Community Structure of Epilithic Moss Mites and Their Response to Environmental Factors in Different Grades of Rocky Desertification Habitats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Site Set-Up and Sample Collection
2.3. Isolation and Identification of Moss Mites
2.4. Data Processing and Calculation
2.4.1. Community Dominance
2.4.2. Biodiversity Index Calculation [49]
2.4.3. Analysis
3. Results
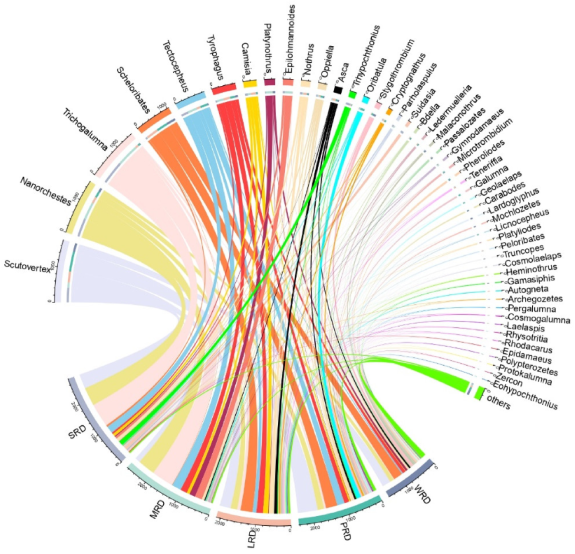
3.1. Community Composition and Distribution of Moss Mites in Different Grades of Rock Desertification
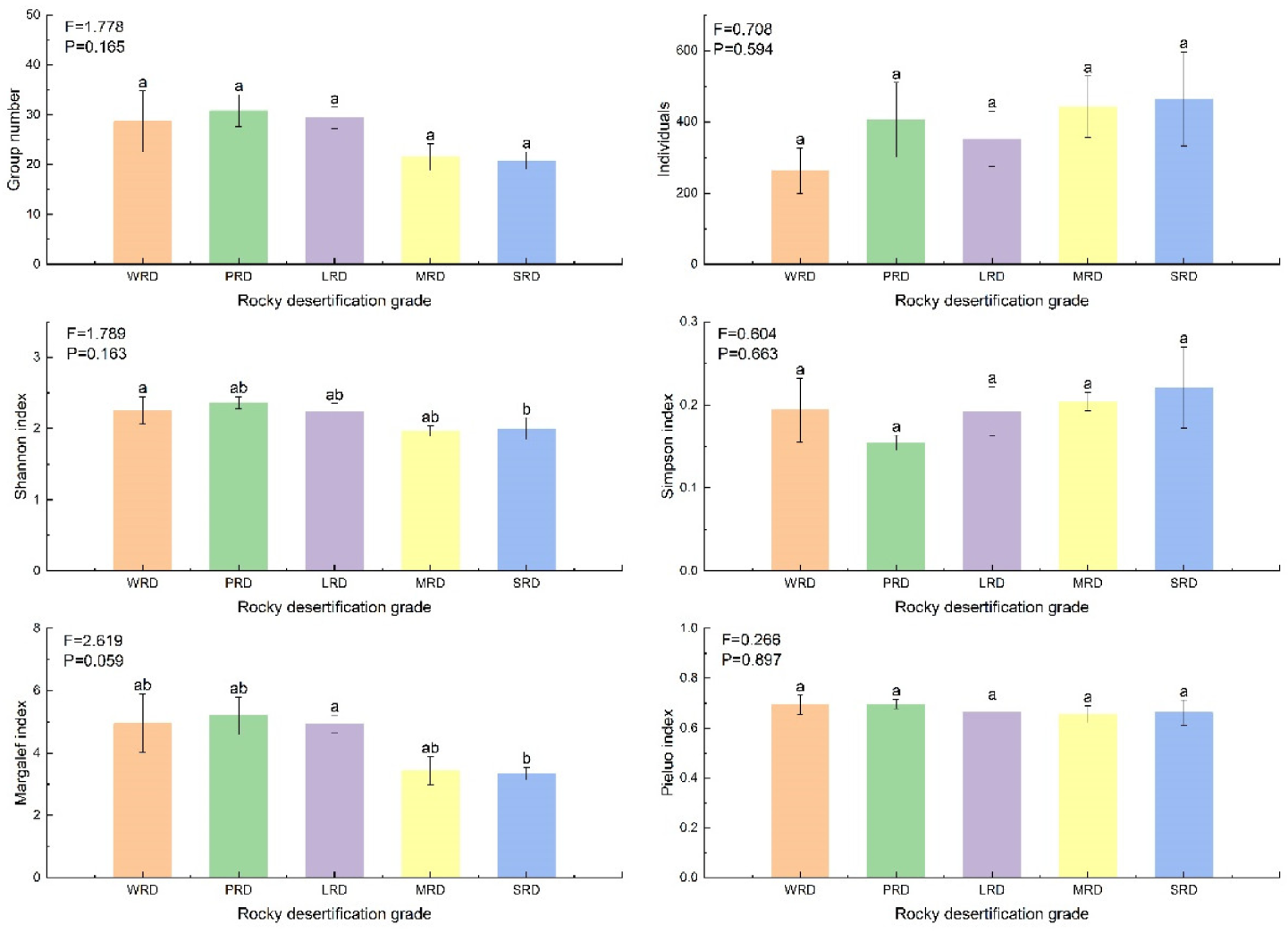
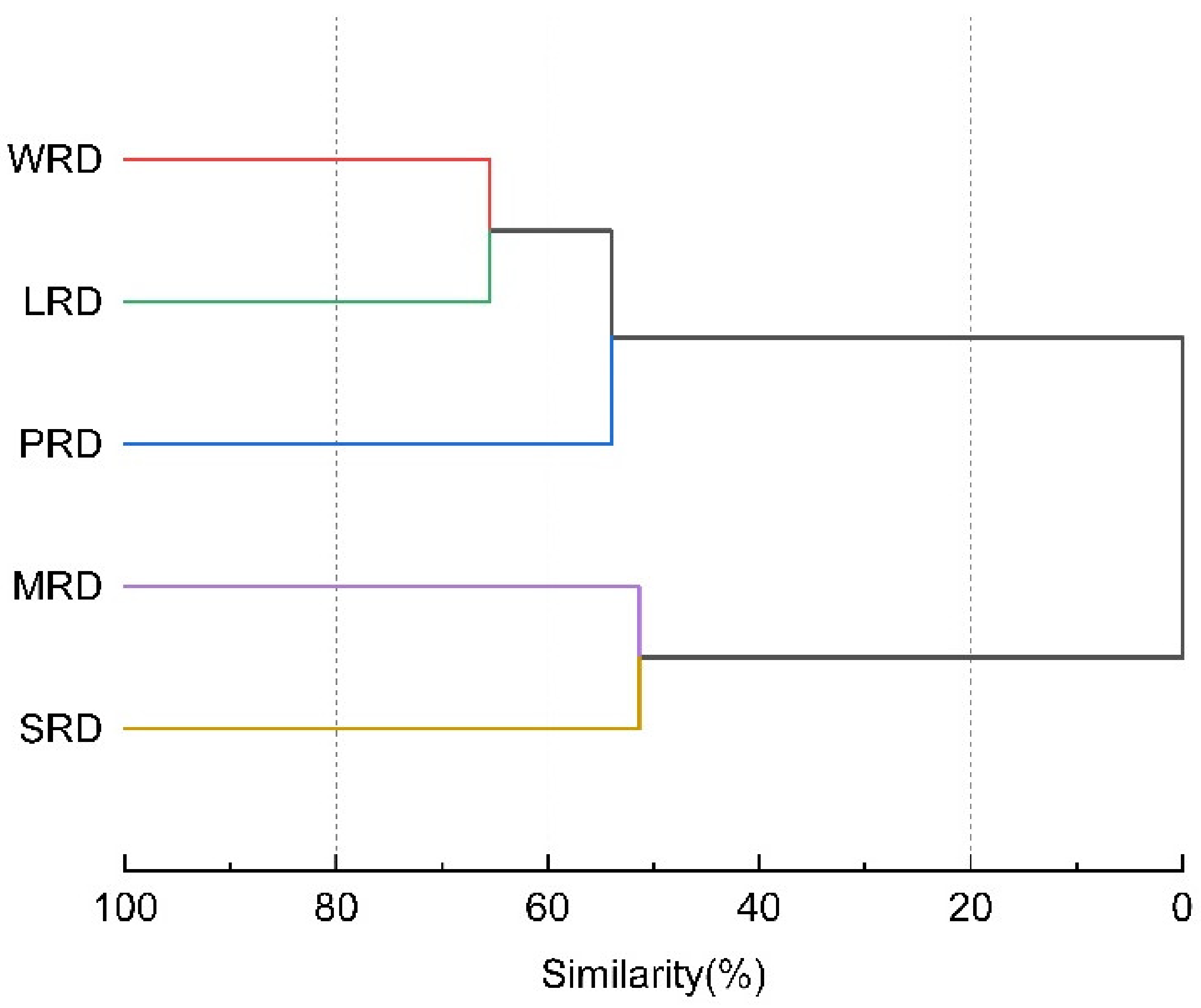
3.2. Diversity and Similarity of Moss Mite Communities in Different Rocky Desertification Grades
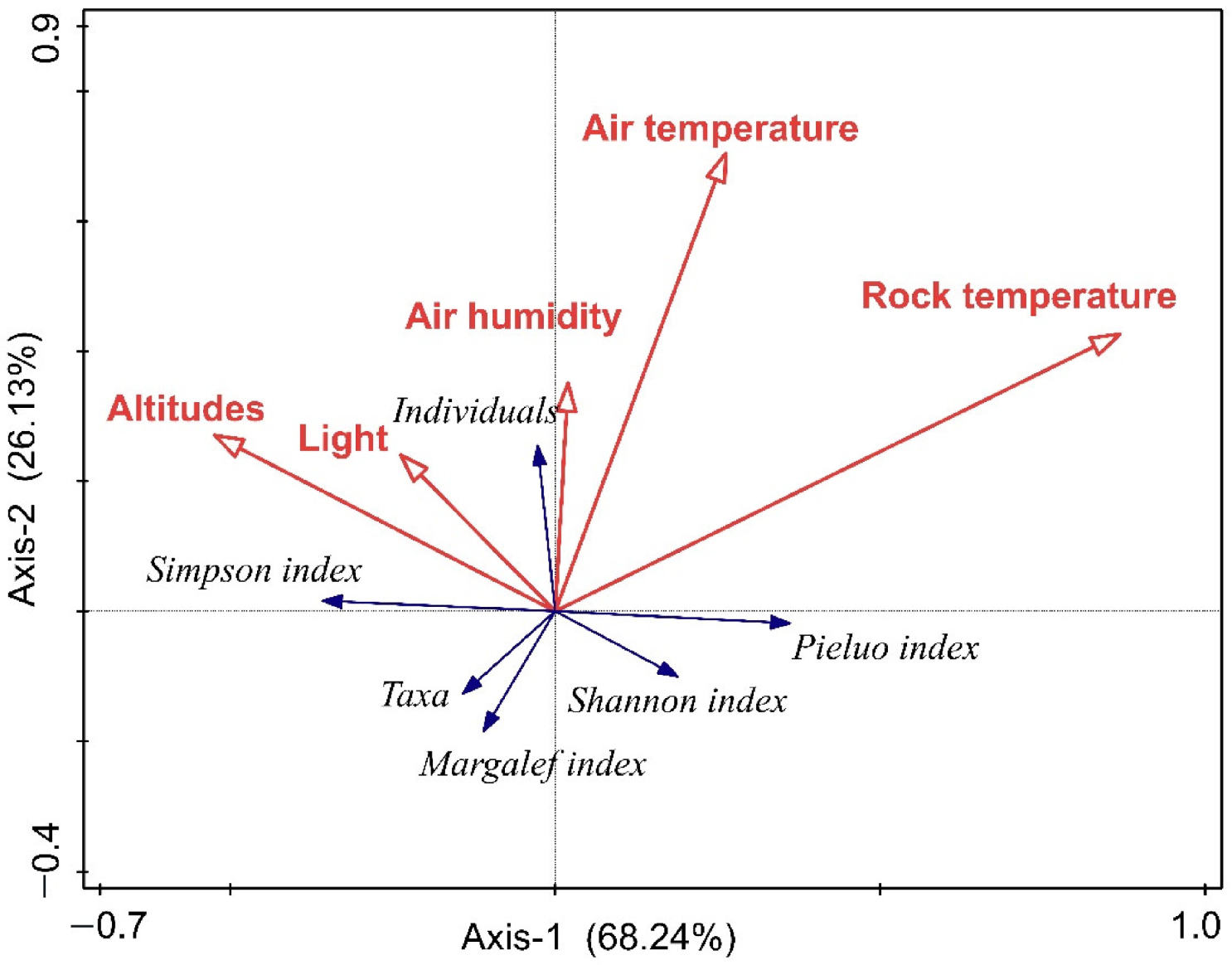
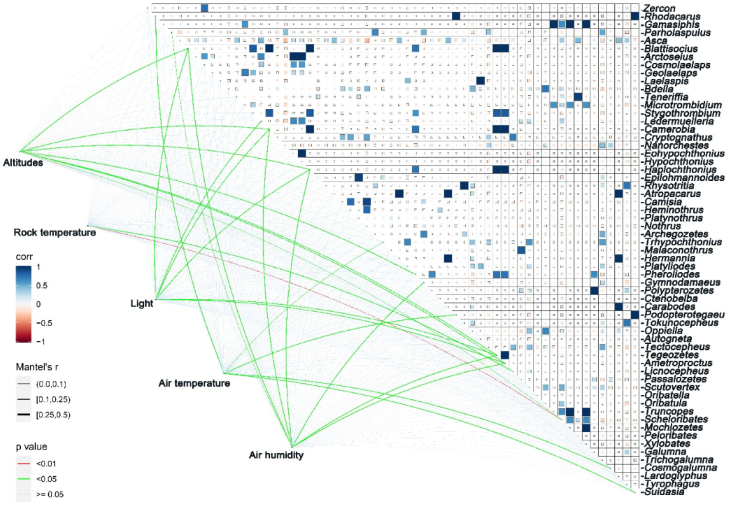
3.3. Response of Epilithic Moss Mites to Environmental Factors in Different Grades of Rocky Desertification
4. Discussion
4.1. Diversity of Epilithic Moss Mites in Different Rock Desertification Grades
4.2. Response of Epilithic Moss Mites to Environmental Factors in Different Rocky Desertification Grades
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Genus | Quantity | |||||
|---|---|---|---|---|---|---|---|
| WRD | PRD | LRD | MRD | SRD | Total | ||
| Trachytidae | Trachytes | 1 (+) | 1 (+) | ||||
| Uropodidae | Uropoda | 1 (+) | 1 (+) | ||||
| Dinychidae | Dinychus | 1 (+) | 1 (+) | ||||
| Epicriidae | Epicrius | 6 (+) | 6 (+) | ||||
| Zerconidae | Metazercon | 1 (+) | 1 (+) | ||||
| Prozercon | 3 (+) | 3 (+) | |||||
| Xenozercon | 1 (+) | 1 (+) | |||||
| Zercon | 2 (+) | 8 (+) | 2 (+) | 12 (+) | |||
| Parasitidae | Cornigamasus | 2 (+) | 2 (+) | ||||
| Noegamasus | 2 (+) | 4 (+) | 4 (+) | 1 (+) | 11 (+) | ||
| Vulgarogamasus | 1 (+) | 1 (+) | |||||
| Veigaiidae | Veigaia | 1 (+) | 2 (+) | 4 (+) | 7 (+) | ||
| Rhodacaridae | Punctoden-rolaelaps | 1 (+) | 1 (+) | ||||
| Gamasellus | 1 (+) | 2 (+) | 3 (+) | ||||
| Dendrolaelaps | 2 (+) | 2 (+) | |||||
| Rhodacarus | 3 (+) | 2 (+) | 2 (+) | 2 (+) | 4 (+) | 13 (+) | |
| Rhodacarellus | 2 (+) | 2 (+) | |||||
| Ologamasidae | Gamasiphis | 12 (++) | 3 (+) | 6 (+) | 2 (+) | 23 (+) | |
| Eviphididae | Alliphis | 2 (+) | 1 (+) | 3 (+) | |||
| Macrochelidae | Macrocheles | 1 (+) | 2 (+) | 2 (+) | 1 (+) | 5 (+) | 11 (+) |
| Parholaspididae | Krantzholaspis | 3 (+) | 3 (+) | ||||
| Parholaspulus | 3 (+) | 1 (+) | 4 (+) | 35 (+++) | 47 (+++) | 90 (++) | |
| Pachylaelapidae | Pachylaelaps | 1 (+) | 1 (+) | ||||
| Ascidae | Asca | 45 (+++) | 53 (+++) | 92 (+++) | 30 (+++) | 6 (+) | 226 (+++) |
| Leioseius | 1 (+) | 1 (+) | |||||
| Neojordensia | 1 (+) | 1 (+) | |||||
| Phytoseiidae | Amblyseius | 1 (+) | 1 (+) | ||||
| Blattisociidae | Blattisocius | 10 (++) | 1 | ||||
| Cheiroseius | 1 (+) | 1 (+) | |||||
| Arctoseius | 8 (+) | 1 (+) | 9 (+) | ||||
| Lasioseius | 1 (+) | 2 (+) | 2 (+) | 5 (+) | |||
| Zerconopsis | 1 (+) | 1 (+) | |||||
| Laelapidae | Cosmolaelaps | 1 (+) | 9 (+) | 1 | 3 (+) | 3 (+) | 26 (+) |
| Geolaelaps | 6 (+) | 29 (+++) | 1 (+) | 6 (+) | 42 (+) | ||
| Laelaspis | 6 (+) | 9 (+) | 1 (+) | 16 (+) | |||
| Ololaelaps | 2 (+) | 2 (+) | 4 (+) | ||||
| Pneumolaelaps | 1 (+) | 1 (+) | |||||
| Hypoaspis | 1 (+) | 1 (+) | |||||
| Labidostomatidae | Labidostoma | 1 (+) | 1 (+) | ||||
| Bdellidae | Bdella | 9 (++) | 35 (+++) | 26 (+++) | 2 (+) | 6 (+) | 78 (++) |
| Teneriffidae | Teneriffia | 4 (+) | 31 (+++) | 11 (+) | 9 (+) | 55 (+) | |
