Differential Hydrological Properties of Forest Litter Layers in Artificial Afforestation of Eroded Areas of Latosol in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Litter Collection
2.3. Laboratory Analyses
2.4. Statistical Analysis
3. Results
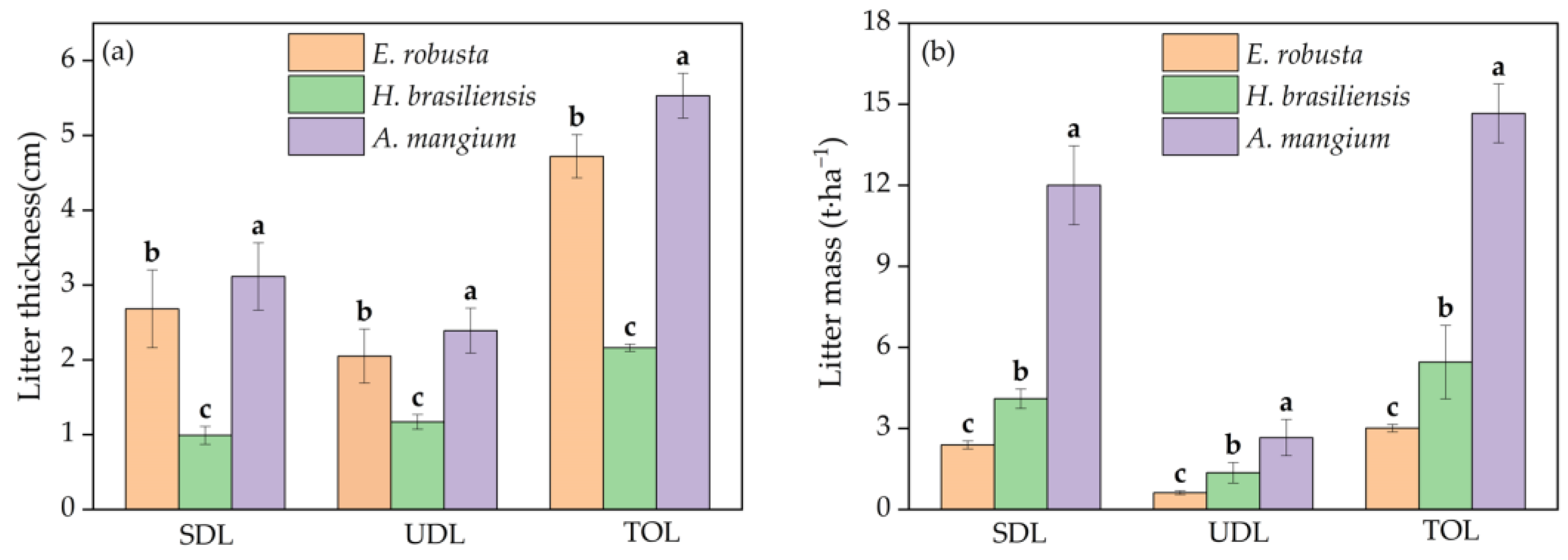
3.1. Variations in Litter Thickness and Mass
3.2. Variations in Rm, Weff, and Wmax
3.3. Variations in Water-Holding Capacity of Litter
3.4. Variations in Litter Water-Absorption Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mackay, D.S.; Band, L.E. Forest ecosystem processes at the watershed scale: Dynamic coupling of distributed hydrology and canopy growth. Hydrol. Process. 1997, 11, 1197–1217. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Zhang, X.; Chen, J.J.; Zhan, F.D.; Guo, X.H.; Zu, Y.Q. Differential water and soil conservation capacity and associated processes in four forest ecosystems in Dianchi Watershed, Yunnan Province, China. J. Soil Water Conserv. 2015, 70, 198–206. [Google Scholar] [CrossRef]
- Acharya, B.S.; Stebler, E.; Zou, C.B. Monitoring litter interception of rainfall using leaf wetness sensor under controlled and field conditions. Hydrol. Process. 2017, 31, 240–249. [Google Scholar] [CrossRef]
- Ilek, A.; Kucza, J.; Szostek, M. The effect of stand species composition on water storage capacity of the organic layers of forest soils. Eur. J. Forest Res. 2015, 134, 187–197. [Google Scholar] [CrossRef]
- Gomyo, M.; Kuraji, K. Effect of the litter layer on runoff and evapotranspiration using the paired watershed method. J. Forest Res. 2016, 21, 306–313. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Tanaka, N.; Gao, T.; Zhu, L.; Zou, C. Hydrological properties of litter layers in mixed forests in Mt. Qinling, China. iForest 2018, 11, 243–250. [Google Scholar] [CrossRef]
- Guevara-Escobar, A.; Gonzalez-Sosa, E.; Ramos-Salinas, M.; Hernandez-Delgado, G.D. Experimental analysis of drainage and water storage of litter layers. Hydrol. Earth Syst. Sci. 2007, 11, 1703–1716. [Google Scholar] [CrossRef]
- Zagyvai-Kiss, K.A.; Kalicz, P.; Szilágyi, J.; Gribovszki, Z. On the specific water holding capacity of litter for three forest ecosystems in the eastern foothills of the Alps. Agr. Forest Meteorol. 2019, 278, 107656. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Xu, Z.; Wang, Y.; Peng, H. Effect of vegetation on soil water retention and storage in a semi-arid alpine forest catchment. J. Arid Land 2013, 5, 207–219. [Google Scholar] [CrossRef]
- Dunkerley, D. Percolation through leaf litter: What happens during rainfall events of varying intensity? J. Hydrol. 2015, 525, 737–746. [Google Scholar] [CrossRef]
- Pang, X.; Bao, W. Effect of substituting plantation species for native shrubs on the water-holding characteristics of the forest floor on the eastern Tibetan Plateau. J Resour. Ecol. 2011, 2, 217–224. [Google Scholar]
