Grasses in Semi-Arid Lowlands—Community Composition and Spatial Dynamics with Special Regard to the Influence of Edaphic Factors
Abstract
1. Introduction
2. Materials and Methods
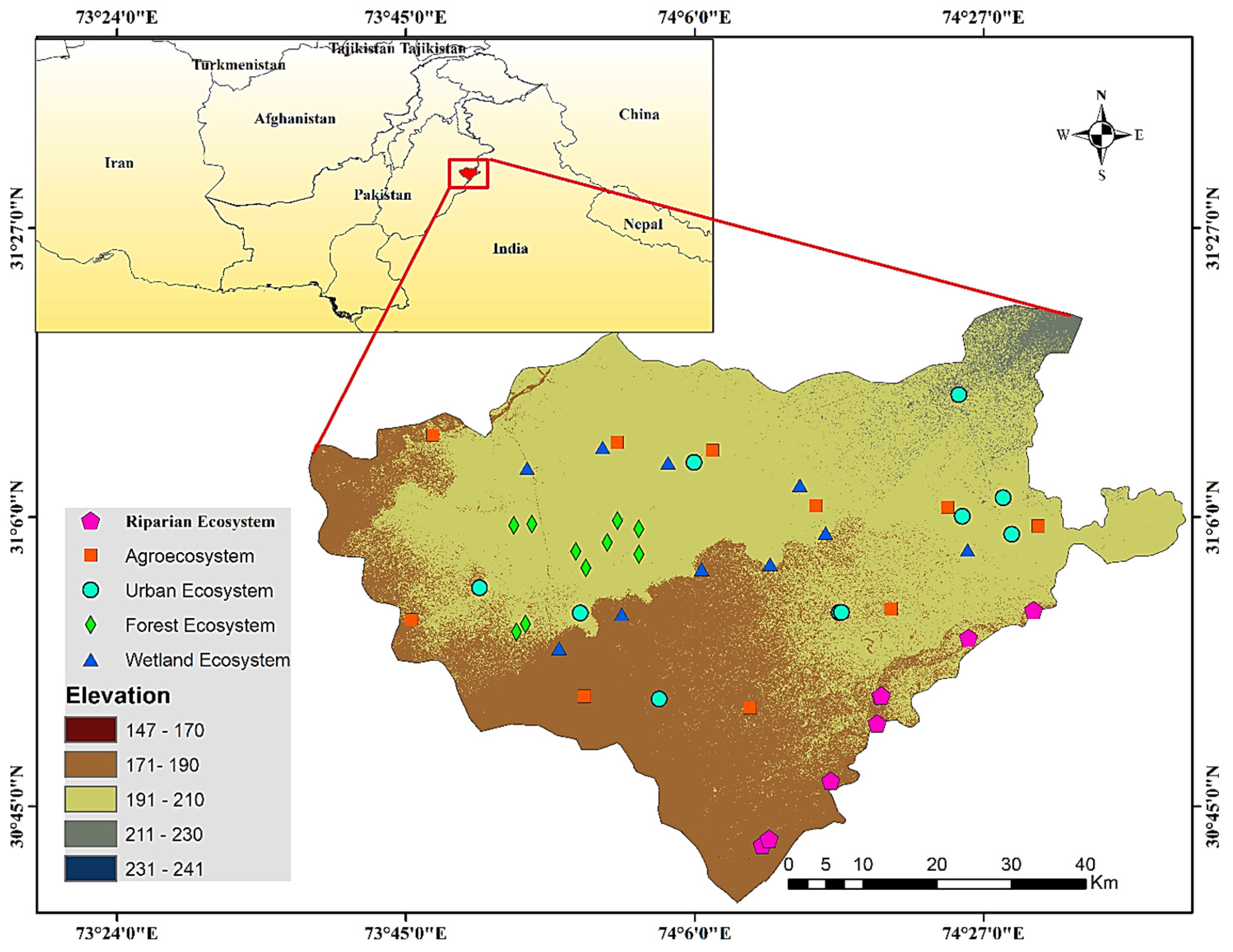
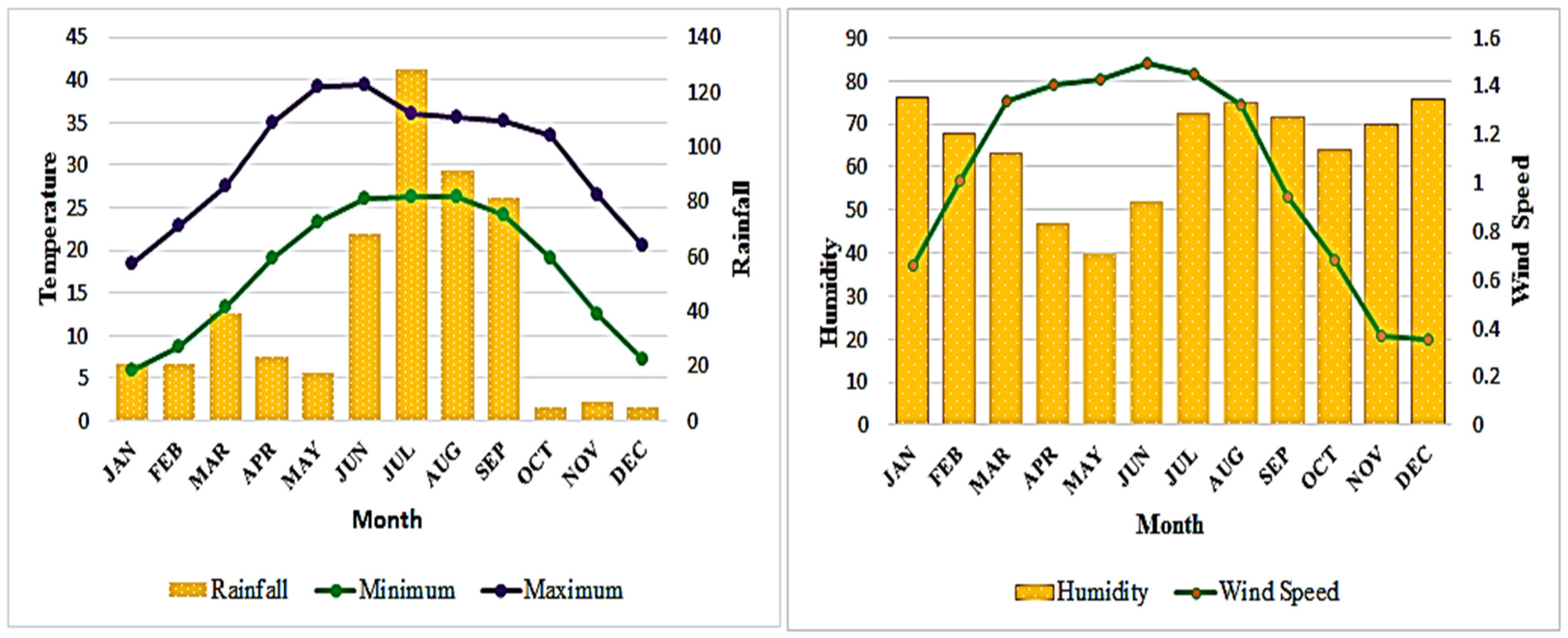
2.1. Study Area
2.2. Field Sampling
2.3. Soil Sampling
2.4. Indicator Species Analysis
2.5. Data Analysis
3. Results
3.1. Diversity and Ecological Traits
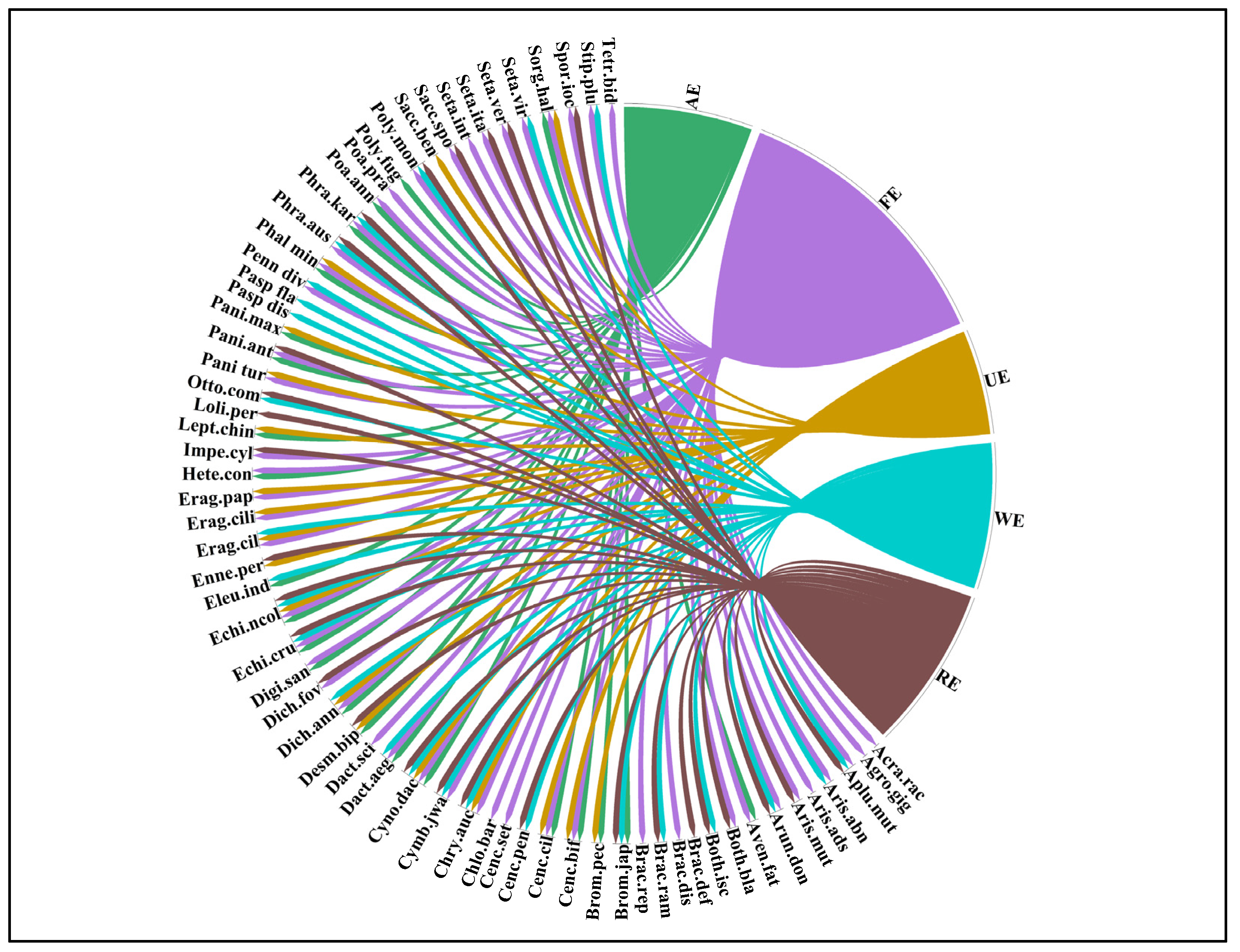
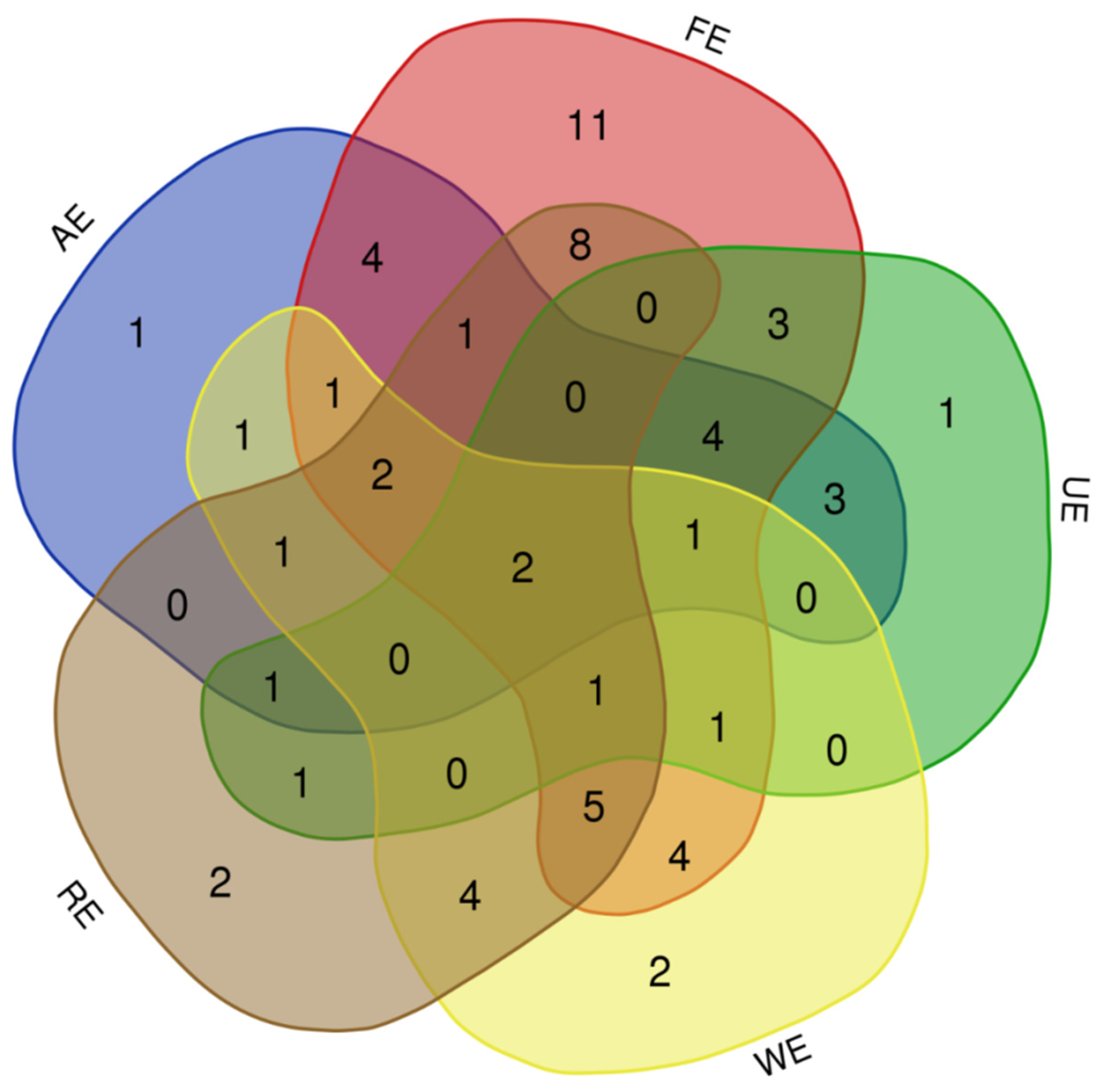
3.2. Species Similarities in Different Ecosystem
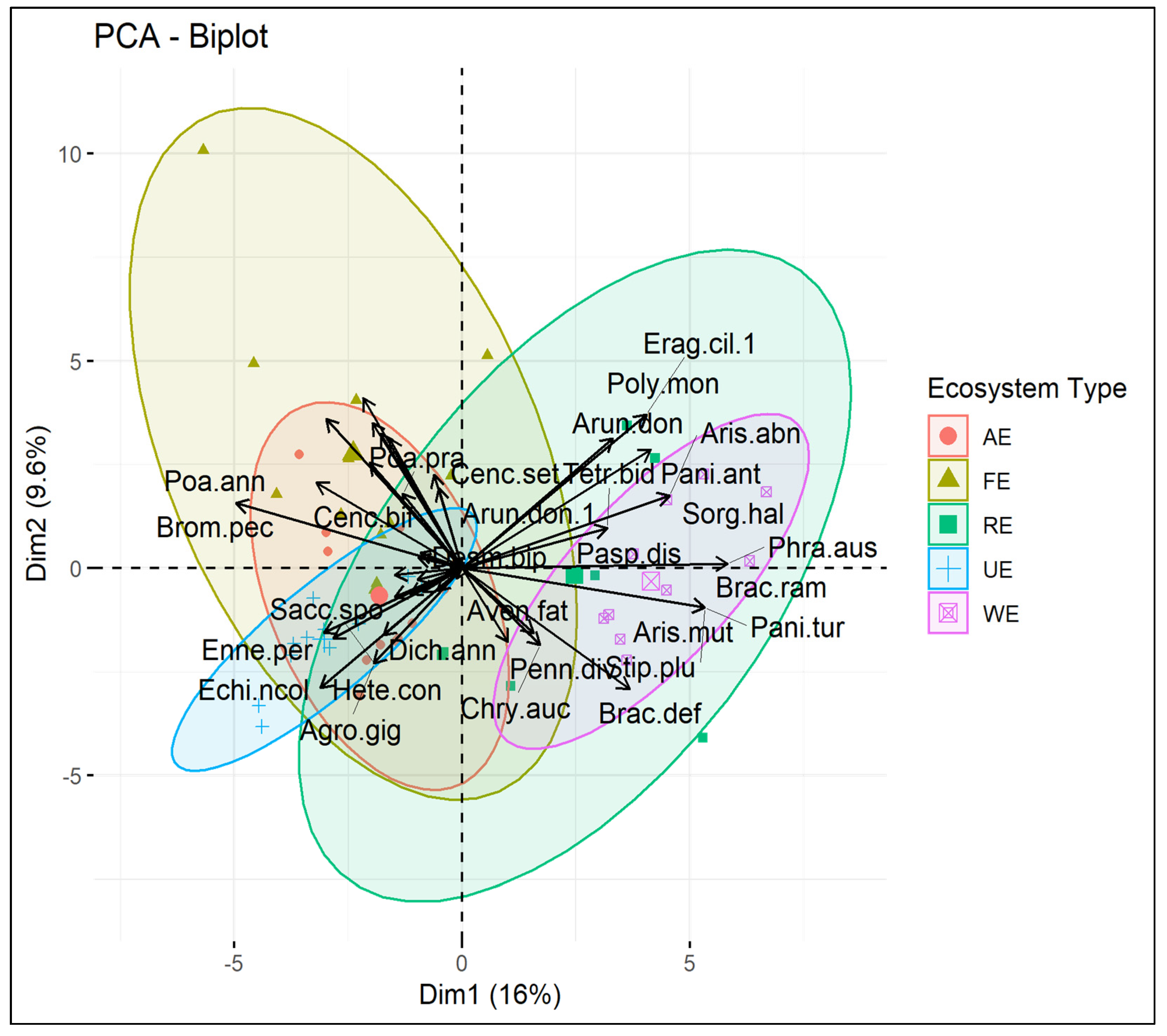
3.3. Principal Component Analysis
3.4. Canonical Correspondence Analysis
3.5. Indicator Species Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Xiong, Q.; Luo, P.; Zhang, Y.; Gu, X.; Lin, B. Direct and indirect effects of environmental factors, spatial constraints, and functional traits on shaping the plant diversity of montane forests. Ecol. Evol. 2020, 10, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.M.; Malik, A.H.; Khuroo, A.A.; Rashid, I. Floristic composition and biological spectrum of keran-a remote valley of Northwestern Himalaya. Acta Ecol. Sin. 2019, 39, 372–379. [Google Scholar] [CrossRef]
- Báez, S.; Fadrique, B.; Feeley, K.; Homeier, J. Changes in tree functional composition across topographic gradients and through time in a tropical montane forest. PLoS ONE 2022, 17, e0263508. [Google Scholar] [CrossRef] [PubMed]
- Hofhansl, F.; Chacón-Madrigal, E.; Fuchslueger, L.; Jenking, D.; Morera-Beita, A.; Plutzar, C.; Silla, F.; Andersen, K.M.; Buchs, D.M.; Dullinger, S. Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.; Oleksyn, J.; Westoby, M.; Walters, M. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Haq, S.M.; Tariq, A.; Li, Q.; Yaqoob, U.; Majeed, M.; Hassan, M.; Fatima, S.; Kumar, M.; Bussmann, R.W.; Moazzam, M.F.U.; et al. Influence of Edaphic Properties in Determining Forest Community Patterns of the Zabarwan Mountain Range in the Kashmir Himalayas. Forests 2022, 13, 1214. [Google Scholar] [CrossRef]
- Rahman, I.U.; Hart, R.E.; Ijaz, F.; Afzal, A.; Iqbal, Z.; Calixto, E.S.; Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Al Arjani, A.B.F.; et al. Environmental variables drive plant species composition and distribution in the moist temperate forests of Northwestern Himalaya, Pakistan. PLoS ONE 2022, 17, e0260687. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Spasojevic, M.J. The edaphic control of plant diversity. Glob. Ecol. Biogeogr. 2020, 29, 1634–1650. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity influences spatial patterns and demographics in forest stands. J. Ecol. 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Concostrina-Zubiri, L.; Prieto, M.; Hurtado, P.; Escudero, A.; Martínez, I. Functional diversity regulates the effects of habitat degradation on biocrust phylogenetic and taxonomic diversities. Ecol. Appl. 2022, 22, e2599. [Google Scholar] [CrossRef]
