How Extraction and Purification Affect MALDI-TOF MS Characterization of Mangrove Condensed Tannins, An Ecologically Important Secondary Metabolites in Coastal Wetland Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mangrove Material Collection and Pretreatment
2.2. Extraction and Purification of Mangrove Condensed Tannins
2.3. Characterization of Condensed Tannin Using MALDI-TOF MS
2.4. Statistical Analysis
3. Results and Discussion
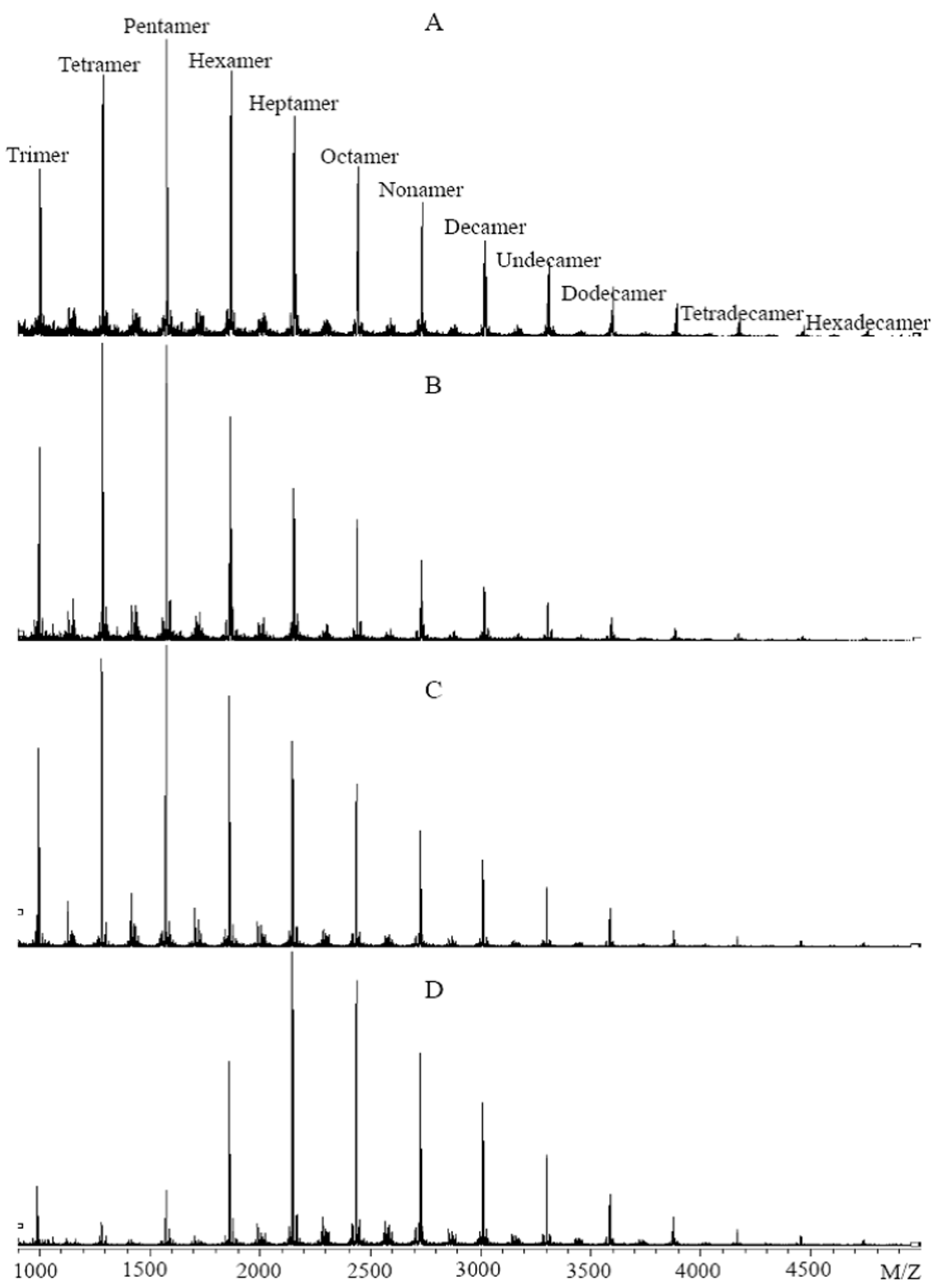
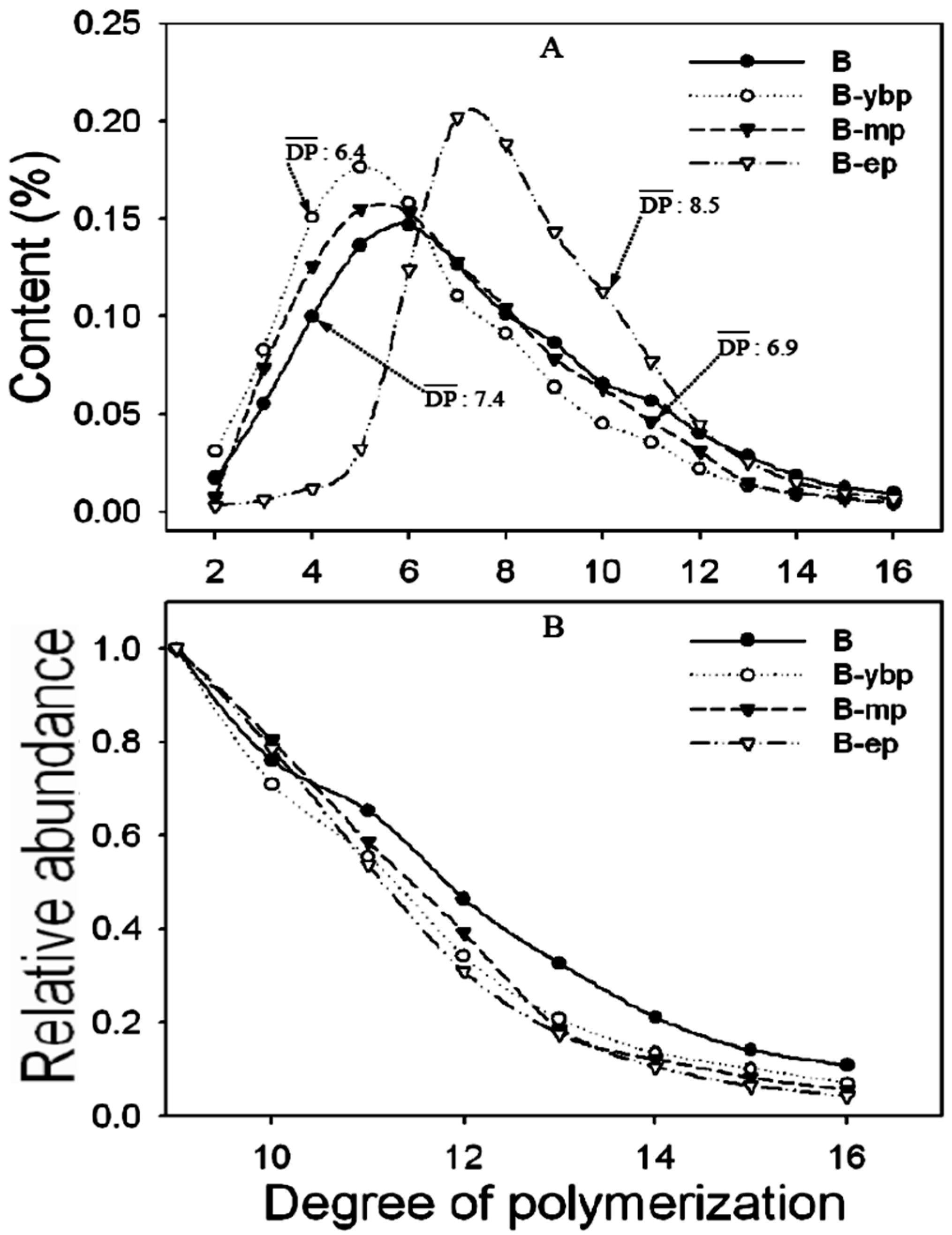
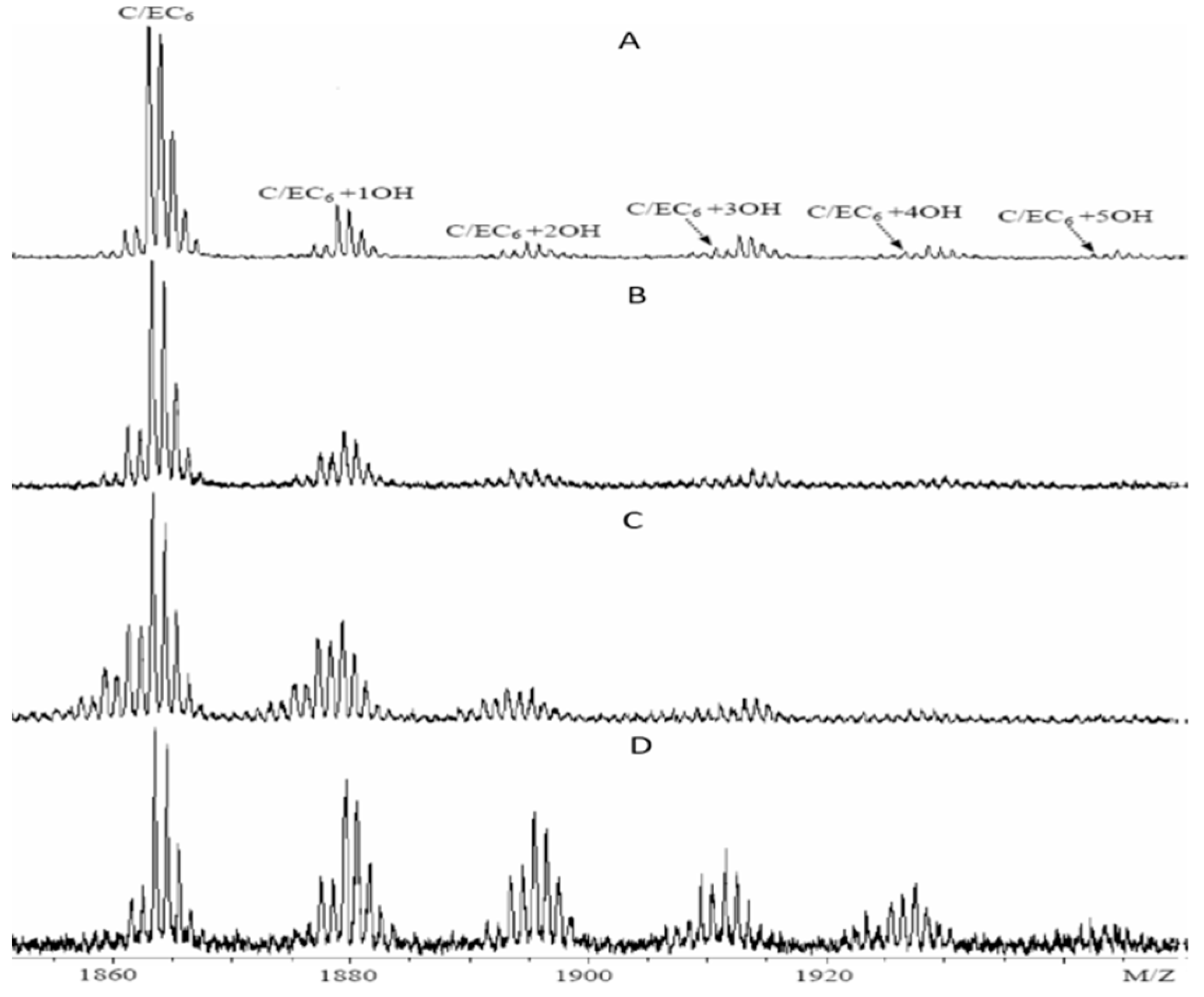
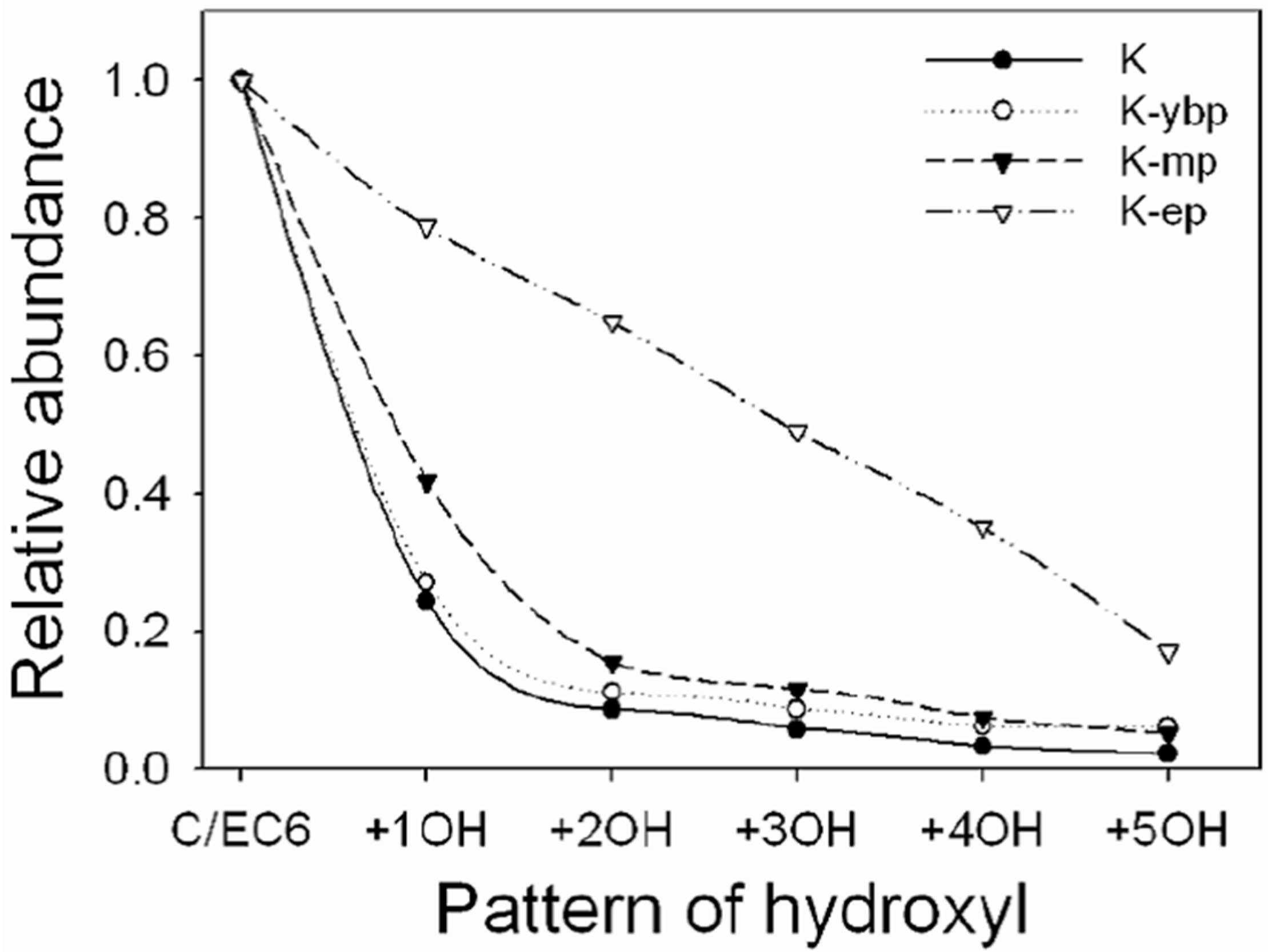
3.1. Effects of Different Extraction Methods on the Results of CT Structure Analysis
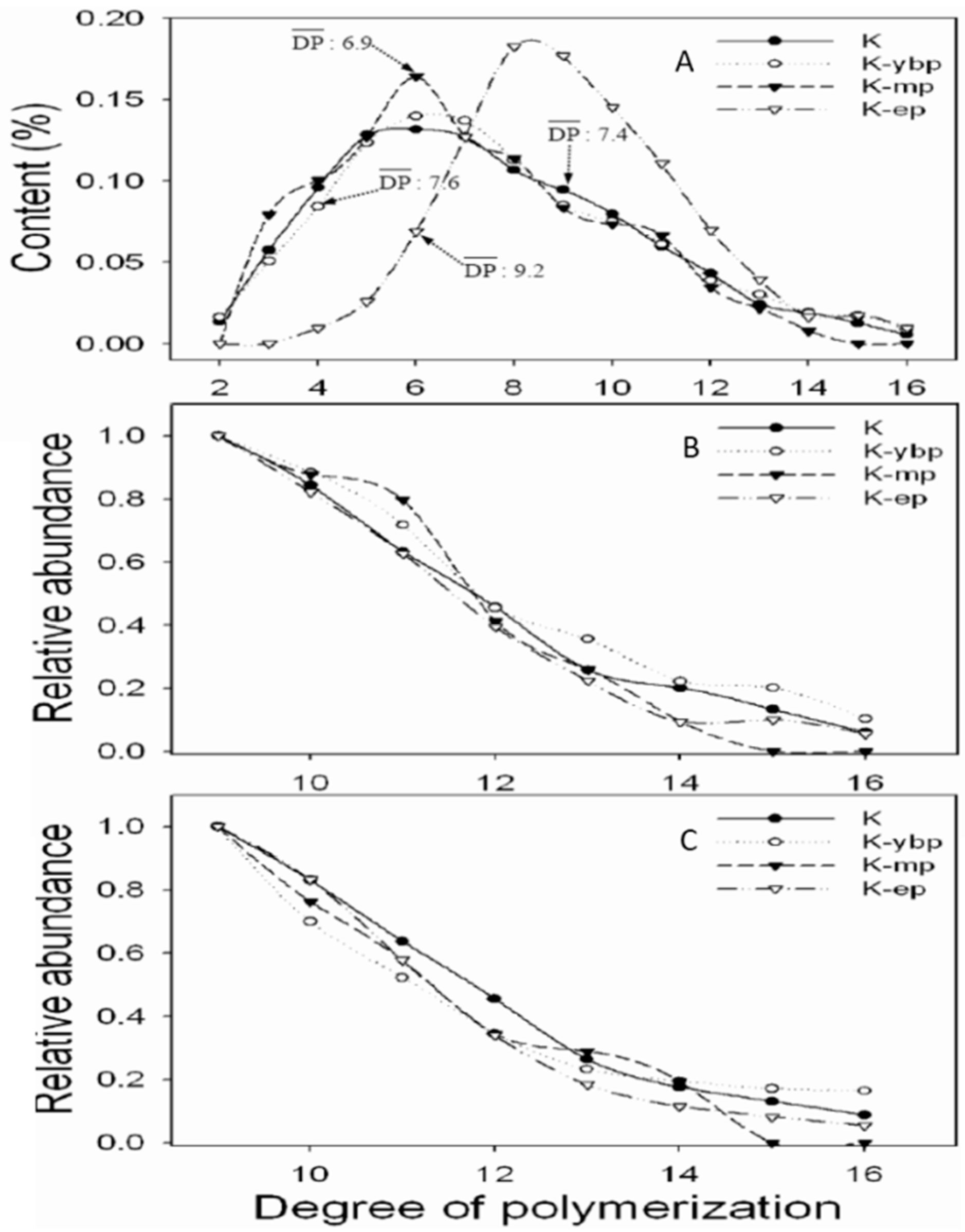
3.2. Effects of Different Purification Methods on the Results of CT Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P. Two-dimensional tannin fingerprints by liquid chromatography tandem mass spectrometry offer a new dimension to plant tannin analyses and help to visualize the tannin diversity in plants. J. Agric. Food Chem. 2018, 66, 9162–9171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Tam, N.F.Y. Polyphenols, tannins and antioxidant activities of eight true mangrove plant species in south China. Plant Soil 2014, 374, 549–563. [Google Scholar] [CrossRef]