| Calyptosto-matidae | Calyptostoma | 1 (+) | 1 (+) | 2 (+) | |||
| Trombidiidae | Allothrombium | 1 (+) | 1 (+) | ||||
| Microtrombidiidae | Echinothrombium | 1 (+) | 1 (+) | ||||
| Microtrombidium | 19 (+++) | 20 (++) | 3 (+) | 13 (+) | 5 (+) | 60 (++) | |
| Stygothrombidi-idae | Stygothrombium | 85 (+++) | 54 (+++) | 17 (++) | 156 (+++) | ||
| Stigmaeidae | Ledermuelleria | 8 (++) | 33 (+++) | 27 (+++) | 68 (++) | ||
| Mecognathidae | Mecognatha | 2 (+) | 2 (+) | ||||
| Camerobiidae | Camerobia | 6 (+) | 6 (+) | ||||
| Barbutiidae | Barbutia | 1 (+) | 1 (+) | ||||
| Cryptognathidae | Cryptognathus | 1 (+) | 47 (+++) | 45 (+++) | 14 (++) | 21 (++) | 128 (+++) |
| Nanorchestidae | Nanorchestes | 118 (+++) | 193 (+++) | 201 (+++) | 520 (++++) | 556 (++++) | 1588 (++++) |
| Brachychthoniidae | Liochthonius | 1 (+) | 1 (+) | 2 (+) | |||
| Mesoplophoridae | Archoplophora | 1 (+) | 1 (+) | ||||
| Hypochthoniidae | Eohypochthonius | 1 | 1 (+) | 1 (+) | 12 (+) | ||
| Hypochthonius | 3 (+) | 1 (+) | 1 (+) | 5 (+) | |||
| Lohmanniidae | Mixacarus | 1 (+) | 1 (+) | ||||
| Meristolohmannia | 1 (+) | 1 (+) | |||||
| Annectacarus | 1 (+) | 1 (+) | |||||
| Haplochthoniidae | Haplochthonius | 3 (+) | 1 (+) | 4 (+) | |||
| Gehypochthoniidae | Gehypochthonius | 1 (+) | 1 (+) | ||||
| Eulohmanniidae | Eulohmannia | 1 (+) | 2 (+) | 3 (+) | |||
| Epilohmanniidae | Epilohmannia | 1 (+) | 1 (+) | ||||
| Epilohmannoides | 5 (+) | 7 (+) | 221 (+++) | 65 (+++) | 298 (+++) | ||
| Euphthiracaridae | Microtritia | 1 (+) | 1 (+) | 1 (+) | 3 (+) | ||
| Rhysotritia | 3 (+) | 3 (+) | 1 (+) | 7 (+) | 1 (+) | 15 (+) | |
| Phthiracaridae | Atropacarus | 7 (+) | 7 (+) | ||||
| Phthiracarus | 2 (+) | 2 (+) | 1 (+) | 2 (+) | 7 (+) | ||
| Stegacarus | 2 (+) | 2 (+) | |||||
| Camisiidae | Camisia | 12 (++) | 43 (+++) | 153 (+++) | 90 (+++) | 81 (+++) | 379 (+++) |
| Heminothrus | 4 (+) | 2 (+) | 15 (++) | 4 (+) | 1 (+) | 26 (+) | |
| Platynothrus | 67 (+++) | 11 (+) | 16 (++) | 178 (+++) | 31 (+++) | 303 (+++) | |
| Nothridae | Nothrus | 23 (+++) | 37 (+++) | 105 (+++) | 32 (+++) | 70 (+++) | 267 (+++) |
| Nanhermanniidae | Nanhermannia | 4 (+) | 4 (+) | ||||
| Trhypochthoniidae | Archegozetes | 3 (+) | 4 (+) | 1 (+) | 3 (+) | 1 | 21 (+) |
| Trhypochthonius | 9 (++) | 20 (++) | 26 (+++) | 6 (+) | 133 (+++) | 194 (+++) | |
| Malaconothridae | Malaconothrus | 47 (+++) | 1 (+) | 1 (+) | 12 (+) | 7 (+) | 68 (++) |
| Trimalaconothrus | 1 (+) | 1 (+) | |||||
| Hermanniidae | Hermannia | 11 (++) | 11 (+) | ||||
| Phyllhermannia | 1 (+) | 1 (+) | 1 (+) | 3 (+) | |||
| Hermanniellidae | Hermanniella | 1 (+) | 1 (+) | ||||
| Liodidae | Liodes | 1 (+) | 1 (+) | 1 (+) | 3 (+) | ||
| Neoliodidae | Platyliodes | 1 (+) | 1 (+) | 18 (++) | 14 (++) | 34 (+) | |
| poroliodes | 1 (+) | 1 (+) | |||||
| Pheroliodidae | Pheroliodes | 32 (+++) | 28 (+++) | 60 (++) | |||
| Licnodamaeidae | Licnodamaeus | 6 (+) | 6 (+) | ||||
| Gymnodamaeidae | Gymnodamaeus | 5 (+) | 4 (+) | 32 (+++) | 22 (++) | 63 (++) | |
| Odontodamaeus | 2 (+) | 2 (+) | |||||
| Licnobelbidae | licnobelba | 2 (+) | 3 (+) | 5 (+) | |||
| Pedrocortesellidae | Pedrocortesella | 1 (+) | 4 (+) | 2 (+) | 7 (+) | ||
| Damaeidae | Belba | 2 (+) | 2 (+) | ||||
| Damaeus | 3 (+) | 3 (+) | |||||
| Epidamaeus | 2 (+) | 2 (+) | 9 (+) | 13 (+) | |||
| Microtegeidae | Microtegeus | 1 (+) | 1 (+) | ||||
| Polypterozetidae | polypterozetes | 1 | 3 (+) | 13 (+) | |||
| Eremulidae | Eremulus | 2 (+) | 2 (+) | ||||