- Vanneste, T.; Govaert, S.; De Kesel, W.; Van Den Berge, S.; Vangansbeke, P.; Meeussen, C.; Brunet, J.; Cousins, S.A.O.; Decocq, G.; Diekmann, M.; et al. Plant diversity in hedgerows and road verges across Europe. J. Appl. Ecol. 2020, 57, 1244–1257. [Google Scholar] [CrossRef]
- Eiserhardt, W.L.; Svenning, J.-C.; Kissling, W.D.; Balslev, H. Geographical ecology of the palms (Arecaceae): Determinants of diversity and distributions across spatial scales. Ann. Bot. 2011, 108, 1391–1416. [Google Scholar] [CrossRef] [PubMed]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez-Olabarria, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Bouchenak-Khelladi, Y.; Verboom, G.A.; Savolainen, V.; Hodkinson, T.R. Biogeography of the grasses (Poaceae): A phylogenetic approach to reveal evolutionary history in geographical space and geological time. Bot. J. Linn. Soc. 2010, 162, 543–557. [Google Scholar] [CrossRef]
- Abbas, Z.; Khan, S.M.; Alam, J.; Abideen, Z.; Ullah, Z. Plant communities and anthropo-natural threats in the Shigar Valley, (Central Karakorum) Baltistan-Pakistan. Pak. J. Bot. 2020, 52, 987–994. [Google Scholar] [CrossRef]
- Slewinski, T.L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A.S.; Distel, R.A.; Didone, N.G. Decomposition and nutrient dynamic of leaf litter and roots from palatable and unpalatable grasses in a semi-arid grassland. Appl. Soil Ecol. 2001, 18, 31–37. [Google Scholar] [CrossRef]
- Sarwar, M.; Khan, M.A.; Iqbal, Z. Status paper feed resources for livestock in Pakistan. Int. J. Agric. Biol. 2002, 4, 186–192. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. J. Sci. 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Porqueddu, C.; Ates, S.; Louhaichi, M.; Kyriazopoulos, A.; Moreno, G.; Del Pozo, A.; Ovalle, C.; Ewing, M.; Nichols, P. Grasslands in ‘old world’and ‘new world’mediterranean-climate zones: Past trends, current status and future research priorities. Grass Forage Sci. 2016, 71, 1–35. [Google Scholar] [CrossRef]
- Moro, M.F.; Silva, I.A.; Araújo FSd Nic Lughadha, E.; Meagher, T.R.; Martins, F.R. The role of edaphic environment and climate in structuring phylogenetic pattern in seasonally dry tropical plant communities. PLoS ONE 2015, 10, e0119166. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.O.; Boldrini, I.I.; Cadenazzi, M.; Pillar, V.D.; Overbeck, G.E. Grassland vegetation sampling-a practical guide for sampling and data analysis. Acta Bot. Croat. 2019, 33, 786–795. [Google Scholar] [CrossRef]
- Haq, S.M.; Singh, B.; Bashir, F.; Farooq, A.J.; Singh, B.; Calixto, E.S. Exploring and understanding the floristic richness, life-form, leaf-size spectra and phenology of plants in protected forests: A case study of Dachigam National Park in Himalaya, Asia. Acta Ecol. Sin. 2021, 41, 479–490. [Google Scholar] [CrossRef]
- Khan, W.; Khan, S.M.; Ahmad, H.; Alqarawi, A.A.; Shah, G.M.; Hussain, M.; Abd_Allah, E. Life forms, leaf size spectra, regeneration capacity and diversity of plant species grown in the Thandiani Forests, district Abbottabad, Khyber Pakhtunkhwa, Pakistan. Saudi J. Biol. Sci. 2018, 25, 94–100. [Google Scholar] [CrossRef]
