A.; Kraushaar, J.; Morsch, A.; Meyer, T. Postexercise Hypotension as a Predictor for Long-Term Training-Induced Blood Pressure Reduction: A Large-Scale Randomized Controlled Trial. Clin. J. Sport Med. 2018, 28, 509–515. [Google Scholar] [CrossRef]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. 2017, 122, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, P.; Campbell, C.S.G.; Sales, M.; Motta-Santos, D.; Moreira, S.; Cunha, V.; Benford, R.; Simões, H. Acute resistance exercise is more effective than aerobic exercise for 24h blood pressure control in type 2 diabetics. Diabetes Metab. 2011, 37, 112–117. [Google Scholar] [CrossRef] [PubMed]
- João, G.A.; Bocalini, D.S.; Rodriguez, D.; Charro, M.A.; Ceschini, F.; Martins, A.; Junior, A.F. Powerlifting sessions promote significant post-exercise hypotension. Rev. Bras. Med. Esporte 2017, 23, 118–122. [Google Scholar] [CrossRef] [Green Version]
- International Paralympic Committee (IPC). World Para Powerlifiting. Rules & Regulations. Available online: https://www.paralympic.org/sites/default/files/document/180215210800620_World%2BPara%2BPowerlifting%2BRules%2Band%2BRegulations_Feb%2B2018_0.pdf (accessed on 13 August 2022).
- De Souza, J.A.; De França, I.S.X. Prevalence of high blood pressure in people with impaired physical mobility: Nursing implications. Rev. Bras. Enferm. 2008, 61, 816–821. [Google Scholar] [CrossRef] [Green Version]
- Mohammedi, K.; Potier, L.; Belhatem, N.; Matallah, N.; Hadjadj, S.; Roussel, R.; Marre, M.; Velho, G. Lower-extremity amputation as a marker for renal and cardiovascular events and mortality in patients with long standing type 1 diabetes. Cardiovasc. Diabetol. 2016, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Taber, C.; Morris, J.; Wagle, J.; Merrigan, J. Accentuated Eccentric Loading in the Bench Press: Considerations for Eccentric and Concentric Loading. Sports 2021, 9, 54. [Google Scholar] [CrossRef]
- Paz, D.A.; Aidar, F.J.; de Matos, D.G.; de Souza, R.F.; da Silva-Grigoletto, M.E.; Tillaar, R.V.D.; Ramirez-Campillo, R.; Nakamura, F.Y.; Costa, M.D.C.; Nunes-Silva, A.; et al. Comparison of Post-Exercise Hypotension Responses in Paralympic Powerlifting Athletes after Completing Two Bench Press Training Intensities. Medicina 2020, 56, 156. [Google Scholar] [CrossRef] [Green Version]
- Aidar, F.J.; Paz, D.A.; Gama, D.d.M.; de Souza, R.F.; Souza, L.M.V.; dos Santos, J.L.; Almeida-Neto, P.F.; Marçal, A.C.; Neves, E.B.; Moreira, O.C.; et al. Evaluation of the Post-Training Hypotensor Effect in Paralympic and Conventional Powerlifting. J. Funct. Morphol. Kinesiol. 2021, 6, 92. [Google Scholar] [CrossRef]
- Nam, G.-B. Exercise, Heart and Health. Korean Circ. J. 2011, 41, 113–121. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin. Proc. 2012, 87, 587–595. [Google Scholar] [CrossRef]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of Different Training Interventions on Heart Rate Variability and Cardiovascular Health and Risk Factors in Young and Middle-Aged Adults: A Systematic Review. Front. Physiol. 2021, 12, 657274. [Google Scholar] [CrossRef] [PubMed]