- Wei, S.D.; Lin, Y.M.; Liao, M.M.; Zhou, H.C.; Li, Y.Y. Characterization and antioxidative properties of condensed tannins from the mangrove plant Aegiceras corniculatum. J. Appl. Polym. Sci. 2012, 124, 2463–2472. [Google Scholar] [CrossRef]
- Zhou, H.C. Biogeochemical Implications of Mangrove Plant Polyphenols and Their Antioxidant Activities. Ph.D. Thesis, Xiamen University, Xiamen, China, 2012. [Google Scholar]
- Zhou, H.C.; Tam, N.F.Y.; Lin, Y.M.; Wei, S.D.; Li, Y.Y. Changes of condensed tannins during decomposition of leaves of Kandelia obovata in a subtropical mangrove swamp in China. Soil Biol. Biochem. 2012, 44, 113–121. [Google Scholar] [CrossRef]
- Zhou, H.C.; Tam, N.F.Y.; Lin, Y.M.; Ding, Z.H.; Chai, W.M.; Wei, S.D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef]
- Lang, T.; Wei, P.; Chen, X.; Fu, Y.; Tam, N.F.Y.; Hu, Z.; Chen, Z.; Li, F.; Zhou, H. Microcosm study on allelopathic effects of leaf litter leachates and purified condensed tannins from Kandelia obovata on germination and growth of Aegiceras corniculatum. Forests 2021, 12, 1000. [Google Scholar] [CrossRef]
- Jiang, S.; Weng, B.S.; Liu, T.; Su, Y.; Liu, J.C.; Lu, H.L.; Yan, C.L. Response of phenolic metabolism to cadmium and phenanthrene and its influence on pollutant translocations in the mangrove plant Aegiceras corniculatum (L.) Blanco (Ac). Ecotoxicol. Environ. Saf. 2017, 141, 290–297. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Laanbroek, H.J. The Effects of condensed tannins derived from senescing rhizophora mangle leaves on carbon, nitrogen and phosphorus mineralization in a Distichlis spicata salt marsh soil. Plant. Soil 2018, 433, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.F.; Laanbroek, H.J. Tannins from senescent Rhizophora mangle mangrove leaves have a distinctive effect on prokaryotic and eukaryotic communities in a Distichlis spicata salt arsh soil. FEMS Microbiol. Ecol. 2020, 96, fiaa148. [Google Scholar] [CrossRef]
- Lang, T.; Tam, N.F.Y.; Hussain, M.; Ke, X.R.; Wei, J.; Fu, Y.J.; Li, M.D.; Huang, X.Z.; Huang, S.Y.; Xiong, Z.J.; et al. Dynamics of heavy metals during the development and decomposition of leaves of Avicennia marina and Kandelia obovata in a subtropical mangrove swamp. Sci. Total Environ. 2022, 855, 158700. [Google Scholar] [CrossRef]
- Kraus, W.; Ngoc, L.H.; Conrad, J.; Klaiber, I.; Reeb, S.; Vogler, B. Investigation of biologicallyactive natural products using online LC-bioassay, LC-NMR, and LC-MS Techniques. Phytochem. Rev. 2002, 1, 409–411. [Google Scholar] [CrossRef]
- Monagas, M.; Quintanilla-Lopez, J.E.; Gomez-Cordoves, C.; Bartolome, B.; Lebron-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharmaceut. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef]