- Pereira, L.C.; Balbinot, L.; Lima, M.T.; Bramorski, J.; Tonello, K.C. Aspects of forest restoration and hydrology: The hydrological function of litter. J. For. Res. 2022, 33, 543–552. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, H.; Xia, Y.; Xiao, J.; Wu, Y. Research on litter hydrology characteristic of typical vegetation in Jinyun mountain in Chongqing City. J. Soil Water Conserv. 2004, 4, 41–44. (In Chinese) [Google Scholar] [CrossRef]
- Keith, D.M.; Johnson, E.A.; Valeo, C. A hillslope forest floor (duff) water budget and the transition to local control. Hydrol. Process. 2010, 24, 2738–2751. [Google Scholar] [CrossRef]
- Neris, J.; Tejedor, M.; Rodríguez, M.; Fuentes, J.; Jiménez, C. Effect of forest floor characteristics on water repellency, infiltration, runoff and soil loss in Andisols of Tenerife (Canary Islands, Spain). Catena 2013, 108, 50–57. [Google Scholar] [CrossRef]
- Sato, Y.; Kumagai, T.o.; Kume, A.; Otsuki, K.; Ogawa, S. Experimental analysis of moisture dynamics of litter layers—The effects of rainfall conditions and leaf shapes. Hydrol. Process. 2004, 18, 3007–3018. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Wang, H.; Jia, G.; Zhao, Y.; Tu, Z.; Deng, W.; Jia, J.; Chen, J. Effects of forest structure on hydrological processes in China. J. Hydrol. 2018, 561, 187–199. [Google Scholar] [CrossRef]
- Llorens, P.; Domingo, F. Rainfall partitioning by vegetation under Mediterranean conditions. A review of studies in Europe. J. Hydrol. 2007, 335, 37–54. [Google Scholar] [CrossRef]
- Wang, B.; Wu, F.; Xiao, S.; Yang, W.; Justine, M.F.; He, J.; Tan, B. Effect of succession gaps on the understory water-holding capacity in an over-mature alpine forest at the upper reaches of the Yangtze River. Hydrol. Process. 2016, 30, 692–703. [Google Scholar] [CrossRef]
- Hua, W.; Jianli, Z.; Lifei, Y.; Lingbin, Y.; Congjun, Y.; Tengyong, L. Study on water conservation capacity of litter from different types of forest in Caohai Basin. Meteorol. Environ. Res. 2013, 4, 17–22+26. [Google Scholar]
- Zhou, Q.; Keith, D.M.; Zhou, X.; Cai, M.; Cui, X.; Wei, X.; Luo, Y. Comparing the water-holding characteristics of broadleaved, coniferous, and mixed forest litter layers in a Karst Region. Mt. Res. Dev. 2018, 38, 210, 220–229. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Lei, Y.; Su, K.; Wang, G.; Ma, H. Hydrological characteristics of litter in different forest succession stages at Liuxihe Watershed, southern China. Front. For. China 2009, 4, 317–322. [Google Scholar] [CrossRef]
