A Review on the Effect of Metakaolin on the Chloride Binding of Concrete, Mortar, and Paste Specimens
Abstract
:1. Introduction
2. Physical Chloride Binding of Specimens Containing MK
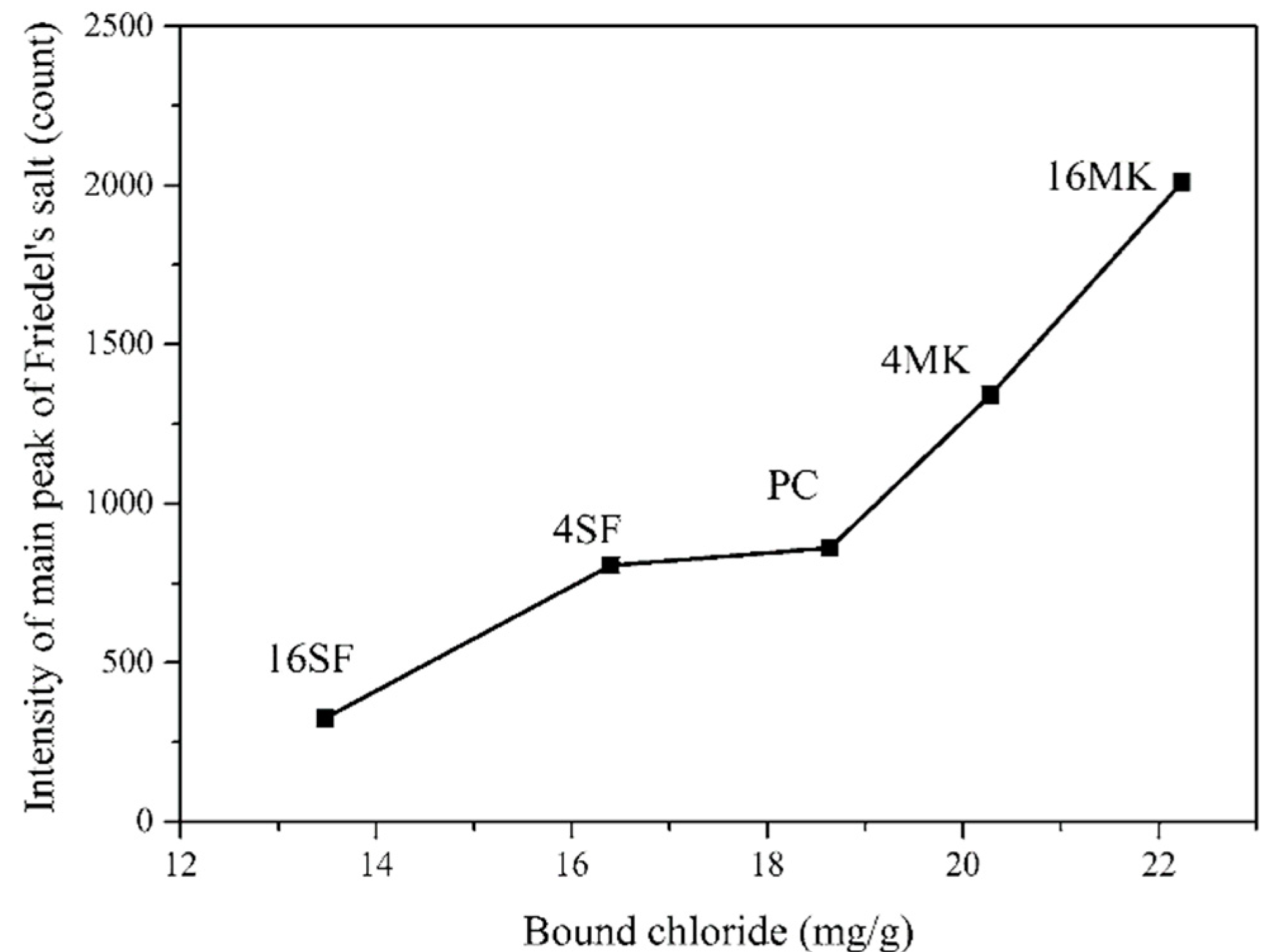
3. Chemical Chloride Binding of Specimens Containing MK
The Effect of MK on Hydrated Cement Products before and after Exposure to NaCl
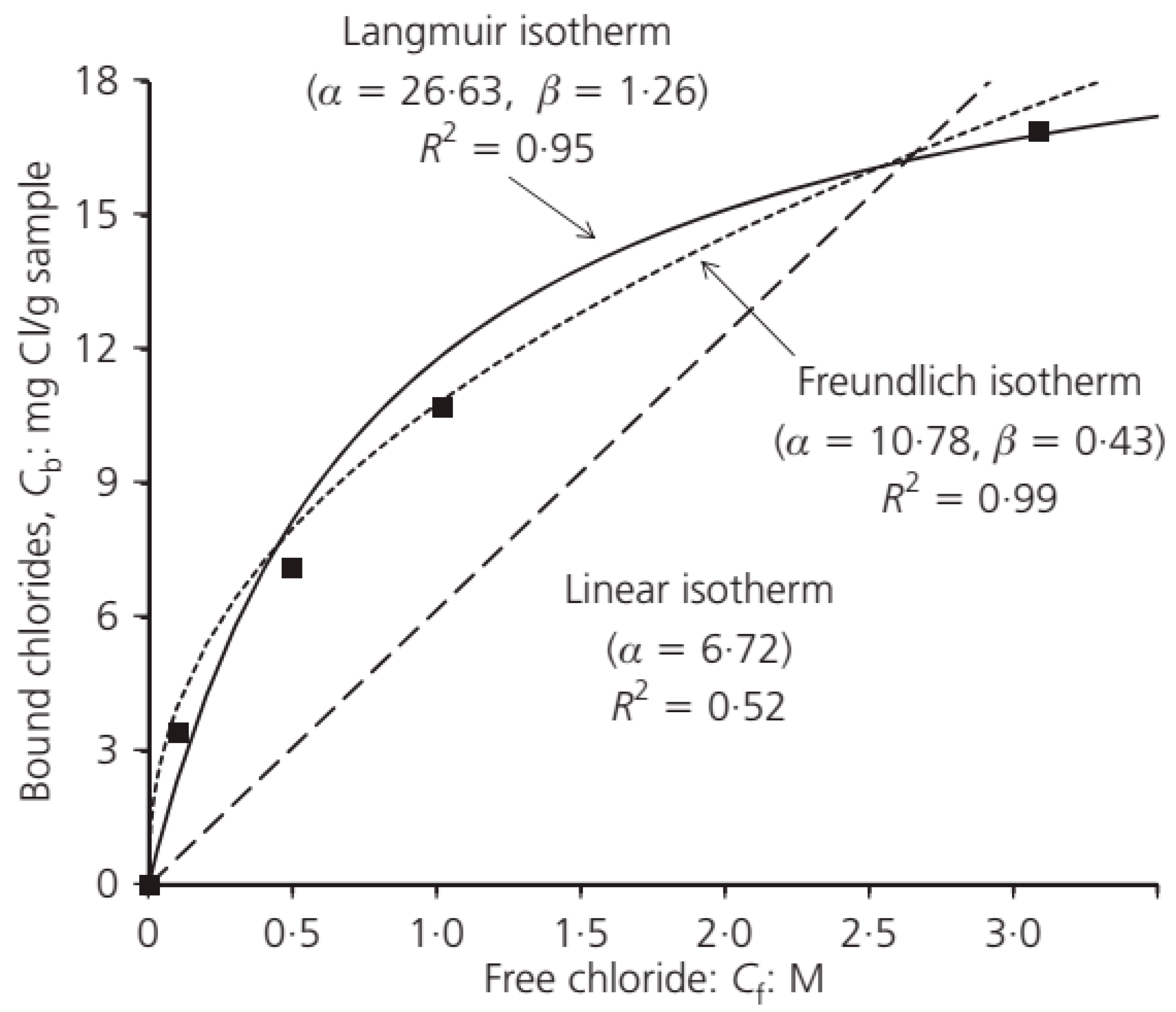
4. Binding Isotherms
5. Other Effective Parameters for Chloride Binding
5.1. Effect of Hydrotalcite
5.2. Effect of Gypsum and Sulfate Ion
5.3. Effect of Carbonation
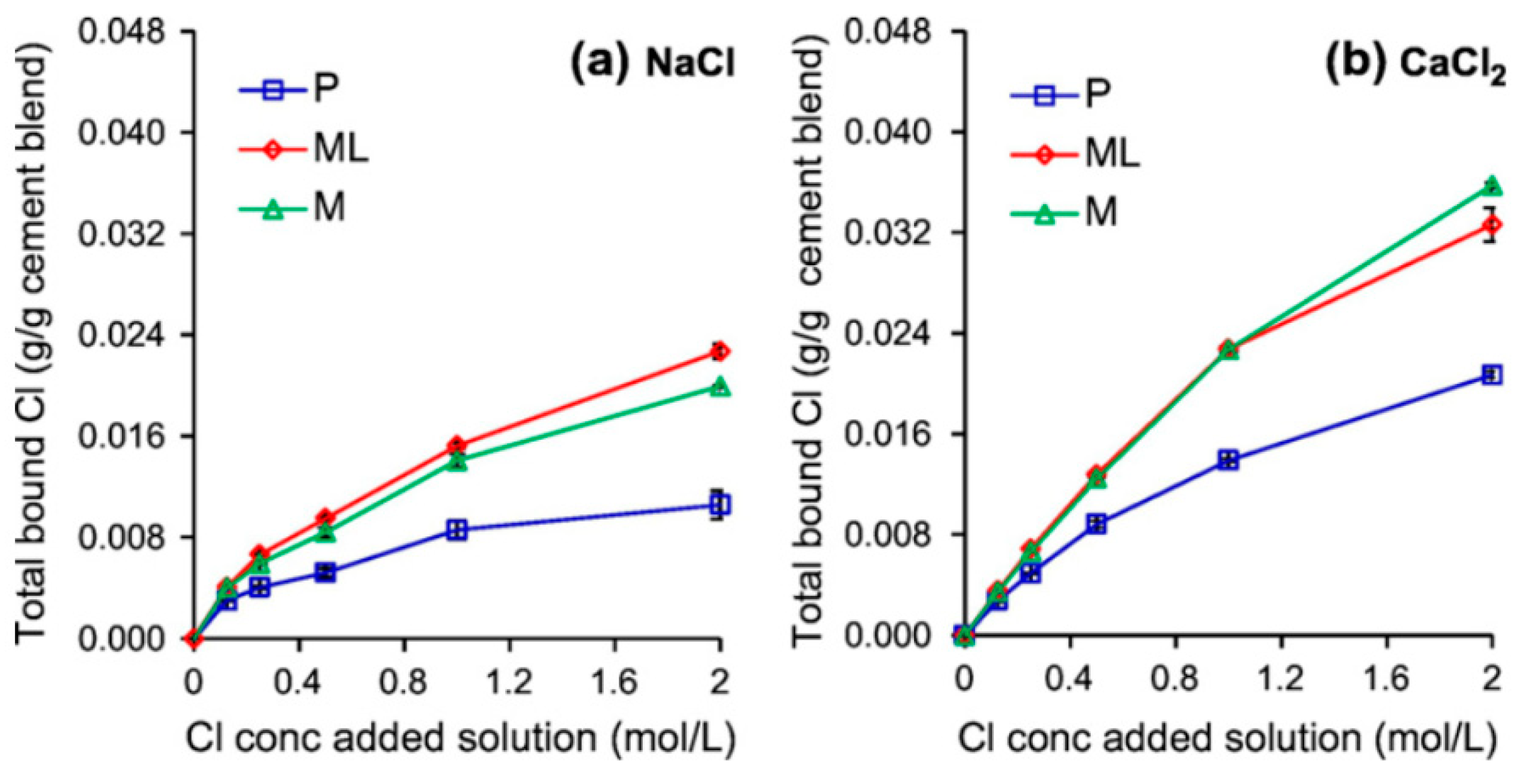
5.4. Chloride Concentration
5.5. Compound Composition of Portland Cement
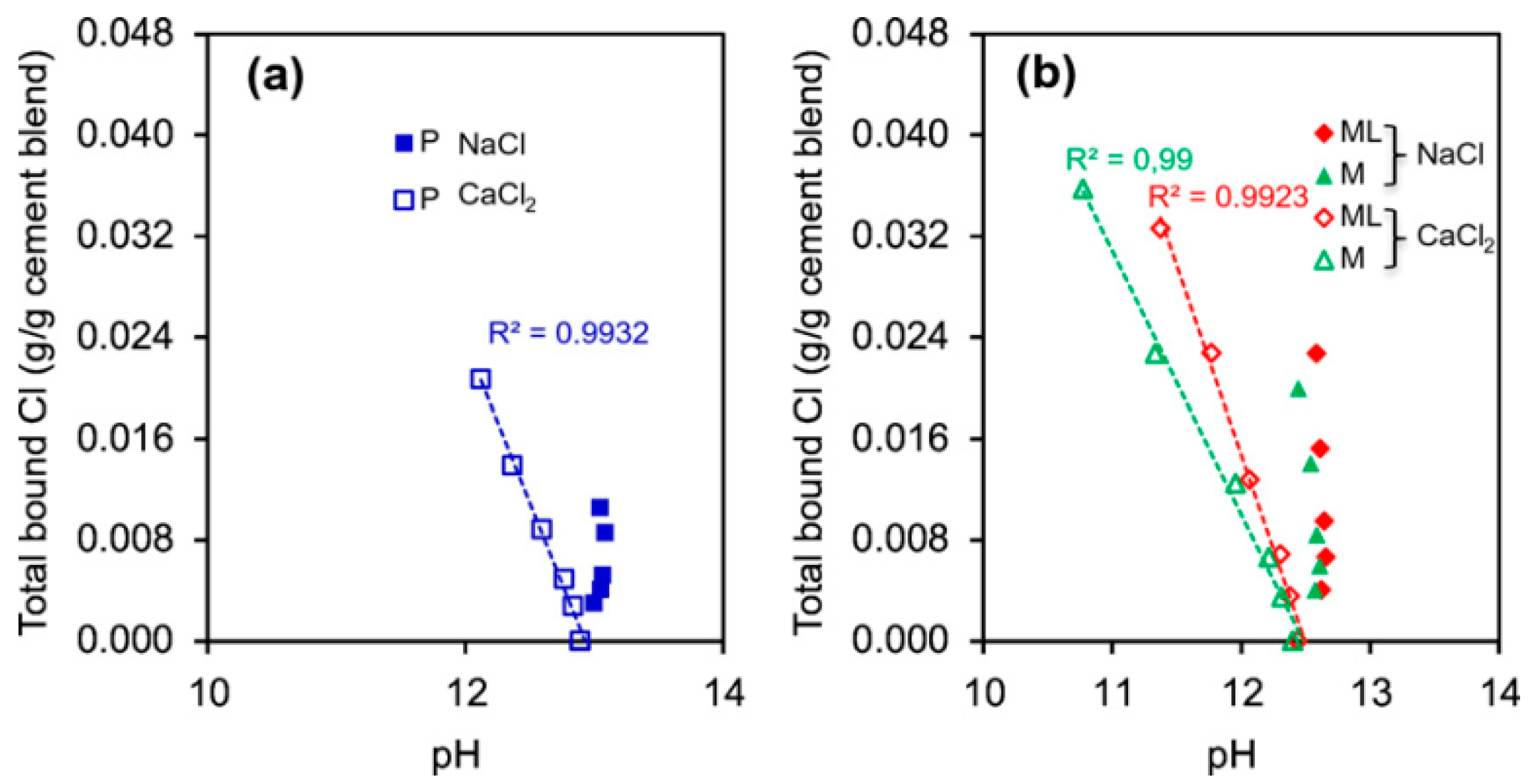
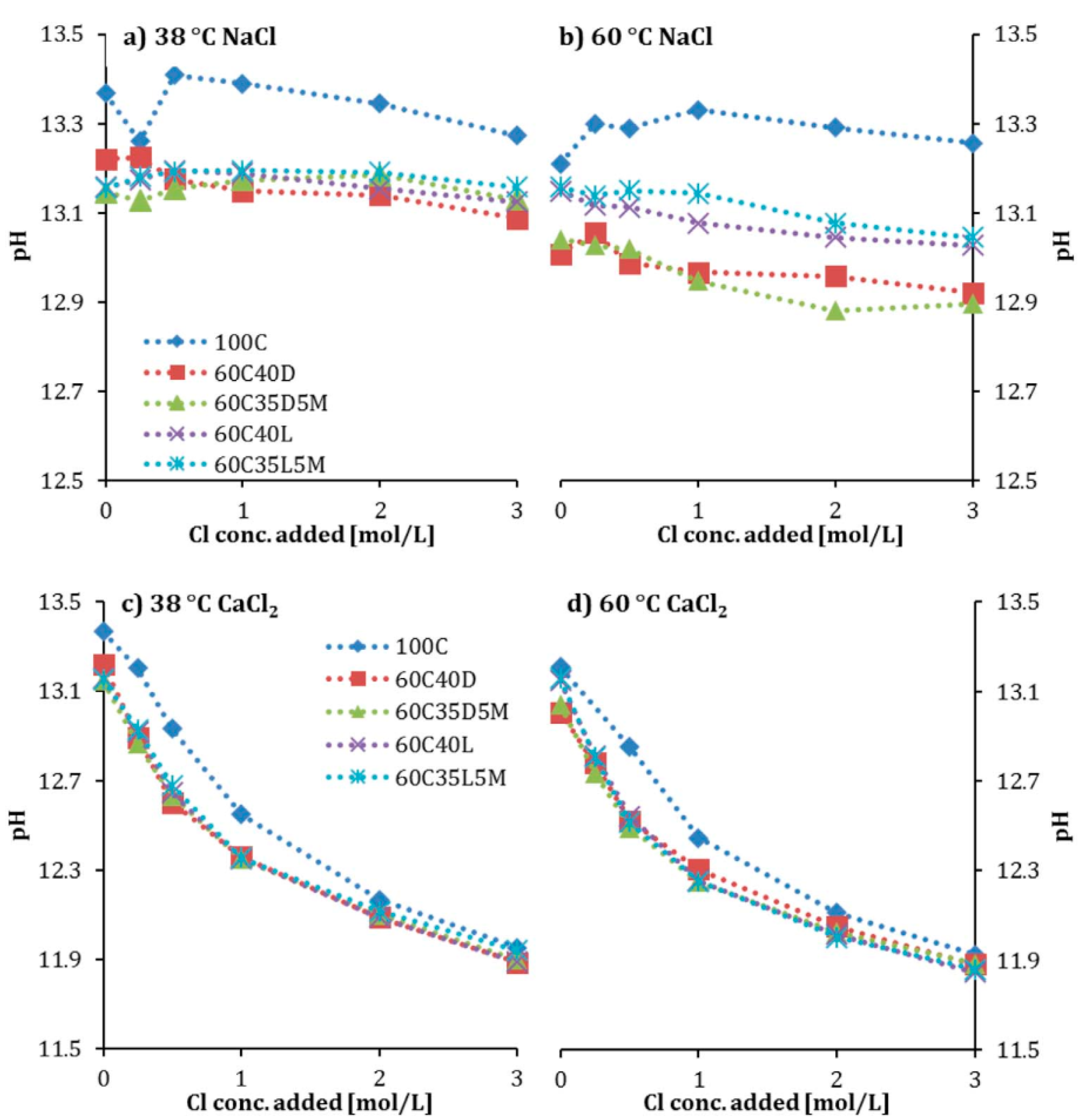
5.6. Hydroxyl Ion (OH−) Concentration and pH
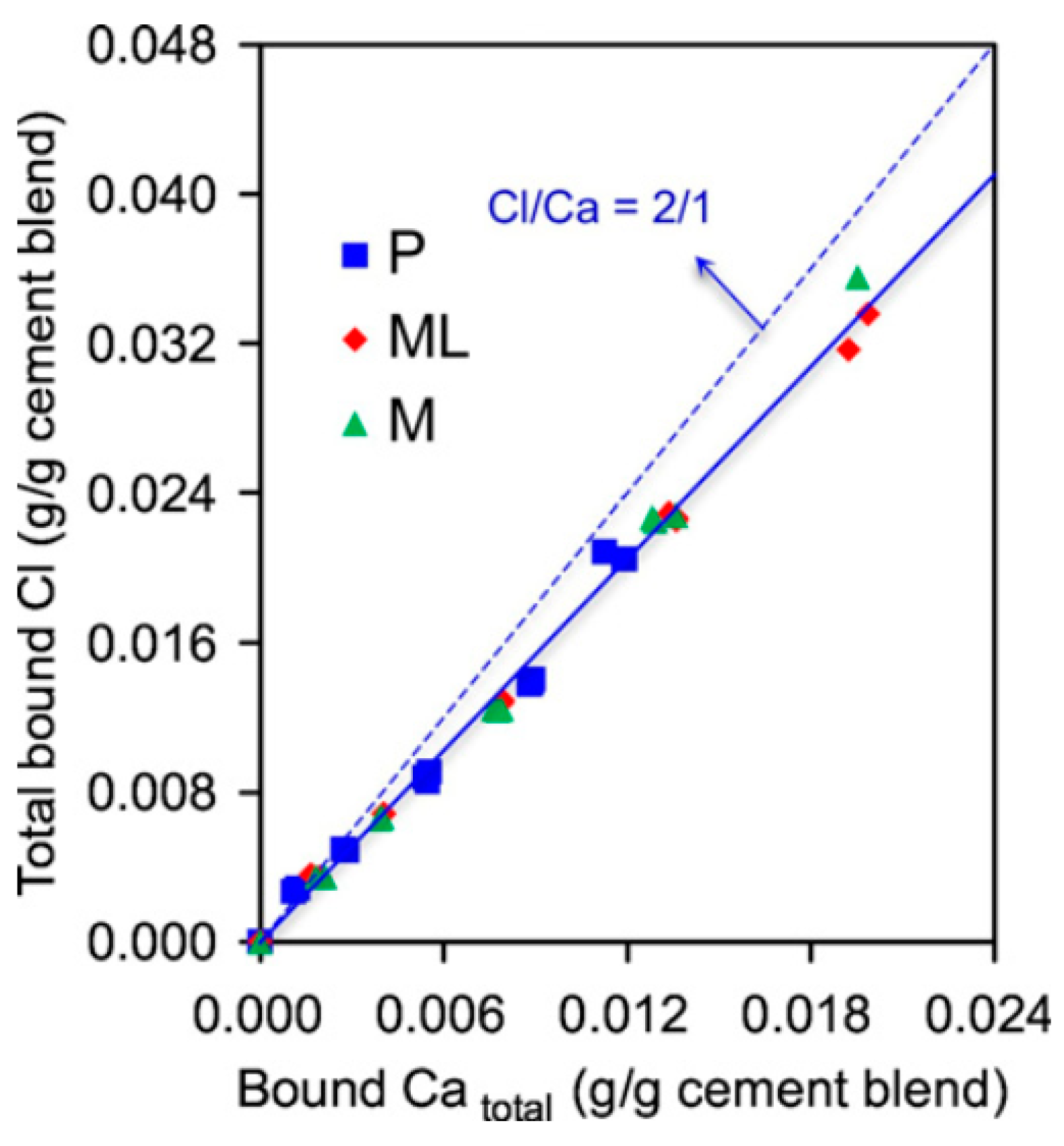
5.7. Cation
5.8. Temperature
5.9. Time and Electrical Field of Accelerated Tests
6. Conclusions
- Chloride binding in specimens containing MK is mostly attributed to chemical chloride binding and Friedel’s salt formation, and the proportion of physical chloride binding is usually lower.
- MK has both negative and positive effects on physical chloride binding. Using MK reduces physical chloride binding by reducing the Ca/Si ratio of CSH, increasing the chain length of CSH, and decreasing the specific surface area of CSH. However, MK increases the physical chloride-binding capacity by increasing the Al/Si ratio of CSH.