- Afzal, R.; Ahmad, H.; Saqib, Z.; Marwat, K.B.; Khan, J. Spatial analysis of vascular flora of Ayubia National Park, Kpk, Pakistan: A classical example of moist temperate himalaya. Pak. J. Bot. 2018, 50, 1499–1508. [Google Scholar]
- Khan, A.M.; Qureshi, R.; Saqib, Z. Multivariate analyses of the vegetation of the Western Himalayan Forests of Muzaffarabad District, Azad Jammu and Kashmir, Pakistan. Ecol. Indic. 2019, 104, 723–736. [Google Scholar] [CrossRef]
- Iqbal, M.; Khan, S.M.; Ahmad, Z.; Hussain, M.; Shah, S.N.; Kamran, S.; Manan, F.; Haq, Z.; Ullah, S. Vegetation classification of the Margalla Foothills, Islamabad under the influence of edaphic factors and anthropogenic activities using modern ecological tools. Pak. J. Bot. 2021, 53, 1831–1843. [Google Scholar] [CrossRef]
- Arshad, F.; Waheed, M.; Iqbal, M.; Fatima, K.; Fatima, K. Ethnobotanical assessment of woody flora of district Kasur (Punjab), Pakistan. Ethnobot. Res. Appl. 2020, 20, 1–13. [Google Scholar]
- Rahman, A.U.; Khan, S.M.; Saqib, Z.; Ullah, Z.; Ahmad, Z.; Ekercin, S.; Mumtaz, A.S.; Ahmad, H. Diversity and abundance of climbers in relation to their hosts and elevation in the monsoon forests of Murree in the Himalayas. Pak. J. Bot. 2020, 52, 601–612. [Google Scholar] [CrossRef]
- Arshad, F.; Waheed, M.; Harun, N.; Fatima, K.; Khan, B.A.; Fatima, K.; Abbas, Z.; Jabeen, S.; Majeed, M. Indigenous farmer’s perception about fodder and foraging species of Semi-arid lowlands of Pakistan: A case study of District Kasur, Pakistan. Taiwania 2022, 67, 510–523. [Google Scholar]
- Adnan, M.; Hölscher, D. Medicinal plants in old-growth, degraded and re-growth forests of NW Pakistan. For. Ecol. Manag. 2011, 261, 2105–2114. [Google Scholar] [CrossRef]
- Rahman, I.U.; Khan, N.; Ali, K. Classification and ordination of understory vegetation using multivariate techniques in the Pinus wallichiana forests of Swat Valley, northern Pakistan. Sci. Nat. 2017, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Bhatti, K.; Amjad, M. Impact of climatic variations on the flowering phenology of plant species in Jhelum district, Punjab, Pakistan. Appl. Ecol. Environ. Res 2021, 19, 3343–3376. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.M.; Ilyas, M.; Alqarawi, A.A.; Ahmad, Z.; Abd_Allah, E.F. Plant species and communities assessment in interaction with edaphic and topographic factors; an ecological study of the Mount Eelum District Swat, Pakistan. Saudi J. Biol. Sci. 2017, 24, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.T.; Hu, Y.Y.; Wolf, A.A.; Han, X.G. Species richness mediates within-species nutrient resorption: Implications for the biodiversity–productivity relationship. J. Ecol. 2019, 107, 2346–2352. [Google Scholar] [CrossRef]
- Rasheed, S.; Khan, S.M.; Ahmad, Z.; Mustafa, G.; Haq, Z.; Shah, H.; Ansari, L.; Jatt, T. Ecological assessment and indicator species analyses of the Cholistan Desert using multivariate statistical tools. Pak. J. Bot. 2022, 54, 683–694. [Google Scholar] [CrossRef]