- Liang, Y.; Li, D.; Lu, X.; Xuan, Y.; Pan, X.; Mu, H.; Shi, D.; Zhang, B. Soil erosion changes over the past five decades in the red soil region of southern China. J. Mt. Sci.-Engl. 2010, 7, 92–99. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, D.; Huang, Z.; Fu, Z.; Liu, J.; Hu, Y. Characteristics of soil and foliar N and P concentrations and stoichiometric ratio along restoration ages of Pinus massoniana plantations in red soils erosion regions of southern China. Chin. J. Appl. Environ. Biol. 2019, 25, 0768–0775. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Q.; Yang, W.; Zhao, J. Characteristics of soil ecological stoichiometry of different vegetation types in ephemeral gully of forestland in Red Soil Region. Res. Soil Water Conserv. 2020, 27, 60–65. (In Chinese) [Google Scholar] [CrossRef]
- Ran, S.; Jin, J. Evolvement and control of vulnerable ecological region—A case study in ongniud banner and aohan banner, inner mongolia. Chin. Geogr. Sci. 2004, 14, 135–141. [Google Scholar] [CrossRef]
- Liu, G.; Shangguan, Z.; Yao, W.; Yang, Q.; Zhao, M.; Dang, X.; Guo, M.; Wang, G.; Wang, B. Ecological Effects of Soil Conservation in Loess Plateau. Bull. Chin. Acad. Sci. 2017, 32, 11–19. (In Chinese) [Google Scholar] [CrossRef]
- Ma, X.; Zhao, C.; Zhu, J. Aggravated risk of soil erosion with global warming—A global meta-analysis. Catena 2021, 200, 105129. [Google Scholar] [CrossRef]
- Kumar, R.; Bhardwaj, A.K.; Rao, B.K.; Vishwakarma, A.K.; Kakade, V.; Dinesh, D.; Singh, G.; Kumar, G.; Pande, V.C.; Bhatnagar, P.R.; et al. Soil loss hinders the restoration potential of tree plantations on highly eroded ravine slopes. J. Soil. Sediments 2021, 21, 1232–1242. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.B.; Grelle, C.E.V.; Rey Benayas, J.M. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 11666. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.T.L.; Herbohn, J.; Gregorio, N.; Pasa, A.; Avela, M.S.; Solano, C.; Moreno, M.O.M.; Almendras-Ferraren, A.; Wills, J.; Shoo, L.P.; et al. Soil organic carbon recovery in tropical tree plantations may depend on restoration of soil microbial composition and function. Geoderma 2019, 353, 70–80. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Yan, M.; Fan, L.; Wang, L. Restoration of soil carbon with different tree species in a post-mining land in eastern Loess Plateau, China. Ecol. Eng. 2020, 158, 106025. [Google Scholar] [CrossRef]
- Lin, Y. Present situation and countermeasures of soil and water conservation in Hainan Province. Soil Water Conserv. China 2015, 3, 7–9. (In Chinese) [Google Scholar] [CrossRef]
- Carnol, M.; Bazgir, M. Nutrient return to the forest floor through litter and throughfall under 7 forest species after conversion from Norway spruce. For. Ecol. Manag. 2013, 309, 66–75. [Google Scholar] [CrossRef]
- Martin, W.K.E.; Timmer, V.R. Capturing spatial variability of soil and litter properties in a forest stand by landform segmentation procedures. Geoderma 2006, 132, 169–181. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Du, J.; Zhang, X.; Di, N. Effects of a broadleaf-oriented transformation of coniferous plantations on the hydrological characteristics of litter layers in subtropical China. Glob. Ecol. Conserv. 2021, 25, e01400. [Google Scholar] [CrossRef]