- In the case of the chemical composition of concrete, some studies support the notion that using MK reduces the ettringite and calcite and increases the strätlingite and monosulfoaluminate. However, monocarboaluminate content remains unchanged or increases a bit.
- In specimens containing MK, monosulfoaluminate (AFm) contributes majorly to Friedel’s salt and ettringite formation with NaCl exposure. However, monocarboaluminate makes limited contribution to Friedel’s salt formation and chloride binding, and its contribution depends on calcium availability. Therefore, the calcium consumption by the pozzolanic reaction of the MK may reduce the contribution of monocarboaluminate to chloride binding. Strätlingite may also contribute to chloride binding in high chloride concentrations. However, ettringite does not seem to make a significant and direct contribution to chloride binding.
- Binding isotherms show the relation between free and binding chlorides. Among binding isotherms, the linear and Langmuir isotherms have acceptable accuracy in low chloride concentrations. However, the BET isotherm is not very popular due to its complexity. Freundlich usually provides the greatest accuracy, especially in conventional sea chloride concentration.
- Using MK also affects other influential parameters for chloride binding, such as hydrotalcite content and carbonation. Furthermore, some other parameters are influential on chloride binding in the presence of MK, such as sulfate ions, pH, temperature, and electrical fields.
- MK usually increases carbonation depth, which negatively affects chloride binding. Other parameters that negatively affect chloride binding are increasing temperature, sulfate ions, applied electrical fields, and increasing pH.
- By augmenting chloride concentration, chloride binding also increases; however, it may reach a plateau and does not increase linearly. Furthermore, in exposure to CaCl2 and MgCl2, chloride binding increases, which may be due to improving adsorption mechanism and increasing positive charge of the surface of CSH.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanein, T.; Thienel, K.-C.; Zunino, F.; Marsh, A.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.; Ben Haha, M.; Avet, F. Clay calcination technology: State-of-the-art review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Munger, J.; Xu, S.; McElroy, M.B.; Hao, J.; Nielsen, C.; Ma, H. CO2 and its correlation with CO at a rural site near Beijing: Implications for combustion efficiency in China. Atmos. Chem. Phys. 2010, 10, 8881–8897. [Google Scholar] [CrossRef] [Green Version]
- Salas, D.A.; Ramirez, A.D.; Rodríguez, C.R.; Petroche, D.M.; Boero, A.J.; Duque-Rivera, J. Environmental impacts, life cycle assessment and potential improvement measures for cement production: A literature review. J. Clean. Prod. 2016, 113, 114–122. [Google Scholar] [CrossRef]
- Homayoonmehr, R.; Ramezanianpour, A.A.; Mirdarsoltany, M. Influence of metakaolin on fresh properties, mechanical properties and corrosion resistance of concrete and its sustainability issues: A review. J. Build. Eng. 2021, 44, 103011. [Google Scholar] [CrossRef]