- Prior, D. Differentiating Athlete’s Heart from Cardiomyopathies—The Right Side. Heart Lung Circ. 2018, 27, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Aidar, F.J.; Dantas, E.F.; Almeida-Neto, P.F.; Neto, F.R.; Garrido, N.D.; Cabral, B.G.; Figueiredo, T.; Reis, V.M. Can Post-Exercise Hemodynamic Response Be Influenced by Different Recovery Methods in Paraplegic Sportsmen? Int. J. Environ. Res. Public Health 2022, 19, 1772. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Eguchi, K.; Reznik, M.E.; Shah, S.S.; Pickering, T.G. Validation of an oscillometric home blood pressure monitor in an end-stage renal disease population and the effect of arterial stiffness on its accuracy. Blood Press. Monit. 2007, 12, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Schutte, R.; Thijs, L.; Asayama, K.; Boggia, J.; Li, Y.; Hansen, T.W.; Liu, Y.-P.; Kikuya, M.; Björklund-Bodegård, K.; Ohkubo, T.; et al. Double Product Reflects the Predictive Power of Systolic Pressure in the General Population: Evidence from 9937 Participants. Am. J. Hypertens. 2013, 26, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.A.; Nerenberg, K.; Daskalopoulou, S.S.; McBrien, K.; Zarnke, K.B.; Dasgupta, K.; Cloutier, L.; Gelfer, M.; Lamarre-Cliche, M.; Milot, A.; et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can. J. Cardiol. 2016, 32, 569–588. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Austin, D.; Mann, B. Powerlifting; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Resende, M.D.A.; Aidar, F.; Resende, R.V.; Reis, G.; Barros, L.D.O.; de Matos, D.; Marçal, A.; de Almeida-Neto, P.; Díaz-De-Durana, A.; Merino-Fernández, M.; et al. Are Strength Indicators and Skin Temperature Affected by the Type of Warm-Up in Paralympic Powerlifting Athletes? Healthcare 2021, 9, 923. [Google Scholar] [CrossRef]
- dos Santos, M.D.M.; Aidar, F.J.; Alejo, A.A.; de Matos, D.G.; de Souza, R.F.; de Almeida-Neto, P.F.; Cabral, B.G.D.A.T.; Nikolaidis, P.T.; Knechtle, B.; Clemente, F.M.; et al. Analysis of Grip Amplitude on Velocity in Paralympic Powerlifting. J. Funct. Morphol. Kinesiol. 2021, 6, 86. [Google Scholar] [CrossRef]
- Cometti, G. Los Metodos Modernos de Musculacion; Paidotribo: Barcelona, Spain, 2007. [Google Scholar]
- Cohen, J. Statistics a power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Wewege, M.A.; Thom, J.M.; Rye, K.-A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, Ş. Exercise and Hypertension. Adv. Exp. Med. Biol. 2020, 1228, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Picón, M.M.; Chulvi, I.M.; Cortell, J.-M.T.; Tortosa, J.; Alkhadar, Y.; Sanchís, J.; Laurentino, G. Acute Cardiovascular Responses after a Single Bout of Blood Flow Restriction Training. Int. J. Exerc. Sci. 2018, 11, 20–31. [Google Scholar]
- Arroyo, E.; Wells, A.J.; Gordon, J.A., 3rd; Varanoske, A.N.; Gepner, Y.; Coker, N.A.; Church, D.D.; Fukuda, D.H.; Stout, J.R.; Hoffman, J.R. Tumor necrosis factor-alpha and soluble TNF-alpha receptor responses in young vs. middle-aged males following eccentric exercise. Exp. Gerontol. 2017, 100, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle: Part I: Cardiopulmonary Emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, E.S.; Asano, R.Y.; Filho, I.G.; Lopes, N.L.; Panelli, P.; Nascimento, D.D.C.; Collier, S.R.; Prestes, J. Acute and Chronic Cardiovascular Response to 16 Weeks of Combined Eccentric or Traditional Resistance and Aerobic Training in Elderly Hypertensive Women: A randomized controlled trial. J. Strength Cond. Res. 2014, 28, 3073–3084. [Google Scholar] [CrossRef]