- Abdullah, M.; Khan, R.A.; Yaqoob, S.; Ahmad, M. Community structure of browse vegetation in Cholistan rangelands of Pakistan. Pak. J. Agri. Sci. 2013, 50, 237–247. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extract and visualize the results of multivariate data analyses. R Package Version 1.0. 5. R package version. 2020; 2020. [Google Scholar]
- Morris, K.A.; Saetre, P.; Norton, U.; Stark, J.M. Plant community effects on soil moisture and nitrogen cycling in a semi-arid ecosystem. Biogeochemistry 2022, 159, 215–232. [Google Scholar] [CrossRef]
- Shaheen, H.; Qureshi, R.; Qaseem, M.F.; Bruschi, P. The fodder grass resources for ruminants: A indigenous treasure of local communities of Thal Desert, Punjab, Pakistan. PLoS ONE 2020, 15, e0224061. [Google Scholar] [CrossRef]
- Majeed, M.; Tariq, A.; Haq, S.M.; Waheed, M.; Anwar, M.M.; Li, Q.; Aslam, M.; Abbasi, S.; Mousa, B.; Jamil, A. A detailed ecological exploration of the distribution patterns of wild poaceae from the Jhelum District (Punjab), Pakistan. Sustainability 2022, 14, 3786. [Google Scholar] [CrossRef]
- Roy, A.; Malaviya, D.; Kaushal, P. Genetic improvement of dominant tropical indian range grasses. Range Manag. Agrofor. 2019, 40, 1–25. [Google Scholar]
- Oliveira, J.R. Alexander Grass Seed Physiology and Production: A Step Towards the Conversion of a Weed Into a Forage Plant. Ph.D. Thesis, Federal Technological University of Paraná, Pato Branco, Brazil, 2017. [Google Scholar]
- Haq, S.M.; Shah, A.A.; Yaqoob, U.; Hassan, M. Floristic quality assessment index of the dagwan stream in Dachigam National Park of Kashmir, Himalaya. Proc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 2021, 91, 657–664. [Google Scholar] [CrossRef]
- Rashid, I.; Haq, S.M.; Lembrechts, J.J.; Khuroo, A.A.; Pauchard, A.; Dukes, J.S. Railways redistribute plant species in mountain landscapes. J. Appl. Ecol. 2021, 58, 1967–1980. [Google Scholar] [CrossRef]
- Haq, S.M.; Malik, Z.A.; Rahman, I.U. Quantification and characterization of vegetation and functional trait diversity of the riparian zones in protected forest of Kashmir Himalaya, India. Nord. J. Bot. 2019, 37. [Google Scholar] [CrossRef]
- Zeb, A.; Iqbal, Z.; Khan, S.M.; Rahman, I.U.; Haq, F.; Afzal, A.; Qadir GIjaz, F. Species diversity, biological spectrum and phenological behaviour of vegetation of Biha Valley (Swat), Pakistan. Acta Ecol. Sin. 2020, 40, 190–196. [Google Scholar] [CrossRef]
- Ali, S.; Shah, S.Z.; Khan, M.S.; Khan, W.M.; Khan, Z.; Hassan, N.; Zeb, U. Floristic list, ecological features and biological spectrum of District Nowshera, Khyber Pakhtunkhwa, Pakistan. Acta Ecol. Sin. 2019, 39, 133–141. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Eller, C.B.; Barros, F.d.V.; Hirota, M.; Brum, M.; Bittencourt, P. Linking plant hydraulics and the fast–slow continuum to understand resilience to drought in tropical ecosystems. New Phytol. 2021, 230, 904–923. [Google Scholar] [CrossRef]