- Qureshi, L.A.; Ali, B.; Ali, A. Combined effects of supplementary cementitious materials (silica fume, GGBS, fly ash and rice husk ash) and steel fiber on the hardened properties of recycled aggregate concrete. Constr. Build. Mater. 2020, 263, 120636. [Google Scholar] [CrossRef]
- Masood, B.; Elahi, A.; Barbhuiya, S.; Ali, B. Mechanical and durability performance of recycled aggregate concrete incorporating low calcium bentonite. Constr. Build. Mater. 2020, 237, 117760. [Google Scholar] [CrossRef]
- Alyousef, R.; Ali, B.; Mohammed, A.; Kurda, R.; Alabduljabbar, H.; Riaz, S. Evaluation of Mechanical and Permeability Characteristics of Microfiber-Reinforced Recycled Aggregate Concrete with Different Potential Waste Mineral Admixtures. Materials 2021, 14, 5933. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Chow, P.; Memon, S. Microstructure, hydration and nanomechanical properties of concrete containing metakaolin. Constr. Build. Mater. 2015, 95, 696–702. [Google Scholar] [CrossRef]
- Poon, C.-S.; Lam, L.; Kou, S.; Wong, Y.-L.; Wong, R. Rate of pozzolanic reaction of metakaolin in high-performance cement pastes. Cem. Concr. Res. 2001, 31, 1301–1306. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Osio-Norgaard, J.; Gevaudan, J.P.; Srubar, W.V., III. A review of chloride transport in alkali-activated cement paste, mortar, and concrete. Constr. Build. Mater. 2018, 186, 191–206. [Google Scholar] [CrossRef]
- IEA. Cement; IEA: Paris, France, 2020. Available online: https://www.iea.org/reports/cement (accessed on 13 November 2022).
