Genetic Characterization and Population Structure of Pea (Pisum sativum L.) by Molecular Markers against Rust (Uromyces viciae-fabae) in Newly Developed Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting Materials and Experimental Design
2.2. DNA Extraction, Amplification and Electrophoresis
2.3. Population Structure and Gene Flow
2.4. Genetic Diversity Analysis and Analysis of Molecular Variance (AMOVA)
3. Results
3.1. Polymorphic Levels of Simple Sequence Repeats Loci
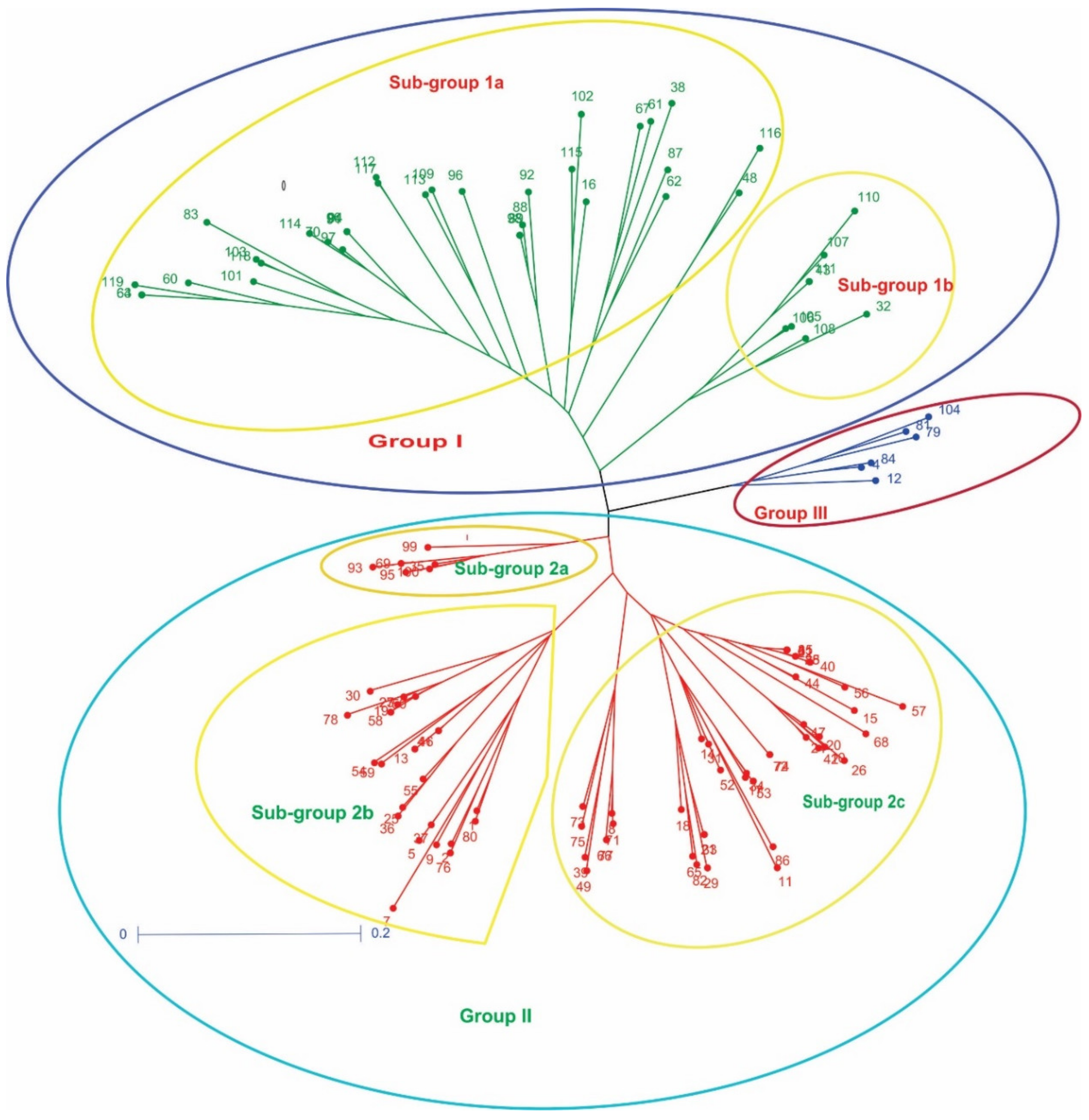
3.2. Genetic Relationship among Pea Germplasm Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, S.; Kumar, A.; Mamidi, S.; McPhee, K. Genetic diversity and population structure among pea (Pisumsativum L.) cultivars as revealed by simple sequence repeat and novel genic markers. Mol. Biotechnol. 2014, 56, 925–938. [Google Scholar] [CrossRef]
- Gali, K.K.; Sackville, A.; Tafesse, E.G.; Lachagari, V.B.; McPhee, K.; Hybl, M.; Warkentin, T.D. Genome-wide association mapping for agronomic and seed quality traits of field pea (Pisumsativum L.). Front. Plant Sci. 2019, 10, 1538. [Google Scholar] [CrossRef]
- Chand, R.; Srivastava, C.P.; Kushwaha, C. Screening technique for pea (Pisum sativum) genotypes against rust disease (Uromyces fabae). Indian J. Agric. Sci. 2014, 74, 166–167. [Google Scholar]
- Duc, G.; Agrama, H.; Bao, S.; Berger, J.; Bourion, V.; De Ron, A.M.; Gowda, C.; Mikic, A.; Millot, D.; Singh, K.; et al. Breeding annual grain legumes for sustainable agriculture: New methods to approach complex traits and target new cultivar ideotypes. Crit. Rev. Plant. Sci. 2015, 34, 381–411. [Google Scholar] [CrossRef]
- Warkentin, T.D.; Smykal, P.; Coyne, C.J.; Weeden, N.; Domoney, C.; Bing, D. Pea (Pisumsativum L.). In Grain Legumes, 1st ed.; De Ron, A.M., Ed.; Springer: New York, NY, USA, 2015; Volume 10, pp. 37–83. [Google Scholar]
- Glaszmann, J.C.; Kilian, B.; Upadhyay, H.D.; Varshney, R.K. Accessing genetic diversity for crop improvement. Curr. Opin. Plant Biol. 2010, 13, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.C.; Rana, M.; Sharma, V.; Nag, A.; Chahota, R.K.; Sharma, T.R. Genetic diversity and structure of pea (Pisumsativum L.) germplasm based on morphological and SSR markers. Plant. Mol. Biol. Rep. 2017, 35, 118–129. [Google Scholar] [CrossRef]