| Eremobelbidae | Eremobelba | 1 (+) | 1 (+) | ||||
| Damaeolidae | Fosseremus | 1 (+) | 1 (+) | 2 (+) | |||
| Ctenobelbidae | Ctenobelba | 3 (+) | 3 (+) | ||||
| Eremaeidae | Eremaeus | 1 (+) | 1 (+) | 2 (+) | |||
| Megeremaeidae | Megeremaeus | 4 (+) | 4 (+) | ||||
| Liacaridae | Liacarus | 1 (+) | 1 (+) | ||||
| Astegistidae | Astegistes | 1 (+) | 1 (+) | ||||
| Cultroribula | 1 (+) | 1 (+) | |||||
| Carabodidae | Carabodes | 29 (+++) | 9 (+) | 1 (+) | 2 (+) | 41 (+) | |
| Odontocepheus | 2 (+) | 1 (+) | 3 (+) | ||||
| Podopterotegae-idae | Podopterotegaeus | 11 (+) | 11 (+) | ||||
| Otocepheidae | Dolicheremaeus | 2 (+) | 2 (+) | ||||
| Tokunocepheidae | Tokunocepheus | 1 (+) | 8 (+) | 9 (+) | |||
| Oppiidae | Lauroppia | 1 (+) | 1 (+) | ||||
| Microppia | 1 (+) | 1 (+) | 2 (+) | ||||
| Oppiella | 37 (+++) | 83 (+++) | 48 (+++) | 44 (+++) | 39 (+++) | 251 (+++) | |
| Oxyoppia | 3 (+) | 3 (+) | |||||
| Goyoppia | 1 (+) | 1 (+) | |||||
| Quadroppiidae | Quadroppia | 9 (++) | 9 (+) | ||||
| Autognetidae | Autogneta | 1 (+) | 19 (++) | 2 (+) | 22 (+) | ||
| Tectocepheidae | Tectocepheus | 36 (+++) | 222 (+++) | 116 (+++) | 280 (++++) | 218 (+++) | 872 (+++) |
| Tegeozetes | 8 (++) | 8 (+) | |||||
| Cymbaeremaeidae | ametroproctus | 2 (+) | 2 (+) | ||||
| Eremellidae | licnocepheus | 2 (+) | 20 (++) | 1 (+) | 3 (+) | 9 (+) | 35 (+) |
| eremella | 4 (+) | 4 (+) | |||||
| Passalozetidae | Passalozetes | 30 (+++) | 1 | 16 (++) | 2 (+) | 6 (+) | 64 (++) |
| Scutoverticidae | Scutovertex | 259 (++++) | 594 (++++) | 329 (++++) | 159 (+++) | 433 (++++) | 1774 (++++) |
| Phenopelopidae | Eupelops | 1 (+) | 1 (+) | ||||
| Peloptulus | 2 (+) | 2 (+) | 4 (+) | ||||
| Achipteriidae | Achipteria | 1 (+) | 1 (+) | ||||
| Parachipteria | 1 (+) | 1 (+) | |||||
| Anachipteria | 1 (+) | 1 (+) | |||||
| Tegoribatidae | Tectoribates | 1 (+) | 1 (+) | ||||
| Oribatellidae | Lamellabates | 1 (+) | 1 (+) | ||||
| Ophidiotrichus | 1 (+) | 1 (+) | |||||
| Oribatella | 3 (+) | 1 (+) | 3 (+) | 7 (+) | |||
| Parakalummidae | Neoribates | 4 (+) | 2 (+) | 2 (+) | 8 (+) | ||
| Oribatulidae | Oribatula | 1 (+) | 130 (+++) | 2 (+) | 24 (++) | 20 (++) | 177 (+++) |
| Zygoribatula | 1 (+) | 7 (+) | 1 (+) | 9 (+) | |||
| Dometorina | 1 (+) | 1 (+) | |||||
| Oripodidae | Truncopes | 22 (+++) | 4 (+) | 2 (+) | 28 (+) | ||
| Scheloribatidae | Scheloribates | 277 (++++) | 345 (++++) | 361 (++++) | 13 (+) | 53 (+++) | 1049 (+++) |
| Mochlozetidae | Mochlozetes | 31 (+++) | 1 (+) | 2 (+) | 4 (+) | 38 (+) | |
| Podoribates | 1 (+) | 1 (+) | |||||
| Unguizetes | 1 (+) | 1 (+) | 2 (+) | ||||
| mochloribatula | 1 (+) | 1 (+) | |||||
| Caloppiidae | Chaunoproctus | 1 (+) | 1 (+) | ||||
| Drymobatidae | drymobates | 1 (+) | 1 (+) | ||||
| Haplozetidae | Haplozetes | 1 (+) | 1 (+) | ||||
| Peloribates | 3 (+) | 4 (+) | 21 (++) | 1 (+) | 29 (+) | ||
| Perxylobates | 8 (++) | 1 (+) | 1 (+) | 1 | |||
| Vilhenabates | 2 (+) | 2 (+) | 1 (+) | 5 (+) | |||
| Indoribates | 1 (+) | 1 (+) | |||||
| Xylobatidae | Xylobates | 10 (++) | 1 (+) | 1 (+) | 12 (+) | ||
| Sellnickiidae | Sellnickia | 1 (+) | 1 (+) | 2 (+) | |||
| Ceratozetidae | Ceratozetes | 1 (+) | 1 (+) | 2 (+) | |||
| Melanozetes | 2 (+) | 1 (+) | 3 (+) | ||||
| Chamobatidae | Chamobates | 3 (+) | 3 (+) | ||||
| Galumnidae | Galumna | 45 (+++) | 2 (+) | 4 (+) | 51 (+) | ||
| Pergalumna | 9 (++) | 3 (+) | 5 (+) | 3 (+) | 2 | ||
| Protokalumna | 5 (+) | 1 (+) | 5 (+) | 2 (+) | 13 (+) | ||