- Jardine, E.C.; Thomas, G.H.; Forrestel, E.J.; Lehmann, C.E.; Osborne, C.P. The global distribution of grass functional traits within grassy biomes. J. Biogeogr. 2020, 47, 553–565. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, L.; Zhang, Z.; Zhang, J.; Cheng, J.; Wei, G.; Lin, Y. Soil microbial diversity during 30 years of grassland restoration on the loess plateau, china: Tight linkages with plant diversity. Land Degrad. Dev. 2019, 30, 1172–1182. [Google Scholar] [CrossRef]
- Provoost, S.; Ampe, C.; Bonte, D.; Cosyns, E.; Hoffmann, M. Ecology, management and monitoring of dune grasslands in Flanders, Belgium. In Proceedings of the Littoral 2002 The Changing Coast, Porto, Portugal, 22–26 September 2002; pp. 11–22. [Google Scholar]
- Segura, C.; Jiménez, M.N.; Fernández-Ondoño, E.; Navarro, F.B. Effects of Afforestation on Plant Diversity and Soil Quality in Semiarid SE Spain. Forests 2021, 12, 1730. [Google Scholar] [CrossRef]
- Bergamin, R.S.; Ascensão, F.; Capinha, C.; Bastazini, V.A.G.; Andrade, B.O.; Boldrini, I.I.; Lezama, F.; Altesor, A.; Perelman, S.; Overbeck, G.E. Native and alien grassland diversity respond differently to environmental and anthropogenic drivers across spatial scales. J. Veg. Sci. 2022, 33, e13133. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Hameed, M.; Ahmad, F.; Sadia, B. Edaphic factors as major determinants of plant distribution of temperate himalayan grasses. Pak. J. Bot. 2016, 48, 567–573. [Google Scholar]
- Filibeck, G.; Sperandii, M.G.; Bazzichetto, M.; Mancini, L.D.; Rossini, F.; Cancellieri, L. Exploring the drivers of vascular plant richness at very fine spatial scale in sub-mediterranean limestone grasszlands (central Apennines, Italy). Biodivers. Conserv. 2019, 28, 2701–2725. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, X.; Awada, T.; Medima-Roldan, E.; Feng, K.; Yue, P.; Lian, J.; Zhao, S.; Cheng, H. Changes of soil bacterial and fungal community structure along a natural aridity gradient in desert grassland ecosystems, inner Mongolia. Catena 2021, 205, 05470. [Google Scholar] [CrossRef]
- Wangchuk, K. Lowland pasture in himalayan highland: Edaphic properties and species composition. J. Mt. Sci. 2016, 13, 455–464. [Google Scholar] [CrossRef]
- Starke, A.; O’Connor, T.; Everson, C.S. Topo-edaphic environment and forest plantation disturbance explain patterns of grassland species richness, composition and structure in an agro-ecological landscape, Maputaland, South Africa. Afr. J. Range Forage Sci. 2021, 38, 206–219. [Google Scholar] [CrossRef]
- Gupta, V.V.; Zhang, B.; Penton, C.R.; Yu, J.; Tiedje, J.M. Diazotroph diversity and nitrogen fixation in summer active perennial grasses in a mediterranean region agricultural soil. Front. Mol. Biosci. 2019, 6, 115. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, Y.; Xiao, N.; Huang, J.; Xiao, H.; Chen, H. Effects of three typical grass cultivation patterns on the community structure of soil mites in rocky desertification control area, Guizhou, China. Environ. Res. Commun. 2022, 4, 045008. [Google Scholar] [CrossRef]
- Soulodre, E.M.; Dhar, A.; Naeth, M.A. Plant community development trends on mixed grass prairie well sites 5 years after reclamation. Ecol. Eng. 2022, 179, 106635. [Google Scholar] [CrossRef]
