Cerium Compounds Coating as a Single Self-Healing Layer for Corrosion Inhibition on Aluminum 3003
Abstract
1. Introduction
2. Experimental Methods and Materials
2.1. Cerium Treatment by Immersion
2.2. Electrochemical Tests
2.3. Characterization
3. Results and Discussion
- Anodic reaction
- 2.
- Cathodic reaction
- 3.
- Active site blocking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, D.; Rubio, E.M.; Lorente-Pedreille, R.M.; Sáenz-Nuño, M.A. Sustainable Processes in Aluminium, Magnesium, and Titanium Alloys Applied to the Transport Sector: A Review. Metals 2021, 12, 9. [Google Scholar] [CrossRef]
- Mavhungu, S.T.; Akinlabi, E.T.; Onitiri, M.A.; Varachia, F.M. Aluminum Matrix Composites for Industrial Use: Advances and Trends. Procedia Manuf. 2017, 7, 178–182. [Google Scholar] [CrossRef]
- Bin Ali, S.; Kamaris, G.S.; Gkantou, M. Flexural Behaviour of Concrete-Filled Double Skin Aluminium Alloy Tubes. Eng. Struct. 2022, 272, 114972. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, C.; Zhou, Z.; Long, H.; Jia, J.; He, L.; Long, Y. Dissimilar Laser Lap Welding of Mg and Al Alloys Using a CoCrFeNi Medium-Entropy Alloy Interlayer. Opt. Laser Technol. 2023, 157, 108639. [Google Scholar] [CrossRef]
- Pan, C.; Li, X.; Mao, J. The Effect of a Corrosion Inhibitor on the Rehabilitation of Reinforced Concrete Containing Sea Sand and Seawater. Materials 2020, 13, 1480. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, Y.; Wang, Y.; Shi, J. TA/Fe(III) Anti-Chloride Coating to Protect Concrete. J. Clean. Prod. 2020, 259, 120922. [Google Scholar] [CrossRef]
- Yuan, A.; Yang, C.; Wang, J.; Chen, L.; Lu, R. Shear Behavior of Epoxy Resin Joints in Precast Concrete Segmental Bridges. J. Bridg. Eng. 2019, 24, 04019009. [Google Scholar] [CrossRef]
- Tian, Y.; Dong, C.; Wang, G.; Cheng, X.; Li, X. The Effect of Nickel on Corrosion Behaviour of High-Strength Low Alloy Steel Rebar in Simulated Concrete Pore Solution. Constr. Build. Mater. 2020, 246, 118462. [Google Scholar] [CrossRef]
- Bakhtiary-Noodeh, M.; Moradian, S.; Ranjbar, Z. Improvement of the Edge Protection of an Automotive Electrocoating in Presence of a Prepared Epoxy-Amine Microgel. Prog. Org. Coat. 2017, 103, 111–125. [Google Scholar] [CrossRef]