| Trichogalumna | 20 (+++) | 60 (+++) | 100 (+++) | 657 (++++) | 712 (++++) | 1549 (++++) | |
| Cosmogalumna | 3 (+) | 1 (+) | 8 (+) | 2 (+) | 5 (+) | 19 (+) | |
| Galumnellidae | Galumnella | 1 (+) | 1 (+) | 2 (+) | |||
| Histiostomatidae | Histiostoma | 3 (+) | 3 (+) | ||||
| Glycyphagidae | Glycyhagus | 2 (+) | 2 (+) | ||||
| Ctenoglyphus | 1 (+) | 1 (+) | |||||
| Lardoglyphidae | Lardoglyphus | 41 (+++) | 41 (+) | ||||
| Acaridae | Tyrophagus | 94 (+++) | 143 (+++) | 195 (+++) | 202 (+++) | 50 (+++) | 684 (+++) |
| Mycetoglyphus | 1 (+) | 1 (+) | |||||
| Caloglyphus | 2 (+) | 2 (+) | |||||
| Rhizoglyphus | 1 (+) | 1 (+) | |||||
| Thyreophagus | 4 (+) | 4 (+) | |||||
| Suidasia | 90 (+++) | 90 (++) | |||||
| Pyoglyphidae | Euroglyphus | 1 (+) | 1 (+) | ||||
| Taxa | 101 | 96 | 79 | 54 | 54 | ||
| Individuals | 1575 | 2432 | 2114 | 2656 | 2786 | 11563 |
References
- Pan, S.; Liang, J.; Chen, W.; Li, J.; Liu, Z. Gray Forecast of Ecosystem Services Value and Its Driving Forces in Karst Areas of China: A Case Study in Guizhou Province, China. Int. J. Environ. Res. Public Health 2021, 18, 12404. [Google Scholar] [CrossRef]
- Guo, B.; Yang, F.; Fan, J.; Lu, Y. The Changes of Spatiotemporal Pattern of Rocky Desertification and Its Dominant Driving Factors in Typical Karst Mountainous Areas under the Background of Global Change. Remote Sens. 2022, 14, 2351. [Google Scholar] [CrossRef]
- Ren, J.; Liu, F.; Luo, Y.; Zhu, J.; Luo, X.Q.; Liu, R. The Pioneering Role of Bryophytes in Ecological Restoration of Manganese Waste Residue Areas, Southwestern China. J. Chem. 2021, 2021, 9969253. [Google Scholar] [CrossRef]
- Wei, C.; Guo, B.; Fan, Y.; Zang, W.; Ji, J. The Change Pattern and Its Dominant Driving Factors of Wetlands in the Yellow River Delta Based on Sentinel-2 Images. Remote Sens. 2022, 14, 4388. [Google Scholar] [CrossRef]
- Tu, N.; Yan, Y.J.; Dai, Q.H.; Ren, Q.Q.; Meng, W.P.; Zhu, L.K.; Cen, L.P. Soil fixation and water retention of rocky moss under typical habitat in a karst rocky desertification area. Acta Ecol. Sin. 2021, 41, 6203–6214. [Google Scholar]
- Wang, Y.T.; Zheng, J.M.; Peng, X.W. A review on ecological functions of bryophytes in extreme environments. Plant Physiol. J. 2022, 58, 101–108. [Google Scholar]
- Lyons, A.; Turner, S.; Ashton, P.A. Management of upland calcareous grasslands for target vascular plant community impacts upon abundance but not diversity of non-target bryophytes. Biodivers. Conserv. 2022, 31, 1023–1036. [Google Scholar] [CrossRef]
- Gavini, S.S.; Suárez, G.M.; Ezcurra, C.; Aizen, M.A. Facilitation of vascular plants by cushion mosses in high-Andean communities. Alpine Bot. 2019, 129, 137–148. [Google Scholar] [CrossRef]
- Nelson, D.R.; Bartels, P.J.; Fegley, S.R. Environmental correlates of tardigrade community structure in mosses and lichens in the Great Smoky Mountains National Park (Tennessee and North Carolina, USA). Zool. J. Linn. Soc. 2019, 188, 913–924. [Google Scholar] [CrossRef]
- Pyszko, P.; Plášek, V.; Drozd, P. Don’t eat where you sleep: Unexpected diversity of food web for beetles feeding on mosses. Insect Conserv. Divers. 2021, 14, 325–334. [Google Scholar] [CrossRef]
- Spieksma, F.T.M. Mite biology. Clin. Rev. Allergy 1990, 8, 31–49. [Google Scholar] [CrossRef]
- Wang, H.F.; Jin, D.C. The review of Chinese acarology. Entomol. Knowl. 2000, 37, 36–41. [Google Scholar]