- Muchuweti, M.; Ndhlala, A.R.; Kasiyamhuru, A. Estimation of the degree of polymerization of condensed tannins of some wild fruits of zimbabwe (Uapaca kirkiana and Ziziphus mauritiana) using the modified vanillin-HCl method. J. Sci. Food Agric. 2005, 85, 1647–1650. [Google Scholar] [CrossRef]
- Nierop, K.G.J.; Preston, C.M.; Verstraten, J.M. Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. a case study using four tannins. Soil Biol. Biochem. 2006, 38, 2794–2802. [Google Scholar] [CrossRef]
- Noferi, M.; Masson, E.; Merlin, A.; Pizzi, A.; Deglise, X. Antioxidant characteristics of hydrolysable and polyflavonoid tannins: An ESR kinetics study. J. Appl. Polym. Sci. 1997, 63, 475–482. [Google Scholar] [CrossRef]
- Noble, A.C. Astringency and bitterness of flavonoid phenols. In Chemistry of Taste: Mechanisms, Behaviorsand Mimics; Given, P., Paredes, D., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 825, pp. 192–201. [Google Scholar]
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 2005, 66, 2248–2263. [Google Scholar] [CrossRef]
- Rejtar, T.; Hu, P.; Juhasz, P.; Campbell, J.M.; Vestal, M.L.; Preisler, J.; Karger, B. Off-line coupling of high-resolution capillary electrophoresis to MALDI-TOF and TOF/TOF MS. J. Prote. Res. 2002, 1, 171–179. [Google Scholar] [CrossRef]