- Akafuah, N.; Poozesh, S.; Salaimeh, A.; Patrick, G.; Lawler, K.; Saito, K. Evolution of the Automotive Body Coating Process—A Review. Coatings 2016, 6, 24. [Google Scholar] [CrossRef]
- Wang, J.; Pang, X.; Jahed, H. Surface Protection of Mg Alloys in Automotive Applications: A Review. AIMS Mater. Sci. 2019, 6, 567–600. [Google Scholar] [CrossRef]
- Ellappan, R.; Arumugam, S. The Effect of Corrosion Inhibitor on Corrosion of Automotive Materials in Biodegradable Engine Oil. IOP Conf. Ser. Mater. Sci. Eng. 2018, 390, 012092. [Google Scholar] [CrossRef]
- Glover, C.F.; Lim, M.L.C.; Scully, J.R. The Effect of Surface Treatment on the Performance of a Zirconium-Based Conversion Coating on AA7075 Automotive Alloys for Protection against Filiform Corrosion. In TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2020; pp. 937–946. [Google Scholar]
- Pearce, C.D. Surface Engineering for Corrosion Protection of Underbonnet and Underbody Automotive Components. Surf. Eng. 1992, 8, 188–192. [Google Scholar] [CrossRef]
- Tiong, U.H.; Clark, G. Impact of Mechanical Strain Environment on Aircraft Protective Coatings and Corrosion Protection. J. Aircr. 2011, 48, 1315–1330. [Google Scholar] [CrossRef]
- Doublet, A.; Kjellberg, M.; Jousselme, B.; Pinault, M.; Deniau, G.; Cornut, R.; Charrier, G. Bifunctional Coatings: Coupling an Organic Adhesion Promoter with an Anticorrosion Inorganic Layer. RSC Adv. 2019, 9, 24043–24049. [Google Scholar] [CrossRef]
- Becker, M. Chromate-Free Chemical Conversion Coatings for Aluminum Alloys. Corros. Rev. 2019, 37, 321–342. [Google Scholar] [CrossRef]
- Majd, M.T.; Davoudi, M.; Ramezanzadeh, M.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. Construction of a Smart Active/Barrier Anti-Corrosion System Based on Epoxy-Ester/Zinc Intercalated Kaolin Nanocontainer for Steel Substrate. Constr. Build. Mater. 2020, 247, 118555. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Yang, W.; Xu, B.; Chen, Y.; Yin, X.; Liu, Y. Superior Conducting Polypyrrole Anti-Corrosion Coating Containing Functionalized Carbon Powders for 304 Stainless Steel Bipolar Plates in Proton Exchange Membrane Fuel Cells. Chem. Eng. J. 2020, 393, 124675. [Google Scholar] [CrossRef]
- Wen, J.; Lei, J.; Chen, J.; Gou, J.; Li, Y.; Li, L. An Intelligent Coating Based on PH-Sensitive Hybrid Hydrogel for Corrosion Protection of Mild Steel. Chem. Eng. J. 2020, 392, 123742. [Google Scholar] [CrossRef]