- Peñailillo, L.; Diaz-Reiher, M.; Gurovich, A.; Flores-Opazo, M. A Short-Term Eccentric HIIT Induced Greater Reduction in Cardio-Metabolic Risk Markers in Comparison With Concentric HIIT in Sedentary Overweight Men. Res. Q. Exerc. Sport 2022, 1–13. [Google Scholar] [CrossRef]
- Duncan, M.J.; Birch, S.L.; Oxford, S.W. The Effect of Exercise Intensity on Postresistance Exercise Hypotension in Trained Men. J. Strength Cond. Res. 2014, 28, 1706–1713. [Google Scholar] [CrossRef] [Green Version]
- Río-Rodríguez, D.; Iglesias-Soler, E.; Del Olmo, M.F. Set Configuration in Resistance Exercise: Muscle Fatigue and Cardiovascular Effects. PLoS ONE 2016, 11, e0151163. [Google Scholar] [CrossRef]
- Whitman, M.; Jenkins, C.; Sabapathy, S.; Adams, L. Comparison of Heart Rate Blood Pressure Product Versus Age-Predicted Maximum Heart Rate as Predictors of Cardiovascular Events During Exercise Stress Echocardiography. Am. J. Cardiol. 2019, 124, 528–533. [Google Scholar] [CrossRef]
- Júnior, F.A.; Gomes, S.G.; Da Silva, F.F.; Souza, P.M.; Oliveira, E.C.; Coelho, D.B.; Nascimento-Neto, R.M.; Lima, W.; Becker, L.K. The effects of aquatic and land exercise on resting blood pressure and post-exercise hypotension response in elderly hypertensives. Cardiovasc. J. Afr. 2020, 31, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.S.V.; Bocchi, E.A.; Guimaraes, G.V.; Padovani, C.R.; Da Silva, M.H.G.G.; Pereira, S.F.; Fontes, R.D. Benefits of exercise training in the treatment of heart failure: Study with a control group. Arq. Bras. Cardiol. 2002, 79, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Rodrigues, K.A.; Brazão, J.C.; César, B.M.; Ozaki, E.H.; Almeida, R.D.S.; Soares, R.J.; Mezêncio, B.; Serrão, J.C.; Amadio, A.C.; De Cerqueira, A.S.O. Does muscle fatigue influence the response of the everter muscles after the simulation of an ankle sprain? Rev. Bras. Med. Esporte 2015, 21, 8–11. [Google Scholar] [CrossRef]
| Features | PP (n = 12) (Mean ± SD) |
|---|---|
| Age (years) | 30.8 ± 10.05 |
| Body mass (kg) | 70.0 ± 16.1 kg |
| Experience (years) | 2.8 ± 1.3 |
| Systolic blood pressure (mmHg) | 125.8 ± 15.7 |
| Diastolic blood pressure (mmHg) | 71.5 ± 12.7 |
| 1RM test (bench press) (kg) | 122.0 ± 38.06 |
| 1RM test/body mass | 1.7 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus, J.B.; Aidar, F.J.; de Souza Leite Junior, J.A.; Menezes, J.L.; Silva, A.F.; Carvutto, R.; Poli, L.; Cataldi, S.; Messina, G.; Banja Fernandes, T.L.; et al. Analysis of Post-Exercise Acute Hemodynamic Sustainability in Different Training Methods in Paralympic Powerlifting Athletes. Sustainability 2022, 14, 14817. https://doi.org/10.3390/su142214817
de Jesus JB, Aidar FJ, de Souza Leite Junior JA, Menezes JL, Silva AF, Carvutto R, Poli L, Cataldi S, Messina G, Banja Fernandes TL, et al. Analysis of Post-Exercise Acute Hemodynamic Sustainability in Different Training Methods in Paralympic Powerlifting Athletes. Sustainability. 2022; 14(22):14817. https://doi.org/10.3390/su142214817
Chicago/Turabian Stylede Jesus, Joseane Barbosa, Felipe J. Aidar, Joilson Alves de Souza Leite Junior, Jainara Lima Menezes, Ana Filipa Silva, Roberto Carvutto, Luca Poli, Stefania Cataldi, Giulia Messina, Tulio Luiz Banja Fernandes, and et al. 2022. "Analysis of Post-Exercise Acute Hemodynamic Sustainability in Different Training Methods in Paralympic Powerlifting Athletes" Sustainability 14, no. 22: 14817. https://doi.org/10.3390/su142214817