- Peters, G.; Andrew, R.; Canadell, J.; Friedlingstein, P.; Jackson, R.; Korsbakken, J.; Le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Change 2020, 10, 3–6. [Google Scholar] [CrossRef]
- Fernandez Pales, A.; Leung, Y. Technology Roadmap-Low-Carbon Transition in the Cement Industry; International Energy Agency: Paris, France, 2018. Available online: https://webstore.iea.org/technologyroadmap-low-carbon-transition-in-the-cement-industry (accessed on 8 November 2022).
- Wei, J.; Cen, K. Empirical assessing cement CO2 emissions based on China’s economic and social development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States; No. FHWA-RD-01-156, R315-01; Federal Highway Administration: Washington, DC, USA, 2002.
- Di Filippo, J.; Karpman, J.; De Shazo, J. The impacts of policies to reduce CO2 emissions within the concrete supply chain. Cem. Concr. Compos. 2019, 101, 67–82. [Google Scholar] [CrossRef]
- Mirdarsoltany, M.; Rahai, A.; Hatami, F.; Homayoonmehr, R.; Abed, F. Investigating Tensile Behavior of Sustainable Basalt–Carbon, Basalt–Steel, and Basalt–Steel-Wire Hybrid Composite Bars. Sustainability 2021, 13, 10735. [Google Scholar] [CrossRef]
- Mirdarsoltany, M.; Abed, F.; Homayoonmehr, R.; Abad, S.V.A.N.K. A Comprehensive Review of the Effects of Different Simulated Environmental Conditions and Hybridization Processes on the Mechanical Behavior of Different FRP Bars. Sustainability 2022, 14, 8834. [Google Scholar] [CrossRef]
- Homayoonmehr, R.; Rahai, A.; Ramezanianpour, A.A. Predicting the chloride diffusion coefficient and surface electrical resistivity of concrete using statistical regression-based models and its application in chloride-induced corrosion service life prediction of RC structures. Constr. Build. Mater. 2022, 357, 129351. [Google Scholar] [CrossRef]
- Gruber, K.; Ramlochan, T.; Boddy, A.; Hooton, R.; Thomas, M. Increasing concrete durability with high-reactivity metakaolin. Cem. Concr. Compos. 2001, 23, 479–484. [Google Scholar] [CrossRef]
- Li, C.; Xiao, K. Chloride threshold, modelling of corrosion rate and pore structure of concrete with metakaolin addition. Constr. Build. Mater. 2021, 305, 124666. [Google Scholar] [CrossRef]
- DorMohammadi, H.; Pang, Q.; Murkute, P.; Árnadóttir, L.; Isgor, O.B. Investigation of iron passivity in highly alkaline media using reactive-force field molecular dynamics. Corros. Sci. 2019, 157, 31–40. [Google Scholar] [CrossRef]
- Furcas, F.E.; Lothenbach, B.; Isgor, O.B.; Mundra, S.; Zhang, Z.; Angst, U.M. Solubility and speciation of iron in cementitious systems. Cem. Concr. Res. 2022, 151, 106620. [Google Scholar] [CrossRef]
- Käthler, C.B.; Aguilar, A.M.; Angst, U.; Elsener, B. A systematic data collection on chloride-induced steel corrosion in concrete to improve service life modelling and towards understanding corrosion initiation. Corros. Sci. 2019, 157, 331–336. [Google Scholar] [CrossRef]
- Cao, Y.; Gehlen, C.; Angst, U.; Wang, L.; Wang, Z.; Yao, Y. Critical chloride content in reinforced concrete—An updated review considering Chinese experience. Cem. Concr. Res. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, Y.; Zhang, J.; Wang, J.; Zhou, X.; Zhang, Y. Randomness of critical chloride concentration of reinforcement corrosion in reinforced concrete flexural members in a tidal environment. Ocean. Eng. 2019, 172, 330–341. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. 2019, 52, 88. [Google Scholar] [CrossRef]
- Yang, L.; Xu, J.; Huang, Y.; Li, L.; Zhao, P.; Lu, L.; Cheng, X.; Zhang, D.; He, Y. Using layered double hydroxides and anion exchange resin to improve the mechanical properties and chloride binding capacity of cement mortars. Constr. Build. Mater. 2021, 272, 122002. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Tian, W.; Wei, J.; Yu, Q. Physically and chemically bound chlorides in hydrated cement pastes: A comparison study of the effects of silica fume and metakaolin. J. Mater. Sci. 2019, 54, 2152–2169. [Google Scholar] [CrossRef]
- Maes, M.; Gruyaert, E.; De Belie, N. Resistance of concrete with blast-furnace slag against chlorides, investigated by comparing chloride profiles after migration and diffusion. Mater. Struct. 2013, 46, 89–103. [Google Scholar] [CrossRef]
- Plusquellec, G.; Nonat, A. Interactions between calcium silicate hydrate (CSH) and calcium chloride, bromide and nitrate. Cem. Concr. Res. 2016, 90, 89–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Liu, L.; She, W.; Yu, J. Experimental and molecular dynamics studies on the transport and adsorption of chloride ions in the nano-pores of calcium silicate phase: The influence of calcium to silicate ratios. Microporous Mesoporous Mater. 2018, 255, 23–35. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Wang, P. Chloride ions transport and adsorption in the nano-pores of silicate calcium hydrate: Experimental and molecular dynamics studies. Constr. Build. Mater. 2016, 126, 991–1001. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Q.; Brouwers, H. In-situ formation of layered double hydroxides (LDHs) in sodium aluminate activated slag: The role of Al-O tetrahedra. Cem. Concr. Res. 2022, 153, 106697. [Google Scholar] [CrossRef]
- Badogiannis, E.; Aggeli, E.; Papadakis, V.; Tsivilis, S. Evaluation of chloride-penetration resistance of metakaolin concrete by means of a diffusion–Binding model and of the k-value concept. Cem. Concr. Compos. 2015, 63, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Yu, R.; Huang, Y.; Cheng, S. Understanding the chloride binding and diffusion behaviors of marine concrete based on Portland limestone cement-alumina enriched pozzolans. Constr. Build. Mater. 2019, 198, 207–217. [Google Scholar] [CrossRef]
- Ferreira, R.; Castro-Gomes, J.; Costa, P.; Malheiro, R. Effect of metakaolin on the chloride ingress properties of concrete. KSCE J. Civ. Eng. 2016, 20, 1375–1384. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Possible states of chloride in the hydration of tricalcium silicate in the presence of calcium chloride. Matériaux Et Constr. 1971, 4, 3–12. [Google Scholar] [CrossRef]