- Rahman, M.; Balu, R.; Dutta, N.K.; Roy Choudhury, N. In Vitro Corrosion Resistance of a Layer-by-Layer Engineered Hybrid Coating on ZK60 Magnesium Alloy. Sustainability 2022, 14, 2459. [Google Scholar] [CrossRef]
- Xiang, S.; Yuan, Y.; Zhang, C.; Chen, J. Effects of Process Parameters on the Corrosion Resistance and Biocompatibility of Ti6Al4V Parts Fabricated by Selective Laser Melting. ACS Omega 2022, 7, 5954–5961. [Google Scholar] [CrossRef] [PubMed]
- Jafar Mazumder, M.A. Global Impact of Corrosion: Occurrence, Cost and Mitigation. Glob. J. Eng. Sci. 2020, 5. [Google Scholar] [CrossRef]
- Bowman, E.; Jacobson, G.; Koch, G.; Varney, J.; Thopson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study. NACE Int. 2016, A-19, 2–6. Available online: http://impact.nace.org/documents/Nace-International-Report.pdf (accessed on 12 October 2022).
- Paz Martínez-Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fernandes, F.A.O.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef]
- Mehdizade, M.; Soltanieh, M.; Eivani, A.R. Investigation of Anodizing Time and Pulse Voltage Modes on the Corrosion Behavior of Nanostructured Anodic Layer in Commercial Pure Aluminum. Surf. Coat. Technol. 2019, 358, 741–752. [Google Scholar] [CrossRef]
- Elabar, D.; La Monica, G.R.; Santamaria, M.; Di Quarto, F.; Skeldon, P.; Thompson, G.E. Anodizing of Aluminium and AA 2024-T3 Alloy in Chromic Acid: Effects of Sulphate on Film Growth. Surf. Coat. Technol. 2017, 309, 480–489. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; He, C.; Lu, M.; Sun, L. Studies on the Sealing Processes of Corrosion Resistant Coatings Formed on 2024 Aluminium Alloy with Tartaric-Sulfuric Anodizing. Surf. Coat. Technol. 2019, 360, 369–375. [Google Scholar] [CrossRef]
- Liu, J.; Fang, X.; Zhu, C.; Xing, X.; Cui, G.; Li, Z. Fabrication of Superhydrophobic Coatings for Corrosion Protection by Electrodeposition: A Comprehensive Review. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125498. [Google Scholar] [CrossRef]
- Yi, P.; Dong, C.; Ao, M.; Xiao, K. A Study on Protection Behavior of Inorganic Coating Prepared by Magnetron Sputtering Technology for Electrochemical Migration Corrosion Failure of SAC305 Alloy. Ceram. Int. 2020, 46, 25568–25575. [Google Scholar] [CrossRef]