- Roeder, K.A.; Benson, B.R.; Weiser, M.D.; Kaspari, M. Testing the role of body size and litter depth on invertebrate diversity across six forests in North America. Ecology 2022, 103, e3601. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Argolo, P.S.; Moraes, G.J.D.; Norton, R.A.; SCHATZ, H. A checklist of the oribatid mite species (Acari: Oribatida) of Brazil. Zootaxa 2017, 4245, 1–89. [Google Scholar] [CrossRef]
- Meyer, S.; Kundel, D.; Birkhofer, K.; Fliessbach, A.; Scheu, S. Soil microarthropods respond differently to simulated drought in organic and conventional farming systems. Ecol. Evol. 2021, 11, 10369–10380. [Google Scholar] [CrossRef]
- NiedbaŁa, W.; Szywilewska-szczykutowicz, A. Ptyctimous mites (Acari, Oribatida) of Victoria (Australia). Zootaxa 2017, 4344, 47–85. [Google Scholar] [CrossRef]
- van der Merwe, S.S.; Swart, V.R.; Bredenhand, E.; Haddad, C.R. Soil-dwelling arthropods as indicators of erosion in a South African grassland habitat. Pedobiologia 2020, 80, 150647. [Google Scholar] [CrossRef]
- Leonov, V.D. The first report on the oribatid mites (Acari: Oribatida) in tundra of the Chunatundra Mountains on the Kola Peninsula, Russia. Acarologia 2020, 60, 722–734. [Google Scholar] [CrossRef]
- Pan, S.; Liang, J.; Wu, H.; Wei, L.; Cai, Y. Effect of urban greening and afforestation on soil microarthropod communities in coastal reclaimed land: Insights from functional traits. Appl. Soil Ecol. 2022, 173, 104391. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Zhou, Z.; Lin, D.; Wu, R.; Zhu, J. Soil Mite Community Structure in Mixed Evergreen and Deciduous Broad-leaved Forest of Fanjingshan. Soils 2018, 50, 687–695. [Google Scholar]
- Manzo, R.M.; Rizzuto, S.; Ruiz, E.V.; MartÍnez, P.A. Oribatid mites (Acari: Oribatida) from the Patagonian steppe, Argentina. Zootaxa 2019, 4686, 241–252. [Google Scholar] [CrossRef]
- Bayartogtokh, B.; Yondon, G. Contribution to the knowledge of soil mites (Acari: Oribatida) in Trans-Altai Gobi desert of Mongolia. Biologia 2019, 74, 1637–1652. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; de Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Agricultural intensification and de-intensification differentially affect taxonomic diversity of predatory mites, earthworms, enchytraeids, nematodes and bacteria. Appl. Soil Ecol. 2012, 57, 39–49. [Google Scholar] [CrossRef]
- Schatz, H.; Fortini, L.; Fusco, T.; Casale, F.; Jacomini, C.; Giulio, A.D. Oribatid mites (Acari, Oribatida) from Parco Naturale delle Alpi Marittime (Piedmont, Italy). Zootaxa 2021, 5082, 501–540. [Google Scholar] [CrossRef]
- Vackova, T.; Pekar, S.; Klimov, P.B.; Hubert, J. Sharing a bed with mites: Preferences of the house dust mite Dermatophagoides farinae in a temperature gradient. Exp. Appl. Acarol. 2021, 84, 755–767. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Mountford, O.J.; Maruşca, T.; Blaj, V.A.; Onete, M. Soil Mite (Acari: Mesostigmata) Communities and Their Relationships with Some Environmental Variables in Experimental Grasslands from Bucegi Mountains in Romania. Insects 2022, 13, 285. [Google Scholar] [CrossRef]
- Wehner, K.; Simons, N.K.; Blüthgen, N.; Heethoff, M. Drought, windthrow and forest operations strongly affect oribatid mite communities in different microhabitats. Glob. Ecol. Conserv. 2021, 30, e1757. [Google Scholar] [CrossRef]
- Julien, K.; Pokou, P.K.; Séka, F.A.; N’da, R.A.; Lagerlöf, J. Edaphic characteristics and environmental impact of rubber tree plantations on soil mite (Acari) communities. Acarologia 2018, 58, 951–962. [Google Scholar]