- Dousti, A.; Beaudoin, J.J.; Shekarchi, M. Chloride binding in hydrated MK, SF and natural zeolite-lime mixtures. Constr. Build. Mater. 2017, 154, 1035–1047. [Google Scholar] [CrossRef]
- Teymouri, M.; Shakouri, M.; Vaddey, N.P. pH-dependent chloride desorption isotherms of Portland cement paste. Constr. Build. Mater. 2021, 312, 125415. [Google Scholar] [CrossRef]
- Jain, A.; Gencturk, B.; Pirbazari, M.; Dawood, M.; Belarbi, A.; Sohail, M.; Kahraman, R. Influence of pH on chloride binding isotherms for cement paste and its components. Cem. Concr. Res. 2021, 143, 106378. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Garcia, R.; de la Rubia, M.; Enriquez, E.; del Campo, A.; Fernandez, J.; Moragues, A. Chloride binding capacity of metakaolin and nanosilica supplementary pozzolanic cementitious materials in aqueous phase. Constr. Build. Mater. 2021, 298, 123903. [Google Scholar] [CrossRef]
- Florea, M.; Brouwers, H. Chloride binding related to hydration products: Part I: Ordinary Portland Cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Alizadeh, R.A. Nanostructure and Engineering Properties of Basic and Modified Calcium-Silicate-Hydrate Systems; University of Ottawa: Ottawa, ON, Canada, 2009; Volume 71. [Google Scholar]
- Beaudoin, J.J.; Ramachandran, V.S.; Feldman, R.F. Interaction of chloride and C–S–H. Cem. Concr. Res. 1990, 20, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Geiker, M.R.; De Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement–metakaolin–limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Brykov, A.; Krasnobaeva, S.; Mokeev, M. Hydration of Portland cement in the presence of highly reactive metakaolin. Mater. Sci. Appl. 2015, 6, 391. [Google Scholar] [CrossRef]
- Qomi, M.A.; Krakowiak, K.; Bauchy, M.; Stewart, K.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.; Gonthier, J.N.; Georget, F.; Scrivener, K.L. Insights on chemical and physical chloride binding in blended cement pastes. Cem. Concr. Res. 2022, 156, 106747. [Google Scholar] [CrossRef]
- Zhao, D.; Khoshnazar, R. Microstructure of cement paste incorporating high volume of low-grade metakaolin. Cem. Concr. Compos. 2020, 106, 103453. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Maraghechi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC3) with various kaolinite contents with respect to chloride transport. Mater. Struct. 2018, 51, 125. [Google Scholar] [CrossRef] [Green Version]
- Babaahmadi, A.; Machner, A.; Kunther, W.; Figueira, J.; Hemstad, P.; De Weerdt, K. Chloride binding in Portland composite cements containing metakaolin and silica fume. Cem. Concr. Res. 2022, 161, 106924. [Google Scholar] [CrossRef]
- Dai, Z.; Tran, T.T.; Skibsted, J. Aluminum Incorporation in the C–S–H phase of white Portland cement–metakaolin blends studied by 27 Al and 29 Si MAS NMR spectroscopy. J. Am. Ceram. Soc. 2014, 97, 2662–2671. [Google Scholar] [CrossRef]
- Love, C.; Richardson, I.; Brough, A. Composition and structure of C–S–H in white Portland cement–20% metakaolin pastes hydrated at 25 C. Cem. Concr. Res. 2007, 37, 109–117. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Iwasa, A.; Nawa, T.; Sato, T.; Kurumisawa, K. Ion-cement hydrate interactions govern multi-ionic transport model for cementitious materials. Cem. Concr. Res. 2010, 40, 1756–1765. [Google Scholar] [CrossRef] [Green Version]
- Henocq, P.; Marchand, J.; Samson, E.; Lavoie, J.-A. Modeling of ionic interactions at the C–S–H surface—Application to CsCl and LiCl solutions in comparison with NaCl solutions. In Proceedings of the 2nd International Symposium on Advances in Concrete through Science and Engineering, RILEM Proceedings, Quebec City, QC, Canada, 11–13 September 2006; RILEM Publications: Lyon, France, 2006. [Google Scholar]
- Lambert, P.; Page, C.; Short, N. Pore solution chemistry of the hydrated system tricalcium silicate/sodium chloride/water. Cem. Concr. Res. 1985, 15, 675–680. [Google Scholar] [CrossRef]
- Talero, R.; Trusilewicz, L. Morphological differentiation and crystal growth form of Friedel’s salt originated from pozzolan and Portland cement. Ind. Eng. Chem. Res. 2012, 51, 12517–12529. [Google Scholar] [CrossRef]
- Gbozee, M.; Zheng, K.; He, F.; Zeng, X. The influence of aluminum from metakaolin on chemical binding of chloride ions in hydrated cement pastes. Appl. Clay Sci. 2018, 158, 186–194. [Google Scholar] [CrossRef]
- Baquerizo, L.G.; Matschei, T.; Scrivener, K.L.; Saeidpour, M.; Wadsö, L. Hydration states of AFm cement phases. Cem. Concr. Res. 2015, 73, 143–157. [Google Scholar] [CrossRef]
- Justnes, H. A review of chloride binding in cementitious systems. Nord. Concr. Res. Publ. 1998, 21, 48–63. [Google Scholar]
- Suryavanshi, A.; Scantlebury, J.; Lyon, S. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Huang, Y.; Yu, R.; Ling, G. Chloride binding behaviors of metakaolin-lime hydrated blends: Influence of gypsum and atmospheric carbonation. Constr. Build. Mater. 2019, 201, 380–390. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M.R. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Zibara, H.; Hooton, R.; Thomas, M.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Chen, J.; Ng, P.; Chu, S.; Guan, G.; Kwan, A. Ternary blending with metakaolin and silica fume to improve packing density and performance of binder paste. Constr. Build. Mater. 2020, 252, 119031. [Google Scholar] [CrossRef]
- Ekolu, S.; Thomas, M.; Hooton, R. Pessimum effect of externally applied chlorides on expansion due to delayed ettringite formation: Proposed mechanism. Cem. Concr. Res. 2006, 36, 688–696. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Huang, Y.; Sun, T.; Duan, P. Properties of coral waste-based mortar incorporating metakaolin: Part II. Chloride migration and binding behaviors. Constr. Build. Mater. 2018, 174, 433–442. [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Saout, G.L.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Yu, R.; Huang, Y. Chloride ingress and binding of coral waste filler-coral waste sand marine mortar incorporating metakaolin. Constr. Build. Mater. 2018, 190, 1069–1080. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; Lothenbach, B.; De Weerdt, K.; Garzón, S.F.; Enemark-Rasmussen, K.; Skibsted, J. Friedel’s salt profiles from thermogravimetric analysis and thermodynamic modelling of Portland cement-based mortars exposed to sodium chloride solution. Cem. Concr. Compos. 2017, 78, 73–83. [Google Scholar] [CrossRef]