- Rodič, P.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Milošev, I. The Effect of Copolymerisation on the Performance of Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA2024-T. Prog. Org. Coat. 2020, 147, 105701. [Google Scholar] [CrossRef]
- Ryu, S.; Kwon, Y.J.; Kim, Y.; Lee, J.U. Corrosion Protection Coating of Three-Dimensional Metal Structure by Electrophoretic Deposition of Graphene Oxide. Mater. Chem. Phys. 2020, 250, 123039. [Google Scholar] [CrossRef]
- Hoche, H.; Pusch, C.; Oechsner, M. Corrosion and Wear Protection of Mild Steel Substrates by Innovative PVD Coatings. Surf. Coat. Technol. 2020, 391, 125659. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Srivastava, V.; Haque, J.; Ebrahimi, B. El Virgin and Chemically Functionalized Amino Acids as Green Corrosion Inhibitors: Influence of Molecular Structure through Experimental and in Silico Studies. J. Mol. Struct. 2020, 1226, 129259. [Google Scholar] [CrossRef]
- Ma, L.; Qiang, Y.; Zhao, W. Designing Novel Organic Inhibitor Loaded MgAl-LDHs Nanocontainer for Enhanced Corrosion Resistance. Chem. Eng. J. 2021, 408, 127367. [Google Scholar] [CrossRef]
- Oh, Y.; Han, C.H.; Wang, M.; Chun, Y.-B.; Han, H.N. Effect of Rare Earth Oxide Addition on Microstructure and Mechanical Properties of Ni-Based Alloy. J. Alloy. Compd. 2021, 853, 156980. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Hu, L.; Zhu, J.; Shi, J.; Guo, D. Rare Earth Insitu-Doped ZIF-67 Derived N Doped C Encapsulated Sm2O3/Co Nanoparticles as Excellent Oxygen Reduction Reaction Catalyst for Al-Air Batteries. J. Power Sources 2021, 482, 229052. [Google Scholar] [CrossRef]
- Azzeddine, H.; Hanna, A.; Dakhouche, A.; Rabahi, L.; Scharnagl, N.; Dopita, M.; Brisset, F.; Helbert, A.L.; Baudin, T. Impact of Rare-Earth Elements on the Corrosion Performance of Binary Magnesium Alloys. J. Alloy. Compd. 2020, 829, 154569. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Cui, Z.; Qi, W.; Wang, J.; Ju, P.; Zhao, Y.; Liu, B.; Zhang, T.; Wang, F. Influence of Rare Earth Element (Y) on Microstructure and Corrosion Behavior of Hot Extrusion AZ91 Magnesium Alloy. Materials 2020, 13, 3651. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Y. Study on Properties of New Mg-Y-Nd-(La+Ce)-Zr Degradable Magnesium Alloy. IOP Conf. Ser. Earth Environ. Sci. 2019, 358, 052059. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Ferreira, M.G.S.; Zheludkevich, M.L.; Terryn, H.; Mol, J.M.C.; Gonzalez-Garcia, Y. Novel and Self-Healing Anticorrosion Coatings Using Rare Earth Compounds; Woodhead Publishing Limited: Sawston, UK, 2014; ISBN 9780857093585. [Google Scholar]
- Hinton, B.R.W.; Arnott, D.R.; Ryan, N.E. Inhibition of Aluminum Alloy Corrosion by Cerous Cations. Met. Forum 1984, 7, 211–217. [Google Scholar]
- Deyab, M.A.; El-Rehim, S.S.A.; Hassan, H.H.; Shaltot, A.M. Impact of Rare Earth Compounds on Corrosion of Aluminum Alloy (AA6061) in the Marine Water Environment. J. Alloy. Compd. 2020, 820, 153428. [Google Scholar] [CrossRef]
- Behrsing, T.; Deacon, G.B.; Junk, P.C. The Chemistry of Rare Earth Metals, Compounds, and Corrosion Inhibitors; Woodhead Publishing Limited: Sawston, UK, 2014; ISBN 9780857093585. [Google Scholar]
- Kong, F.; Ying, Y.; Lu, S. Heavy Metal Pollution Risk of Desulfurized Steel Slag as a Soil Amendment in Cycling Use of Solid Wastes. J. Environ. Sci. 2023, 127, 349–360. [Google Scholar] [CrossRef]
- Al-Swadi, H.A.; Usman, A.R.A.; Al-Farraj, A.S.; Al-Wabel, M.I.; Ahmad, M.; Al-Faraj, A. Sources, Toxicity Potential, and Human Health Risk Assessment of Heavy Metals-Laden Soil and Dust of Urban and Suburban Areas as Affected by Industrial and Mining Activities. Sci. Rep. 2022, 12, 8972. [Google Scholar] [CrossRef] [PubMed]
- Yuk, S.; Jung, J.; Song, K.-Y.; Wook Lee, D.; Lee, D.-H.; Choi, S.; Doo, G.; Hyun, J.; Kwen, J.; Young Kim, J.; et al. Addressing the Detrimental Effect of CeO2 Radical Scavenger on the Durability of Polymer Electrolyte Membrane Fuel Cells. Chem. Eng. J. 2023, 452, 139061. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Lv, X.; Huang, G.; Xu, W.; Li, X.; Jia, H. Pt/CeO2 Coated with Polyoxometallate Chainmail to Regulate Oxidation of Chlorobenzene without Hazardous by-Products. J. Hazard. Mater. 2023, 441, 129925. [Google Scholar] [CrossRef]
- Mendez, J.A.C.; Vong, Y.M.; Bueno, J.d.J.P. Cerium and Other Rare Earth Salts as Corrosion Inhibitors—A Review. Prot. Met. Phys. Chem. Surf. 2022, 58, 801–810. [Google Scholar] [CrossRef]