- Arshad, F.; Waheed, M.; Fatima, K.; Harun, N.; Iqbal, M.; Fatima, K.; Umbreen, S. Predicting the Suitable Current and Future Potential Distribution of the Native Endangered Tree Tecomella undulata (Sm.) Seem. in Pakistan. Sustainability 2022, 14, 7215. [Google Scholar] [CrossRef]
- Wang, Y.; Lehnert, L.W.; Holzapfel, M.; Schultz, R.; Heberling, G.; Görzen, E.; Meyer, H.; Seeber, E.; Pinkert, S.; Ritz, M.; et al. Multiple indicators yield diverging results on grazing degradation and climate controls across Tibetan pastures. Ecol. Indic. 2018, 93, 1199–1208. [Google Scholar] [CrossRef]
- Nafeesa, Z.; Haq, S.M.; Bashir, F.; Gaus, G.; Mazher, M.; Anjum, M.; Rasool, A.; Rashid, N. Observations on the floristic, life-form, leaf-size spectra and habitat diversity of vegetation in the Bhimber Hills of Kashmir himalayas. Acta Ecol. Sin. 2021, 41, 228–234. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.; Abd_Allah, E.; Ul Haq, Z.; Alshahrani, T.; Alqarawi, A.; Ur Rahman, I.; Iqbal, M.; Abdullah–Ahmad, H. Assessment of plant communities and identification of indicator species of an ecotonal forest zone at Durand Line, District Kurram, Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6375–6396. [Google Scholar] [CrossRef]


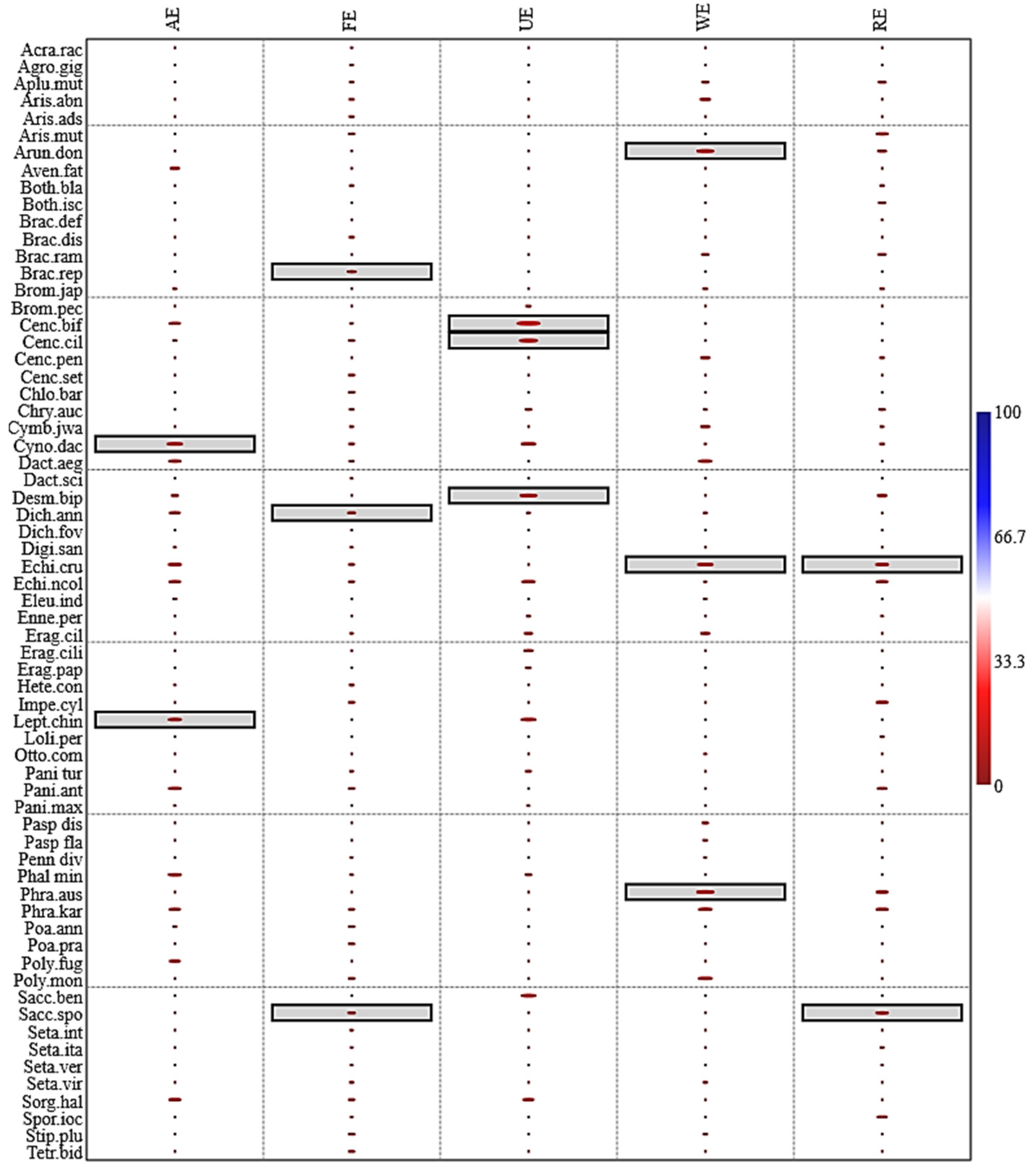
| Species | Nativity | Leaf Spectra | Life Form | Indicator Species Analysis with p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| AE | FE | UE | WE | RE | ||||
| Acrachne racemosa (Heyne ex Roem. and Schult.) Ohwi | Native | Nap | Th | 1 | 0.6475 | 1 | 1 | 1 |
| Agrostis gigantea Roth | Native | Mip | Hc | 1 | 0.4726 | 1 | 1 | 1 |
| Apluda mutica L. | Native | Nap | Hc | 1 | 0.3977 | 1 | 0.1843 | 0.2014 |
| Aristida abnormis Chiov. | Native | Mip | Th | 1 | 0.3555 | 1 | 0.1096 | 1 |
| Aristida adscensionis L. | Native | Mip | Th | 1 | 0.3565 | 1 | 1 | 1 |
| Aristida mutabilis Trin. and Rupr. | Native | Mip | Th | 1 | 0.1417 | 1 | 1 | 0.062 |
| Arundo donax L. | Native | Map | Hc | 1 | 0.7051 | 1 | 0.0315 | 0.1613 |
| Avena fatua L. | Native | Nap | Th | 0.1953 | 0.6724 | 1 | 1 | 1 |
| Bothriochloa bladhii (Retz.) S.T. Blake | Native | Mip | Th | 1 | 0.4167 | 1 | 1 | 0.277 |
| Bothriochloa ischaemum (L.) Keng | Native | Mip | Th | 1 | 1 | 1 | 0.3876 | 0.2147 |
| Brachiaria deflexa (Schumach.) C.E. Hubb. ex Robyns | Native | Mip | Th | 1 | 1 | 1 | 1 | 0.3774 |
| Brachiaria distachya (L.) Stapf | Native | Mip | Th | 1 | 0.306 | 1 | 1 | 1 |
| Brachiaria ramosa (L.) Stapf | Native | Mip | Th | 1 | 1 | 1 | 0.1648 | 0.1857 |
| Brachiaria reptans (L.) C.A. Gardner and C.E. Hubb. | Native | Mip | Th | 1 | 0.0182 | 1 | 1 | 1 |
| Bromus japonicus Thunb. | Native | Mip | Hc | 0.2381 | 1 | 1 | 0.2092 | 0.2913 |
| Bromus pectinatus Thunb. | Introduced | Mip | Hc | 0.3298 | 1 | 0.2228 | 1 | 1 |
| Cenchrus biflorus Roxb. | Native | Nap | Hc | 0.1406 | 0.5435 | 0.016 | 1 | 1 |
| Cenchrus ciliaris L. | Native | Nap | Th | 0.2296 | 0.2333 | 0.0299 | 1 | 1 |
| Cenchrus pennisetiformis Steud. | Native | Nap | Hc | 1 | 1 | 1 | 0.1246 | 0.2561 |