- Caruso, T.; Schaefer, I.; Monson, F.; Keith, A.M. Oribatid mites show how climate and latitudinal gradients in organic matter can drive large-scale biodiversity patterns of soil communities. J. Biogeogr. 2019, 46, 611–620. [Google Scholar] [CrossRef]
- Buch, A.C.; Sautter, K.D.; Marques, E.D.; Silva-Filho, E.V. Ecotoxicological assessment after the world’s largest tailing dam collapse (Fundão dam, Mariana, Brazil): Effects on oribatid mites. Environ. Geochem. Health 2020, 42, 3575–3595. [Google Scholar] [CrossRef]
- Yahya, M.; Afzal, M.; Majeed, M.Z.; Sarwar, I.; Shehzad, K.; Luqman, M.; Shahzad, S.M. Differential Impact of Land-Use, Season and Soil Characteristics on the Abundance of Edaphic Springtails (Insecta: Collembola) and Mites (Arachnida: Acari). Pak. J. Zool. 2020, 52, 1483–1491. [Google Scholar] [CrossRef]
- Lindo, Z.; Gonzalez, A. The Bryosphere: An Integral and Influential Component of the Earth’s Biosphere. Ecosystems 2010, 13, 612–627. [Google Scholar] [CrossRef]
- Khaustov, A.A. New species and records of mites of the family Stigmaeidae (Acari: Prostigmata) collected from mosses in Southern Chile. Acarologia 2016, 56, 639–679. [Google Scholar] [CrossRef][Green Version]
- Lindo, Z.; Winchester, N. Out on a limb: Microarthropod and microclimate variation in coastal temperate rainforest canopies. Insect Conserv. Divers. 2013, 6, 513–521. [Google Scholar] [CrossRef]
- Kreuzinger-Janik, B.; Traunspurger, W.; Majdi, N. Who feeds on whom in semi-aquatic moss ecosystems? Food Webs 2022, 32, e237. [Google Scholar] [CrossRef]
- Moquin, S.A.; Garcia, J.R.; Brantley, S.L.; Takacs-Vesbach, C.D.; Shepherd, U.L. Bacterial diversity of bryophyte-dominant biological soil crusts and associated mites. J. Arid. Environ. 2012, 87, 110–117. [Google Scholar] [CrossRef]
- Xiong, K.N.; Li, P.; Zhou, Z.F. Remote Sensing of Karst Desertification: Typical GIS Study; Geological Press: Beijing, China, 2002. [Google Scholar]
- Zha, G.C.; Liang, L.R.; Zhou, C.Q. Community structure and diversity of arthropods in moss soil. Acta Ecol. Sin. 2003, 23, 1057–1062. [Google Scholar]
- Xiao, L.M.; Zhang, W.; Wang, C.Y.; Hu, P.L.; Chen, Y.K.; Wang, K.L. Functional traits of bryophytes and their response and adaptation to soil factors in different vegetation restoration methods in a typical karst area. Acta Ecol. Sin. 2022, 42, 873–891. [Google Scholar]
- Omar, A. Collection of soil mites and preparation of slide specimens. Bull. Biol. 2012, 47, 57–59. [Google Scholar]
- Wang, R.H.; Liu, Q.Y.; Wang, X.Y.; Zhao, Z.Y.; Dou, Y.J. Responses of Soil Mite Community Diversity to Altitude Gradients in Luya Mountain, China. J. Shanxi Univ. 2022, 45, 1138–1150. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009; p. 807. [Google Scholar]
- Li, L.S.; Li, Y.R. Acarology; Chongqing Publishing Group: Chongqing, China, 1988; p. 520. [Google Scholar]
- Baker, E.W.; Camin, J.H. Acarina Subfamily Search; Shanghai People’s Publishing House: Shanghai, China, 1975; p. 236. [Google Scholar]
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998; p. 756. [Google Scholar]
- Yin, S.G.; Bei, N.X.; Chen, W.P. Soil Gamasid Mites in Northeast China; China Agricultural Press: Beijing, China, 2013. [Google Scholar]
- Li, C.P.; Shen, Z.P. Introduction to Acaroid Mites in China; Science Press: Beijing, China, 2016; p. 568. [Google Scholar]