- Dong, J.; Feng, X.; Jia, J.; Shi, B.; Wu, Y.; Cao, B. Annealing Free CeO2 Electron Transport Layer for Efficient Perovskite Solar Cells. J. Solid State Chem. 2023, 317, 123661. [Google Scholar] [CrossRef]
- Pollard, K.D.; Jenkins, H.A.; Puddephatt, R.J. Chemical Vapor Deposition of Cerium Oxide Using the Precursors [Ce(Hfac) 3 (Glyme)]. Chem. Mater. 2000, 12, 701–710. [Google Scholar] [CrossRef]
- Anderson, K.W.; Kaufman, J.; Gilbert, J. ASM Handbook®, Volume 02B—Properties and Selection of Aluminum Alloys; Anderson, K.W., Kaufman, J., Gilbert, J., Eds.; ASM International: Almere, The Netherlands, 2019. [Google Scholar]
- Baboian, R. Corrosion Testing. In Nace Corrosion Engineer’s Reference Book; Nace International: Houston, TX, USA, 2016; p. 153. ISBN 978-1-57590-321-7. [Google Scholar]
- Chira, A.; Bucur, B.; Radu, G.-L. Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion. Materials 2017, 10, 235. [Google Scholar] [CrossRef]
- Garcia, S.J.; Mol, J.M.C.; Muster, T.H.; Hughes, A.E.; Mardel, J.; Miller, T.; Markley, T.; Terryn, H.; Wit, J.H.W.d. Advances in the Selection and Use of Rare-Earth-Based Inhibitors for Self-Healing Organic Coatings. Self-Heal. Prop. New Surf. Treat. 2011, 58, 148–184. [Google Scholar]
- Cecílio, P.; Duarte, R.G.; Simões, A.M.; Montemor, M.F.; Ferreira, M.G.S. The Effect of Cerium Nitrate on the Corrosion Behaviour of Electrogalvanised Steel Substrates, Evaluated by XPS and SVET. In Innovative Pre-Treatment Techniques to Prevent Corrosion of Metallic Surfaces; Woodhead Publishing: Sawston, UK, 2007; pp. 110–118. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, W.C.; Ma, M.; Jia, Y.P.; Liu, J.P.; Zhang, T.Q. Microstructure, Wear and Corrosion Behavior of Nano-CeO2 Doped Diamond-like Carbon (DLC) Composite Films. Diam. Relat. Mater. 2022, 126, 109087. [Google Scholar] [CrossRef]
- Li, G.; Chai, S.; Zhang, G.; Liu, J.; Zhang, Y. Journal of Environmental Chemical Engineering Deactivation Characteristics of Ce-Modified Cu-Based Carbon Materials for Catalytic Wet Air Oxidation of Phenol Wastewater. J. Environ. Chem. Eng. 2022, 10, 108228. [Google Scholar] [CrossRef]
- Jain, S.; Shah, J.; Negi, N.S.; Sharma, C.; Kotnala, R.K. Significance of Interface Barrier at Electrode of Hematite Hydroelectric Cell for Generating Ecopower by Water Splitting. Int. J. Energy Res. 2019, 43, 4743–4755. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Shimizu, F.M.; Mastelaro, V.R.; M’Peko, J.C.; Lin, L.; Oliveira, O.N. UV-Assisted Chemiresistors Made with Gold-Modified ZnO Nanorods to Detect Ozone Gas at Room Temperature. Microchim. Acta 2019, 186, 418. [Google Scholar] [CrossRef]