| Cenchrus setiger Vahl. | Native | Nap | Hc | 1 | 0.1372 | 1 | 1 | 1 |
| Chloris barbata Sw. | Introduced | Nap | Th | 1 | 0.0927 | 1 | 1 | 1 |
| Chrysopogon aucheri (Boiss.) Stapf | Native | Nap | Th | 1 | 0.3667 | 0.1813 | 0.2915 | 0.2289 |
| Cymbopogon jwarancusa (Jones) Schult. | Native | Nap | Hc | 1 | 0.4349 | 1 | 0.1545 | 0.3372 |
| Cynodon dactylon (L.) Pers. | Native | Lep | Th | 0.016 | 0.3072 | 0.0942 | 0.3685 | 0.2554 |
| Dactyloctenium aegyptium (L.) Willd. | Native | Nap | Th | 0.0928 | 0.3488 | 1 | 0.0963 | 1 |
| Dactyloctenium scindicum Boiss | Native | Nap | Th | 1 | 0.5851 | 1 | 1 | 1 |
| Desmostachya bipinnata (L.) Stapf | Native | Map | Hc | 0.2167 | 1 | 0.0466 | 1 | 0.1582 |
| Dichanthim fovelatum (Del.) Roberty | Introduced | Lep | Th | 0.1823 | 0.0311 | 0.2614 | 0.2805 | 1 |
| Dichanthium annulatum (Forssk.) Stapf | Native | Lep | Th | 1 | 0.7005 | 1 | 1 | 0.431 |
| Digitaria sanguinalis (L.) Scop. | Native | Mip | Th | 0.2837 | 0.5638 | 1 | 1 | 1 |
| Echinochloa colona (L.) Link | Native | Mip | Hc | 0.065 | 0.2772 | 1 | 0.0466 | 0.0175 |
| Echinochloa crus-galli (L.) P.Beauv. | Native | Mip | Hc | 0.1179 | 0.2664 | 0.1087 | 0.3391 | 0.1112 |
| Eleusine indica (L.) Gaertn. | Native | Mip | Th | 0.2688 | 1 | 1 | 0.302 | 1 |
| Enneapogon persicus Boiss. | Native | Mip | Th | 1 | 1 | 0.2426 | 1 | 0.359 |
| Eragrostis cilianensis (All.) Janch. | Native | Mip | Th | 1 | 0.5226 | 0.145 | 0.1581 | 1 |
| Eragrostis ciliaris (L.) R. Br. | Native | Mip | Th | 1 | 0.6554 | 0.1407 | 1 | 1 |
| Eragrostis papposa (Roem. and Schult.) Stued. | Native | Mip | Th | 1 | 0.7454 | 0.2233 | 1 | 1 |
| Heteropogon contortus L.) P. Beauv.ex Roem. and Schult. | Introduced | Mip | Th | 0.313 | 0.3917 | 1 | 1 | 1 |
| Imperata cylindrica (L.) Raeusch. | Introduced | Mip | Th | 1 | 0.1666 | 1 | 1 | 0.0858 |
| Leptochloa chinensis (L.) Nees | Native | Map | Hc | 0.0322 | 1 | 0.067 | 1 | 1 |
| Lolium persicum Boiss. and Hohen. ex Boiss | Introduced | Mip | Hc | 1 | 1 | 1 | 1 | 0.2988 |
| Ottochloa compressa (Forssk.) Hilu | Native | Map | Hc | 1 | 1 | 1 | 0.3335 | 0.4395 |
| Panicum antidotale Retz. | Native | Map | Hc | 1 | 0.4553 | 0.1993 | 1 | 1 |
| Panicum maximum Jacq. | Native | Map | Hc | 0.0773 | 0.1397 | 1 | 1 | 0.154 |
| Panicum turgidum Forssk. | Introduced | Mip | Hc | 0.3208 | 1 | 0.2853 | 1 | 1 |
| Paspalidium flavidum (Retz.) A. Camus | Introduced | Mip | Hc | 1 | 1 | 1 | 0.2021 | 1 |
| Paspalum distichum L. | Introduced | Mip | Ge | 1 | 1 | 1 | 0.2268 | 1 |
| Pennisetum divisum (Forssk. ex J.F. Gmel.) Henrard | Native | Mip | Th | 1 | 0.5644 | 1 | 0.3552 | 1 |
| Phalaris minor Retz. | Native | Map | Th | 0.0657 | 0.5328 | 0.1842 | 1 | 1 |
| Phragmites australis (Cav.) Trin. ex Steud | Introduced | Map | Hc | 1 | 0.7417 | 1 | 0.0145 | 0.09 |
| Phragmites karka (Retz.) Trin. ex Steud. | Introduced | Map | Ge | 0.157 | 0.2518 | 1 | 0.0977 | 0.0641 |
| Poa annua L. | Native | Mip | Th | 0.2644 | 0.1765 | 1 | 1 | 1 |
| Poa pratensis L. | Native | Mip | Th | 1 | 0.2196 | 1 | 1 | 1 |