- Ishida, Y.; Kitagawa, K.; Goto, K.; Ohtani, H. Solid sampling technique for direct detection of condensed tannins in bark by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Sp. 2005, 19, 706–710. [Google Scholar] [CrossRef]
- Wei, S.D.; Zhou, H.C.; Lin, Y.M. Antioxidant activities of extract and fractions from the hypocotyls of the mangrove plant Kandelia candel. Int. J. Mol. Sci. 2010, 11, 4080–4093. [Google Scholar] [CrossRef]
- Dunford, N.; Imak, S.; Jonnala, R. Pressurized solvent extraction of policosanol from wheat straw, germ and bran. Food Chem. 2010, 119, 1246–1249. [Google Scholar] [CrossRef]
- Nawaz, H.; Aslam Shad, M.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar]
- Maie, N.; Behrens, A.; Knicker, H.; Kogel-Knabner, I. Changes in the structure and protein binding ability of condensed tannins during decomposition of fresh needles and leaves. Soil Biol. Biochem. 2003, 35, 577–589. [Google Scholar] [CrossRef]
- Chen, Y.M.; Hagerman, A.E. Characterization of soluble non-covalent complexes between bovine serum albumin and beta-1,2,3,4,6-penta-O-galloyl-D-glucopyranose by MALDI-TOF MS. J. Agric. Food Chem. 2004, 52, 4008–4011. [Google Scholar] [CrossRef] [PubMed]
- Krueger, C.G.; Dopke, N.C.; Treichel, P.M.; Folts, J.; Reed, J.D. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of polygalloyl polyflavan-3-ols in grape seed extract. J. Agric. Food Chem. 2000, 48, 1663–1667. [Google Scholar] [CrossRef]