- Major, G.H.; Avval, T.G.; Moeini, B.; Pinto, G.; Shah, D.; Jain, V.; Carver, V.; Skinner, W.; Gengenbach, T.R.; Easton, C.D.; et al. Assessment of the Frequency and Nature of Erroneous X-ray Photoelectron Spectroscopy Analyses in the Scientific Literature. J. Vac. Sci. Technol. A 2020, 38, 061204. [Google Scholar] [CrossRef]
- Trabelsi, W.; Cecilio, P.; Ferreira, M.G.S.; Montemor, M.F. Electrochemical Assessment of the Self-Healing Properties of Ce-Doped Silane Solutions for the Pre-Treatment of Galvanised Steel Substrates. Prog. Org. Coat. 2005, 54, 276–284. [Google Scholar] [CrossRef]
- Davoodi, A.; Pan, J.; Leygraf, C.; Norgren, S. Multianalytical and In Situ Studies of Localized Corrosion of EN AW-3003 Alloy—Influence of Intermetallic Particles. J. Electrochem. Soc. 2008, 155, C138. [Google Scholar] [CrossRef]
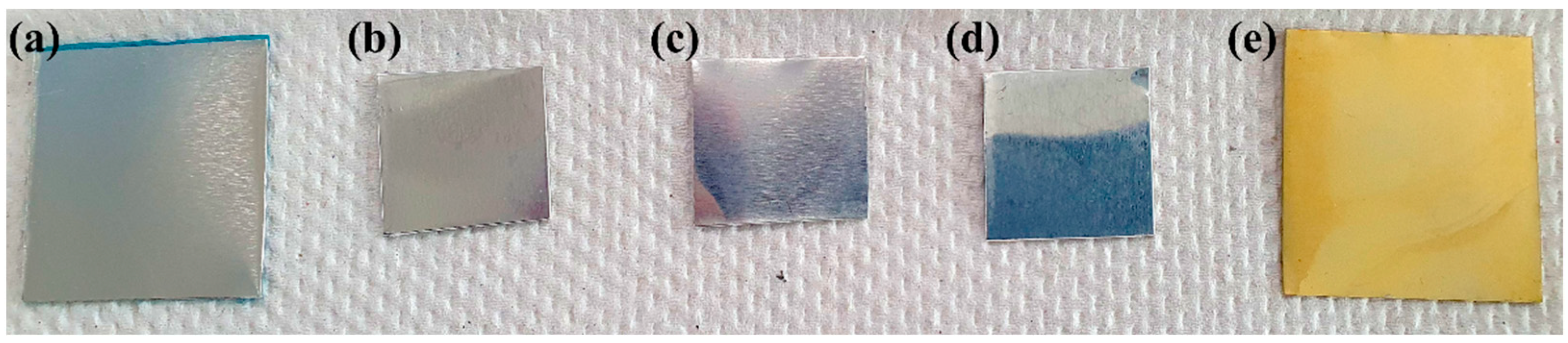

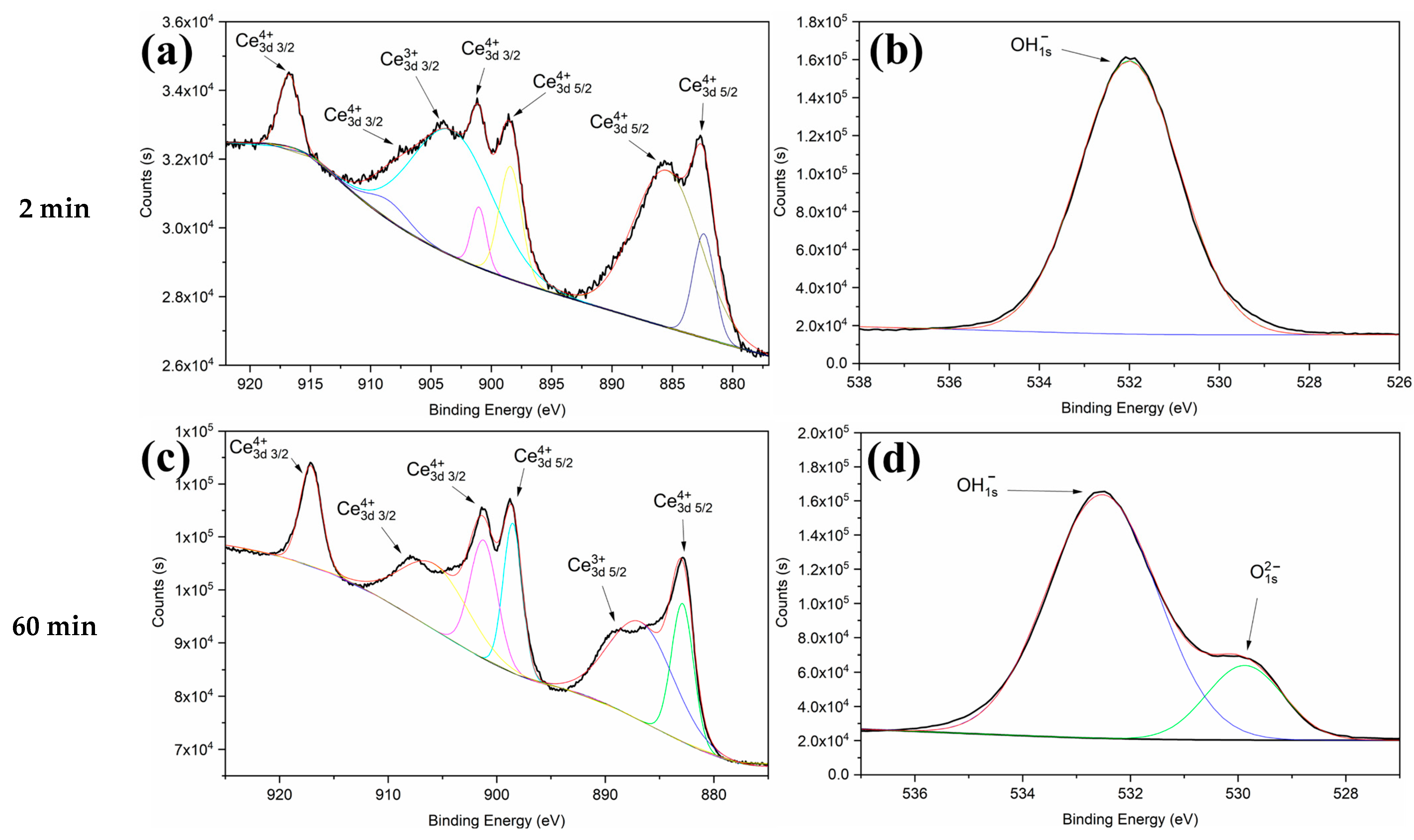
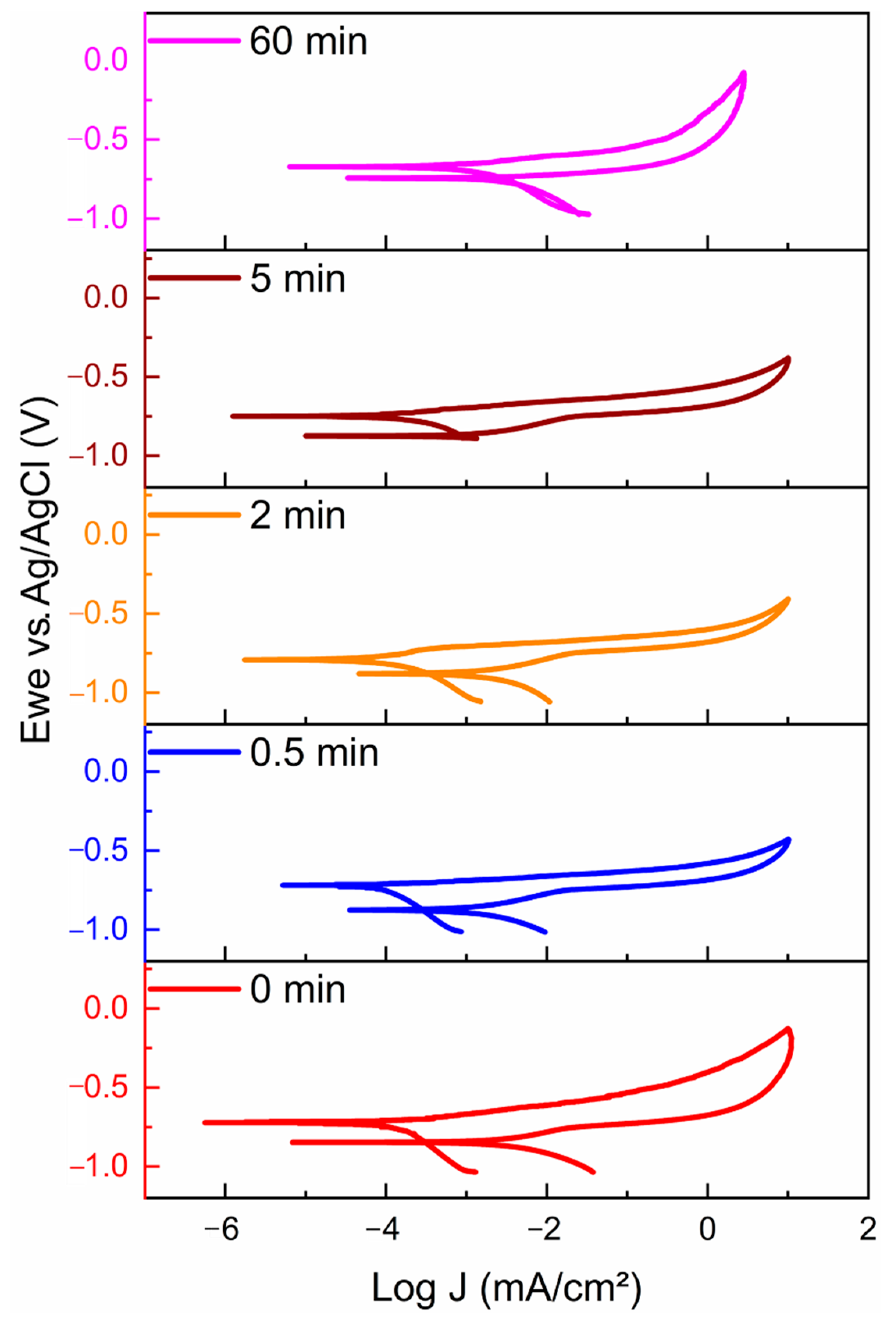



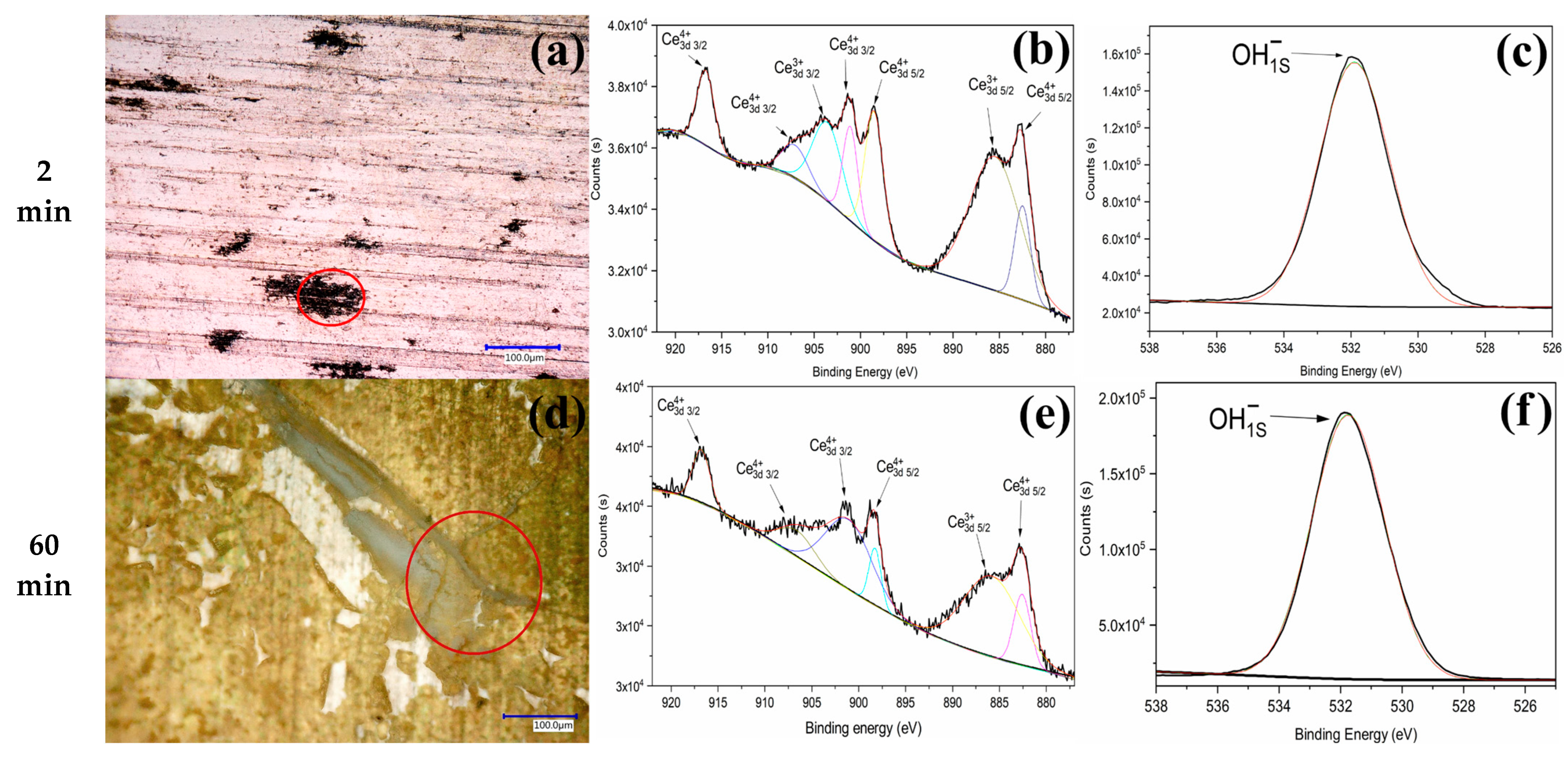
| Time (min) | Al (wt%) | C (wt%) | O (wt%) | Mn (wt%) | Ce (wt%) |
|---|---|---|---|---|---|
| 0 | 92.07 | 5.58 | 1.15 | 1.20 | - |
| 0.5 | 87.82 | 9.07 | 2.13 | 0.77 | 0.22 |
| 2 | 84.07 | 9.84 | 2.88 | 1.46 | 1.75 |
| 5 | 86.37 | 6.77 | 3.67 | 1.36 | 1.84 |
| 60 | 63.20 | 9.06 | 10.22 | - | 17.52 |
| Time (min) | Ce3d Scan | Atomic % | O1s Scan | Atomic % |
|---|---|---|---|---|
| 2 min | Ce3+ | 72.45154 | OH− | 100 |
| Ce4+ | 27.54846 | O2− | - | |
| 60 min | Ce3+ | 43.50885 | OH− | 82.41873 |
| Ce4+ | 66.49118 | O2− | 17.58127 |
| Time (min) | Ecorr (mV) | Epit (mV) | Icorr (μA/cm2) | Corrosion Rate (mmpy) |
|---|---|---|---|---|
| 0 | −734.971 ± 22.39 | Ecorr = Epit | 0.304 ± 0.05 | 0.00164 ± 0.0002 |
| 0.5 | −753.144 ± 32.52 | Ecorr = Epit | 0.314 ± 0.34 | 0.00074 ± 0.0003 |
| 2 | −805.145 ± 32.49 | −690. 2 ± 27.63 | 0.098 ± 0.05 | 0.00053 ± 0.0003 |
| 5 | −747.411 ± 33.89 | Ecorr = Epit | 0.140 ± 0.10 | 0.00076 ± 0.0005 |
| 60 | −676.997 ± 45.95 | Ecorr = Epit | 1.471 ± 0.59 | 0.02262 ± 0.0196 |
| Time (min) | Rs (Ω) | CPE | Rct (Ω) | % Inhibition | |