- Dong, H.; Yang, C.; Su, C.; Cao, H. Litter and Soil Hydrological Effects of Five No-commercial Forests in Dongguan. J. Soil Water Conserv. 2021, 35, 144–149+160. (In Chinese) [Google Scholar] [CrossRef]
- Levia, D.F.; Bollinger, W.C.; Hrabik, R.A. Evaporation of intercepted precipitation from fruit litter of Liquidambar styraciflua L. (sweetgum) in a clearing as a function of meteorological conditions. Int. J. Biometeorol. 2005, 49, 325–331. [Google Scholar] [CrossRef]
- Sun, X.; Onda, Y.; Otsuki, K.; Kato, H.; Hirata, A.; Gomi, T. The effect of strip thinning on tree transpiration in a Japanese cypress (Chamaecyparis obtusa Endl.) plantation. Agric. Forest Meteorol. 2014, 197, 123–135. [Google Scholar] [CrossRef]




| Vegetation Type | Stand Age (a) | Average Tree Height (m) | Average Breast Diameter (cm) | Canopy Coverage | Slope (◦) |
|---|---|---|---|---|---|
| E. robusta | 22 | 16.90 ± 2.10 | 20.10 ± 5.30 | 0.75 | 2~5 |
| H. brasiliensis | 23 | 15.50 ± 1.70 | 13.90 ± 2.70 | 0.82 | 2~5 |
| A. mangium | 22 | 15.00 ± 1.20 | 16.70 ± 3.50 | 0.85 | 2~5 |
| Variable | Factor | df | SS | MS | F | p |
|---|---|---|---|---|---|---|
| Litter thickness | tree | 2.84 | 46.41 | 23.20 | 196.35 | <0.001 *** |
| layer | 1.84 | 3.521 | 3.52 | 29.79 | <0.001 *** | |
| tree × layer | 2.84 | 3.84 | 1.92 | 16.24 | <0.001 *** | |
| Litter mass | tree | 2.84 | 566.67 | 283.34 | 590.01 | <0.001 *** |
| layer | 1.84 | 479.92 | 479.92 | 999.36 | <0.001 *** | |
| tree × layer | 2.84 | 254.33 | 127.16 | 264.80 | <0.001 *** | |
| Total thickness | tree | 2.42 | 92.81 | 46.41 | 734.19 | <0.001 *** |
| Total mass | tree | 2.42 | 1132.99 | 566.49 | 1087.02 | <0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Z.; Chen, S.; Ruan, D.; Chen, Z.; Huang, Y.; Chen, J. Differential Hydrological Properties of Forest Litter Layers in Artificial Afforestation of Eroded Areas of Latosol in China. Sustainability 2022, 14, 14869. https://doi.org/10.3390/su142214869
Tu Z, Chen S, Ruan D, Chen Z, Huang Y, Chen J. Differential Hydrological Properties of Forest Litter Layers in Artificial Afforestation of Eroded Areas of Latosol in China. Sustainability. 2022; 14(22):14869. https://doi.org/10.3390/su142214869
Chicago/Turabian StyleTu, Zhihua, Suyi Chen, Dongshuo Ruan, Zexian Chen, Yanping Huang, and Jinhui Chen. 2022. "Differential Hydrological Properties of Forest Litter Layers in Artificial Afforestation of Eroded Areas of Latosol in China" Sustainability 14, no. 22: 14869. https://doi.org/10.3390/su142214869
APA StyleTu, Z., Chen, S., Ruan, D., Chen, Z., Huang, Y., & Chen, J. (2022). Differential Hydrological Properties of Forest Litter Layers in Artificial Afforestation of Eroded Areas of Latosol in China. Sustainability, 14(22), 14869. https://doi.org/10.3390/su142214869