- Lin, D.D.; Chen, H.; Chen, H.; Liu, P.P.; Liu, Q.S. Soil mite community structure in the evergreen, broad-leaved forest of Fanjing mountain, China. Chin. J. Appl. Environ. Biol. 2018, 24, 1185–1194. [Google Scholar]
- Zhang, J.T. Numerical Ecology; Science Press: Beijing, China, 2018; p. 492. [Google Scholar]
- Huang, B.; David, A.T.H.; Φyvind, H. Introduction to PAST, a comprehensive statistics software package for paleontological data analysis. Acta Palaeontol. Sin. 2013, 52, 161–181. [Google Scholar]
- Ren, Y.L.; Dong, C.B.; Shao, Q.Y.; Zhang, Z.Y.; Liang, Z.Q.; Han, Y.F. Application of redundancy analysis in microbial ecology. J. Mt. Agric. Biol. 2022, 41, 41–48. [Google Scholar]
- Shang, Y.; Wu, X.; Wang, X.; Wei, Q.; Ma, S.; Sun, G.; Zhang, H.; Wang, L.; Dou, H.; Zhang, H. Factors affecting seasonal variation of microbial community structure in Hulun Lake, China. Sci. Total Environ. 2022, 805, 150294. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Zhang, L.; Wu, J.; Li, S.; Zhu, G.; Sun, L. Comparison of Characteristics of Microbial Community Structure in Sewage Treatment Plants of High Altitude Area and Low Altitude Area. Environ. Eng. 2022, 40, 66–73. [Google Scholar]
- Shen, X.C.; Zhang, B.S.; Zhang, F.; Liu, X.T. Worldwide distribution and multivariate similarity clustering analysis of spiders. Acta Ecol. Sin. 2013, 33, 6795–6802. [Google Scholar] [CrossRef]
- O’Neill, K.P.; Godwin, H.W.; Jiménez-Esquilín, A.E.; Battigelli, J.P. Reducing the dimensionality of soil microinvertebrate community datasets using Indicator Species Analysis: Implications for ecosystem monitoring and soil management. Soil Biol. Biochem. 2010, 42, 145–154. [Google Scholar] [CrossRef]
- Guzmán, C.; Aguilar-Fenollosa, E.; Sahún, R.M.; Boyero, J.R.; Vela, J.M.; Wong, E.; Jaques, J.A.; Montserrat, M. Temperature-specific competition in predatory mites: Implications for biological pest control in a changing climate. Agric. Ecosyst. Environ. 2016, 216, 89–97. [Google Scholar] [CrossRef]
- Manzo, R.M.; Dadamia, M.M.; Rizzuto, S. New records of oribatid mites (Acari: Oribatida) from a Patagonian forest affected by wildfire in Argentina. Rev. Mex. Biodivers. 2021, 92, 923468. [Google Scholar] [CrossRef]
- Brunetti, C.; Siepel, H.; Convey, P.; Fanciulli, P.P.; Nardi, F.; Carapelli, A. Overlooked Species Diversity and Distribution in the Antarctic Mite Genus Stereotydeus. Diversity 2021, 13, 506. [Google Scholar] [CrossRef]
- Cordes, P.H.; Maraun, M.; Schaefer, I. Dispersal patterns of oribatid mites across habitats and seasons. Exp. Appl. Acarol. 2022, 86, 173–187. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, Y.; Xiao, N.; Huang, J.; Xiao, H.; Chen, H. Effects of three typical grass cultivation patterns on the community structure of soil mites in rocky desertification control area, Guizhou, China. Environ. Res. Commun. 2022, 4, 45008. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Q.; Xiao, N.; Huang, J.; Gong, T.; Fei, Y.; Shi, Z.; Chen, H. Characteristics of Soil Mites Communities Structure under Vegetation Vertical Gradient in the Shibing World Natural Heritage Property, China. Forests 2022, 13, 598. [Google Scholar] [CrossRef]
- Davidson, B.; Groner, E. An arthropod community beyond the dry limit of plant life. J. Arid Land 2021, 13, 629–638. [Google Scholar] [CrossRef]
- Nielsen, U.N.; King, C.K. Abundance and diversity of soil invertebrates in the Windmill Islands region, East Antarctica. Polar Biol. 2015, 38, 1391–1400. [Google Scholar] [CrossRef]