|---|---|---|---|---|---|
| T | P | ||||
| 0 | 7.10 ± 1.37 | 5.99 × 10−6 ± 1.28 × 10−6 | 0.91 ± 0.007 | 52,712.67 ± 4303 | - |
| 0.5 | 6.96 ± 0.26 | 8.97 × 10−6 ± 3.27 × 10−6 | 0.93 ± 0.01 | 34,384.5 ± 5197.94 | −53.30 |
| 2 | 8.10 ± 1.73 | 3.77 × 10−6 ± 5.21 × 10−6 | 0.47 ± 0.6 | 98,687 ± 38,895 | 46.58 |
| 5 | 7.84 ± 0.91 | 8.68 × 10−6 ± 2.73 × 10−6 | 0.94 ± 0.02 | 54,616 ± 4971.58 | 3.84 |
| 60 | 16.58 ± 10.69 | 2.15 × 10−5 ± 4.5 × 10−6 | 0.97 ± 0.004 | 5850.33 ± 1700.79 | −801.02 |
| Time (min) | σE | σI | Rn (Ω) | Ip | Type of Corrosion |
|---|---|---|---|---|---|
| 0 | 0.00186 | 2762 × 10−8 | 82,407.23 ± 14,417 | 0.58± 0.6 | Localized |
| 0.5 | 0.00152 | 3.29 × 10−8 | 46,101.33 ± 5252.19 | 0.6 ± 0.4 | Localized |
| 2 | 0.00130 | 1379 × 10−8 | 112,155.48 ± 26,074 | 0.49 ± 0.2 | Localized |
| 5 | 0.00177 | 3.85 × 10−8 | 31,491 ± 7942.50 | 0.3 ± 0.04 | Localized |
| 60 | 0.00080 | 17,547 × 10−7 | 6446.98 ± 5083 | 0.2 ± 0.06 | Localized |
| Time (min) | Ce3d Scan | Atomic % | O1s Scan | Atomic % |
|---|---|---|---|---|
| 2 min | Ce3+ | 54.66105 | OH− | 100 |
| Ce4+ | 45.33894 | O2− | - | |
| 60 min | Ce3+ | 39.04718 | OH− | 100 |
| Ce4+ | 60.95282 | O2− | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello Mendez, J.A.; Pérez Bueno, J.d.J.; Meas Vong, Y.; Portales Martínez, B. Cerium Compounds Coating as a Single Self-Healing Layer for Corrosion Inhibition on Aluminum 3003. Sustainability 2022, 14, 15056. https://doi.org/10.3390/su142215056
Cabello Mendez JA, Pérez Bueno JdJ, Meas Vong Y, Portales Martínez B. Cerium Compounds Coating as a Single Self-Healing Layer for Corrosion Inhibition on Aluminum 3003. Sustainability. 2022; 14(22):15056. https://doi.org/10.3390/su142215056
Chicago/Turabian StyleCabello Mendez, José Antonio, José de Jesús Pérez Bueno, Yunny Meas Vong, and Benjamín Portales Martínez. 2022. "Cerium Compounds Coating as a Single Self-Healing Layer for Corrosion Inhibition on Aluminum 3003" Sustainability 14, no. 22: 15056. https://doi.org/10.3390/su142215056
APA StyleCabello Mendez, J. A., Pérez Bueno, J. d. J., Meas Vong, Y., & Portales Martínez, B. (2022). Cerium Compounds Coating as a Single Self-Healing Layer for Corrosion Inhibition on Aluminum 3003. Sustainability, 14(22), 15056. https://doi.org/10.3390/su142215056