| Polypogon fugax Nees ex Steud. | Native | Map | Th | 0.1853 | 1 | 1 | 1 | 1 |
| Polypogon monspeliensis (L.) Desf. | Introduced | Map | Th | 1 | 0.0602 | 1 | 0.0578 | 0.4273 |
| Saccharum bengalense Retz. | Native | Map | Hc | 1 | 1 | 0.0764 | 1 | 1 |
| Saccharum spontaneum L. | Native | Map | Hc | 1 | 0.0472 | 1 | 1 | 0.0294 |
| Setaria intermedia Roem. and Schult. | Native | Mip | Th | 1 | 0.4935 | 1 | 1 | 1 |
| Setaria italica (L.) P. Beauv | Native | Mip | Th | 1 | 0.6285 | 1 | 1 | 0.3222 |
| Setaria verticillata (L.) P. Beauv. | Native | Mip | Th | 1 | 0.6305 | 1 | 1 | 0.3675 |
| Setaria viridis (L.) P. Beauv. | Native | Mip | Th | 1 | 0.4228 | 1 | 0.2572 | 1 |
| Sorghum halepense (L.) Pers. | Native | Mip | Ge | 0.1063 | 0.1995 | 0.1157 | 1 | 1 |
| Sporobolus ioclados (Trin.) Nees | Native | Mip | Hc | 1 | 0.6308 | 1 | 1 | 0.1196 |
| Stipagrostis plumosa Munro ex T. Anderson | Native | Nap | Hc | 1 | 0.0785 | 1 | 0.2634 | 1 |
| Tetrapogon bidentatus Pilg. | Introduced | Map | Hc | 1 | 0.2076 | 1 | 1 | 1 |
| Axes | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Eigen values | 0.313 | 0.206 | 0.155 | 0.103 |
| Species-environment associations | 0.691 | 0.68 | 0.688 | 0.569 |
| Accumulative percentage variance of wild grass species data | 4.7 | 7.8 | 10.1 | 11.7 |
| Accumulative percentage variance of species–environment relation | 30.6 | 50.7 | 65.9 | 76 |
| Total inertia | 6.638 | |||
| Sum of all eigen values | 6.638 | |||
| Sum of all canonical eigenvalues | 1.021 | |||
| Monte Carlo Test | ||||
| Test of significance of first canonical axis: eigenvalue | 0.586 | |||
| F-ratio | 2.034 | |||
| p-value | 0.0210 | |||
| Test of significance of total canonical axes; Trace | 1.021 | |||
| F-ratio | 1.323 | |||
| p-value | 0.0350 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waheed, M.; Haq, S.M.; Arshad, F.; Bussmann, R.W.; Iqbal, M.; Bukhari, N.A.; Hatamleh, A.A. Grasses in Semi-Arid Lowlands—Community Composition and Spatial Dynamics with Special Regard to the Influence of Edaphic Factors. Sustainability 2022, 14, 14964. https://doi.org/10.3390/su142214964
Waheed M, Haq SM, Arshad F, Bussmann RW, Iqbal M, Bukhari NA, Hatamleh AA. Grasses in Semi-Arid Lowlands—Community Composition and Spatial Dynamics with Special Regard to the Influence of Edaphic Factors. Sustainability. 2022; 14(22):14964. https://doi.org/10.3390/su142214964
Chicago/Turabian StyleWaheed, Muhammad, Shiekh Marifatul Haq, Fahim Arshad, Rainer W. Bussmann, Muhammad Iqbal, Najat A. Bukhari, and Ashraf Atef Hatamleh. 2022. "Grasses in Semi-Arid Lowlands—Community Composition and Spatial Dynamics with Special Regard to the Influence of Edaphic Factors" Sustainability 14, no. 22: 14964. https://doi.org/10.3390/su142214964
APA StyleWaheed, M., Haq, S. M., Arshad, F., Bussmann, R. W., Iqbal, M., Bukhari, N. A., & Hatamleh, A. A. (2022). Grasses in Semi-Arid Lowlands—Community Composition and Spatial Dynamics with Special Regard to the Influence of Edaphic Factors. Sustainability, 14(22), 14964. https://doi.org/10.3390/su142214964