- Krueger, C.G.; Vestling, M.M.; Reed, J.D. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of heteropolyflavan-3-ols and glucosylated heteropolyflavans in sorghum [Sorghum Bicolor (L.) Moench]. J. Agric. Food Chem. 2003, 51, 538–543. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkhonen, J. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Butler, L.G.; Price, M.L.; Brotherton, J.E. Vanillin assay for proanthocyanidins (condensed tannins): Modification of the solvent for estimation of the degree of polymerization. J. Agric. Food Chem. 1982, 30, 1087–1089. [Google Scholar] [CrossRef]
- Lin, Y.M.; Liu, J.W.; Xiang, P.; Lin, P.; Ye, G.F.; Sternberg, L.D.S.L. Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River Estuary, Fujian, China. Biogeochemistry 2006, 78, 343–359. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Sun, H.M.; Li, N.Y.; Lu, Y.J.; Shen, Z.D.; Jing, X.S.; Xiang, M.; Shen, X.; Chen, S.L. Multiple signaling networks of extracellular atp, hydrogen peroxide, calcium, and nitric oxide in the mediation of root ion fluxes in secretor and non-secretor mangroves under salt stress. Aquat. Bot. 2014, 119, 33–43. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Condensed tannin purification and characterization of tannin-associated proteins. J. Agric. Food Chem. 1980, 28, 947–952. [Google Scholar] [CrossRef]