- Hirao, H.; Yamada, K.; Takahashi, H.; Zibara, H. Chloride binding of cement estimated by binding isotherms of hydrates. J. Adv. Concr. Technol. 2005, 3, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Hooton, R.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Luping, T.; Nilsson, L.-O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.; Zhu, H.; Li, W.; Xin, M.; Liu, Y.; Guo, Y. Study on chloride binding capability of coal gangue based cementitious materials. Constr. Build. Mater. 2018, 167, 649–656. [Google Scholar] [CrossRef]
- Ipavec, A.; Vuk, T.; Gabrovšek, R.; Kaučič, V. Chloride binding into hydrated blended cements: The influence of limestone and alkalinity. Cem. Concr. Res. 2013, 48, 74–85. [Google Scholar] [CrossRef]
- Fu, C.; Jin, X.; Jin, N. Modeling of Chloride Ions Diffusion in Cracked Concrete. In Earth and Space 2010; American Society of Civil Engineers: Reston, VA, USA, 2010; pp. 3579–3589. [Google Scholar]
- Martın-Pérez, B.; Zibara, H.; Hooton, R.; Thomas, M. A study of the effect of chloride binding on service life predictions. Cem. Concr. Res. 2000, 30, 1215–1223. [Google Scholar] [CrossRef]
- Dousti, A.; Shekarchi, M. Effect of exposure temperature on chloride-binding capacity of cementing materials. Mag. Concr. Res. 2015, 67, 821–832. [Google Scholar] [CrossRef]
- Kayali, O.; Khan, M.; Ahmed, M.S. The role of hydrotalcite in chloride binding and corrosion protection in concretes with ground granulated blast furnace slag. Cem. Concr. Compos. 2012, 34, 936–945. [Google Scholar] [CrossRef]
- Ye, H.; Jin, X.; Chen, W.; Fu, C.; Jin, N. Prediction of chloride binding isotherms for blended cements. Comput. Concr. 2016, 17, 655–672. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Haha, M.B.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Chloride-binding capacity of hydrotalcite in cement pastes containing dolomite and metakaolin. Cem. Concr. Res. 2018, 107, 163–181. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Haha, M.B.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Limitations of the hydrotalcite formation in Portland composite cement pastes containing dolomite and metakaolin. Cem. Concr. Res. 2018, 105, 1–17. [Google Scholar] [CrossRef]
- Taylor, R.; Richardson, I.; Brydson, R. Composition and microstructure of 20-year-old ordinary Portland cement–ground granulated blast-furnace slag blends containing 0 to 100% slag. Cem. Concr. Res. 2010, 40, 971–983. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Žemlička, M.; Kuzielova, E.; Kuliffayova, M.; Tkacz, J.; Palou, M.T. Study of hydration products in the model systems metakaolin–lime and metakaolin–lime–gypsum. Ceram Silik 2015, 59, 283–291. [Google Scholar]
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Long term performance of chloride binding capacity in fly ash concrete in a marine environment. Constr. Build. Mater. 2010, 24, 1352–1357. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Shui, Z.; Li, Q.; Han, J. Experimental investigation on chloride diffusion and binding in concrete containing metakaolin. Corros. Eng. Sci. Technol. 2014, 49, 282–286. [Google Scholar] [CrossRef]
- Babu, U.R.; Kondraivendhan, B. Application of Statistics to the Analysis of Corrosion Data for Rebar in Metakaolin Concrete. In Proceedings of the International Conference on Emerging Trends in Engineering (ICETE), Hyderabad, India, 22–23 March 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 162–169. [Google Scholar]
- Yang, L.; Yu, H.; Ma, H.; Zhou, P. Deterioration of high performance hybrid fibers reinforced expansive concrete exposed to magnesium sulfate solution. In Proceedings of the International Conference on Transportation Engineering 2009, Chengdu, China, 25–27 July 2009; pp. 2614–2619. [Google Scholar]
- Olivier, T. Prediction of Chloride Penetration into Satuarated Concrete-Multi-Species Approach. Ph.D. Thesis, Department of Building Materials Chalmers University of Technology, Gothenburg, Sweden, 2000. [Google Scholar]
- Saillio, M.; Baroghel-Bouny, V.; Barberon, F. Chloride binding in sound and carbonated cementitious materials with various types of binder. Constr. Build. Mater. 2014, 68, 82–91. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z. Chemical Equilibrium Modeling and Experimental Measurement of Solubility for Friedel’s Salt in the Na− OH− Cl− NO3− H2O Systems up to 200 °C. Ind. Eng. Chem. Res. 2010, 49, 8949–8958. [Google Scholar] [CrossRef]
- Page, C.; Vennesland, Ø. Pore solution composition and chloride binding capacity of silica-fume cement pastes. Matériaux Constr. 1983, 16, 19–25. [Google Scholar] [CrossRef]
- Labbez, C.; Pochard, I.; Jönsson, B.; Nonat, A. CSH/solution interface: Experimental and Monte Carlo studies. Cem. Concr. Res. 2011, 41, 161–168. [Google Scholar] [CrossRef]
- Liu, W.; Cui, H.; Dong, Z.; Xing, F.; Zhang, H.; Lo, T.Y. Carbonation of concrete made with dredged marine sand and its effect on chloride binding. Constr. Build. Mater. 2016, 120, 1–9. [Google Scholar] [CrossRef]
- Damidot, D.; Atkins, M.; Kindness, A.; Glasser, F. Sulphate attack on concrete: Limits of the AFt stability domain. Cem. Concr. Res. 1992, 22, 229–234. [Google Scholar] [CrossRef]