- Chen, H.; Jin, D.C.; Chen, H.; Wang, P.J.; Zhou, Z.; Lin, D.D. Differences in soil mite communities under different modes of vegetation restoration in an intense rocky desertification area, Guizhou, China. Acta Ecol. Sin. 2018, 38, 7045–7056. [Google Scholar]
- Chen, H.; Jin, D.C.; Wen, Z.H. Differences in soil mite communities in Karst Areas with different degrees of rocky desertification. Chin. J. Appl. Entomol. 2018, 55, 711–724. [Google Scholar]
- Xiao, H.; Yang, Y.W.; Chi, Y.K.; Chen, H. Characteristics of and differences among soil mite community structures in rocky desertification areas abandoned in various years. Fresen. Environ. Bull. 2022, 31, 2267–2275. [Google Scholar]
- Han, Y.R.; Xue, Q.Q.; Song, H.J.; Qi, J.Y.; Gao, R.H.; Cui, S.P.; Men, L.N.; Zhang, Z.W. Diversity and influencing factors of flower-visiting insects in the Yanshan area. Biodivers. Sci. 2022, 30, 48–59. [Google Scholar] [CrossRef]
- Kamczyc, J.; Dyderski, M.K.; Horodecki, P.; Jagodziński, A.M. Temperature and precipitation affect seasonal changes in mite communities (Acari: Mesostigmata) in decomposing litter of broadleaved and coniferous temperate tree species. Ann. For. Sci. 2022, 79, 12. [Google Scholar] [CrossRef]
- Vissa, S.; Soderberg, D.N.; Hofstetter, R.W. Field Translocation of Mountain Pine Beetles Suggests Phoretic Mite Communities Are Locally Adapted, and Mite Populations Respond Variably to Climate Warming. Insects 2021, 12, 131. [Google Scholar] [CrossRef]
- Wehner, K.; Heethoff, M.; Brückner, A. Seasonal fluctuation of oribatid mite communities in forest microhabitats. Peerj 2018, 6, e4863. [Google Scholar] [CrossRef]
- Jakšová, P.; Ľuptáčik, P.; Miklisová, D.; Horváthová, F.; Hlavatá, H. Oribatida (Acari) communities in arable soils formed under waterlogged conditions: The influence of a soil moisture gradient. Biologia 2020, 75, 243–257. [Google Scholar] [CrossRef]
- Badejo, M.A.; Akinwole, P.O. Microenvironmental preferences of oribatid mite species on the floor of a tropical rainforest. Exp. Appl. Acarol. 2006, 40, 145–156. [Google Scholar] [CrossRef]
| WRD | PRD | LRD | MRD | SRD | |
|---|---|---|---|---|---|
| WRD | 0.417 | 0.429 | 0.348 | 0.325 | |
| PRD | 0.417 | 0.496 | 0.339 | 0.389 | |
| LRD | 0.429 | 0.496 | 0.385 | 0.415 | |
| MRD | 0.348 | 0.339 | 0.385 | 0.421 | |
| SRD | 0.325 | 0.389 | 0.415 | 0.421 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yin, X.; Gong, T.; Liu, Y.; Chen, H. Community Structure of Epilithic Moss Mites and Their Response to Environmental Factors in Different Grades of Rocky Desertification Habitats. Sustainability 2022, 14, 14860. https://doi.org/10.3390/su142214860
Liu W, Yin X, Gong T, Liu Y, Chen H. Community Structure of Epilithic Moss Mites and Their Response to Environmental Factors in Different Grades of Rocky Desertification Habitats. Sustainability. 2022; 14(22):14860. https://doi.org/10.3390/su142214860
Chicago/Turabian StyleLiu, Wenjun, Xiumei Yin, Tong Gong, Ying Liu, and Hu Chen. 2022. "Community Structure of Epilithic Moss Mites and Their Response to Environmental Factors in Different Grades of Rocky Desertification Habitats" Sustainability 14, no. 22: 14860. https://doi.org/10.3390/su142214860
APA StyleLiu, W., Yin, X., Gong, T., Liu, Y., & Chen, H. (2022). Community Structure of Epilithic Moss Mites and Their Response to Environmental Factors in Different Grades of Rocky Desertification Habitats. Sustainability, 14(22), 14860. https://doi.org/10.3390/su142214860