- Mane, C.; Sommerer, N.; Yalcin, T.; Cheynier, V.; Cole, R.B.; Fulcrand, H. Assessment of the molecular weight distribution of tannin fractions through MALDI-TOF MS analysis of protein-tannin complexes. Anal. Chem. 2007, 79, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Hagerman, A.E.; Zhao, Y.; Johnson, S. Methods for determination of condensed and hydrolyzable tannins. In Antinutrients and Phytochemicals in Food; Shahidi, F., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 662, pp. 209–222. [Google Scholar]
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F.Y. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Z.; Yuan, Z. Characteristics of lipid extraction from Chlorella sp. Cultivated in outdoor raceway ponds with mixture of ethyl acetate and ethanol for biodiesel production. Bioresour. Technol. 2015, 191, 433–437. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H. A new cellulose purification approach for higher degree of polymerization: Modeling, optimization and characterization. Carbohydr. Polym. 2016, 152, 280–286. [Google Scholar] [CrossRef]
- Lessa Belone, M.C.; Kokko, M.; Sarlin, E. Degradation of common polymers in sewage sludge purification process developed for microplastic analysis. Environ. Pollut. 2021, 269, 116235. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, T.; Xiang, P.; Li, M.; Chen, Z.; Li, F.; Jiang, M.; Zhou, H. How Extraction and Purification Affect MALDI-TOF MS Characterization of Mangrove Condensed Tannins, An Ecologically Important Secondary Metabolites in Coastal Wetland Ecosystem. Sustainability 2022, 14, 14960. https://doi.org/10.3390/su142214960
Lang T, Xiang P, Li M, Chen Z, Li F, Jiang M, Zhou H. How Extraction and Purification Affect MALDI-TOF MS Characterization of Mangrove Condensed Tannins, An Ecologically Important Secondary Metabolites in Coastal Wetland Ecosystem. Sustainability. 2022; 14(22):14960. https://doi.org/10.3390/su142214960
Chicago/Turabian StyleLang, Tao, Ping Xiang, Mingdang Li, Zhiteng Chen, Fenglan Li, Mingguo Jiang, and Haichao Zhou. 2022. "How Extraction and Purification Affect MALDI-TOF MS Characterization of Mangrove Condensed Tannins, An Ecologically Important Secondary Metabolites in Coastal Wetland Ecosystem" Sustainability 14, no. 22: 14960. https://doi.org/10.3390/su142214960
APA StyleLang, T., Xiang, P., Li, M., Chen, Z., Li, F., Jiang, M., & Zhou, H. (2022). How Extraction and Purification Affect MALDI-TOF MS Characterization of Mangrove Condensed Tannins, An Ecologically Important Secondary Metabolites in Coastal Wetland Ecosystem. Sustainability, 14(22), 14960. https://doi.org/10.3390/su142214960