- Gabrisova, A.; Havlica, J.; Sahu, S. Stability of calcium sulphoaluminate hydrates in water solutions with various pH values. Cem. Concr. Res. 1991, 21, 1023–1027. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, L.; Chen, Y.; Xu, J.; Mo, L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- De Weerdt, K.; Orsáková, D.; Geiker, M.R. The impact of sulphate and magnesium on chloride binding in Portland cement paste. Cem. Concr. Res. 2014, 65, 30–40. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- AASHTO. Designation: T 358-15—Standard Method of Test for Surface Resistivity Indication of Concrete’s Ability to Resist Chloride Ion Penetration; American Association of State Highway and Transportation Officials: Washington, DC, USA, 2015. [Google Scholar]
- Shahidan, S.; Tayeh, B.A.; Jamaludin, A.; Bahari, N.; Mohd, S.; Ali, N.Z.; Khalid, F. Physical and Mechanical Properties of Self-Compacting Concrete Containing Superplasticizer and Metakaolin; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012004. [Google Scholar]
- Hassan, Z. Binding of External Chloride by Cement Pastes; University of Toronto: Toronto, ON, Canada, 2001. [Google Scholar]
- Plusquellec, G.; Nonat, A.; Pochard, I. Anion uptake by calcium silicate hydrate. In Proceedings of the 32nd Cement and Concrete Science Conference, Belfast, Northern Ireland, 17–18 September 2012; pp. 17–18. [Google Scholar]
- Vieira, D.R.; Moreira, A.L.R.; Calmon, J.L.; Dominicini, W.K. Service life modeling of a bridge in a tropical marine environment for durable design. Constr. Build. Mater. 2018, 163, 315–325. [Google Scholar] [CrossRef]
- Dousti, A.; Rashetnia, R.; Ahmadi, B.; Shekarchi, M. Influence of exposure temperature on chloride diffusion in concretes incorporating silica fume or natural zeolite. Constr. Build. Mater. 2013, 49, 393–399. [Google Scholar] [CrossRef]
- Rostam, S. Service Life Design of Concrete Structures-a Challenge to Designers as Well as to Owners. Asian J. Civ. Eng. 2005, 6, 423–445. [Google Scholar]
- Panesar, D.; Chidiac, S. Effect of cold temperature on the chloride-binding capacity of cement. J. Cold Reg. Eng. 2011, 25, 133–144. [Google Scholar] [CrossRef]
- Wowra, O.; Setzer, M.; Setzer, M.; Auberg, R. Sorption of chlorides on hydrated cements and C3S pastes. In Frost Resistance of Concrete; CRC Press: Boca Raton, FL, USA, 1997; pp. 147–153. [Google Scholar]
- Hussain, S.E. Effect of temperature on pore solution composition in plain cements. Cem. Concr. Res. 1993, 23, 1357–1368. [Google Scholar] [CrossRef]
- Larsen, C. Chloride Binding in Concrete-Effect of Surrounding Environment and Concrete Composition. Dr. Ing. Thesis, NTNU, Trondheim, Norway, 1998. [Google Scholar]
- Larsen, C.K. Effect of Type of Aggregate, Temperature and Drying/Rewetting on Chloride Binding and Pore Solution Composition; RILEM: Bagneux, France, 1997; Volume 27. [Google Scholar]
- Nguyen, T.; Lorente, S.; Carcasses, M. Effect of the environment temperature on the chloride diffusion through CEM-I and CEM-V mortars: An experimental study. Constr. Build. Mater. 2009, 23, 795–803. [Google Scholar] [CrossRef]
- Wilson, W.; Georget, F.; Scrivener, K. Unravelling chloride transport/microstructure relationships for blended-cement pastes with the mini-migration method. Cem. Concr. Res. 2021, 140, 106264. [Google Scholar] [CrossRef]
- Gluth, G.J.; Arbi, K.; Bernal, S.A.; Bondar, D.; Castel, A.; Chithiraputhiran, S.; Dehghan, A.; Dombrowski-Daube, K.; Dubey, A.; Ducman, V. RILEM TC 247-DTA round robin test: Carbonation and chloride penetration testing of alkali-activated concretes. Mater. Struct. 2020, 53, 21. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Chloride-binding isotherms in concrete submitted to non-steady-state migration experiments. Cem. Concr. Res. 1999, 29, 1799–1806. [Google Scholar] [CrossRef]
- Ollivier, J.; Arsenault, J.; Truc, O.; Marchand, J. Determination of chloride binding isotherms from migration tests. In Proceedings of the Mario Collepardi Symposium on Advances in Concrete Science and Technology, Rome, Italy, 7 October 1997; pp. 198–217. [Google Scholar]







| Replacement Level | Portland Cement–Metakaolin Pastes | Reference Pastes | ||||
|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 10% | 20% | 30% | |
| Total chloride | 1.29 | 1.11 | 0.61 | 1.03 | 0.90 | 0.66 |
| Free chloride | 0.15 | 0.11 | 0.10 | 0.27 | 0.26 | 0.27 |
| Bound chloride | 1.15 | 1.00 | 0.51 | 0.76 | 0.63 | 0.38 |
| Chloride calculated by Friedel’s salt content | 1.30 | 0.93 | 0.08 | 0.50 | 0.35 | 0.23 |
| Isotherm | Relation |
|---|---|
| Linear binding isotherm | |
| Langmuir isotherm | |
| Freundlich binding isotherm | |
| Brunauer, Emmett, Teller (BET) isotherm |
| Reference | Range of Temperature | Effect of Increasing Temperature |
|---|---|---|
| Wowra and Setzer [119] | 0–40 °C | Increase in bound chloride content (due to faster reaction rates at higher temperature) |
| Hussain and Rasheeduzzafar [120] | 20–70 °C | Increased free chloride |
| CK Larsen 1998 [121] and CK Larsen [122] | 20–80 °C | Increase of the chloride concentration of the pore solution |
| TS Nguyen et al. [123] | 5–35 °C (Cl concentration: 0–20 g/L) | No effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homayoonmehr, R.; Ramezanianpour, A.A.; Moodi, F.; Ramezanianpour, A.M.; Gevaudan, J.P. A Review on the Effect of Metakaolin on the Chloride Binding of Concrete, Mortar, and Paste Specimens. Sustainability 2022, 14, 15022. https://doi.org/10.3390/su142215022
Homayoonmehr R, Ramezanianpour AA, Moodi F, Ramezanianpour AM, Gevaudan JP. A Review on the Effect of Metakaolin on the Chloride Binding of Concrete, Mortar, and Paste Specimens. Sustainability. 2022; 14(22):15022. https://doi.org/10.3390/su142215022
Chicago/Turabian StyleHomayoonmehr, Reza, Ali Akbar Ramezanianpour, Faramarz Moodi, Amir Mohammad Ramezanianpour, and Juan Pablo Gevaudan. 2022. "A Review on the Effect of Metakaolin on the Chloride Binding of Concrete, Mortar, and Paste Specimens" Sustainability 14, no. 22: 15022. https://doi.org/10.3390/su142215022
APA StyleHomayoonmehr, R., Ramezanianpour, A. A., Moodi, F., Ramezanianpour, A. M., & Gevaudan, J. P. (2022). A Review on the Effect of Metakaolin on the Chloride Binding of Concrete, Mortar, and Paste Specimens. Sustainability, 14(22), 15022. https://doi.org/10.3390/su142215022