- Singh, S.; Singh, B.; Sharma, V.R.; Kumar, M.; Sirohi, U. Assessment of Genetic Diversity and Population Structure in Pea (Pisumsativum L.) Germplasm based on Morphological Traits and SSR Markers. Legum. Res. 2021, 45, 683–688. [Google Scholar]
- Baranger, A.; Aubert, G.; Arnau, G.; Lainé, A.L.; Deniot, G.; Potier, J.; Weinachter, C.; Weinachter, C.; Lallemand, J.; Burstin, J. Genetic diversity within Pisumsativum using protein-and PCR-based markers. Theor. Appl. Genet. 2004, 108, 1309–1321. [Google Scholar] [CrossRef]
- Gupta, P.K.; Varshney, R.K. The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat. Euphytica 2000, 113, 163–185. [Google Scholar] [CrossRef]
- Burstin, J.; Deniot, G.; Potier, J.; Weinachter, C.; Aubert, G.; Barranger, A. Microsatellite polymorphism in Pisumsativum. Plant Breed. 2001, 120, 311–317. [Google Scholar] [CrossRef]
- Ford, R.; Roux, K.L.; Itman, C.; Brouwer, J.B.; Taylor, P.W. Diversity analysis and genotyping in Pisum with sequence tagged microsatellite site (STMS) primers. Euphytica 2002, 124, 397–405. [Google Scholar] [CrossRef]
- Loridon, K.; McPhee, K.; Morin, J.; Dubreuil, P.; Pilet-Nayel, M.L.; Aubert, G.; Burstin, J. Microsatellite marker polymorphism and mapping in pea (Pisum sativum L.). Theor. Appl. Genet. 2005, 111, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, R.; Yuan, M.; Tao, A.; Xu, J.; Lin, L.; Fang, P.; Qi, J. Genetic diversity and DNA fingerprinting in jute (Corchorus spp.) based on SSR markers. Crop J. 2015, 3, 416–422. [Google Scholar] [CrossRef]
- Saha, D.; Rana, R.S.; Chakraborty, S.; Datta, S.; Kumar, A.A.; Chakraborty, A.K.; Karmakar, P.G. Development of a set of SSR markers for genetic polymorphism detection and interspecific hybrid jute breeding. Crop J. 2017, 5, 416–429. [Google Scholar] [CrossRef]
- Saha, D.; Rana, R.S.; Das, S.; Datta, S.; Mitra, J.; Cloutier, S.J.; You, F.M. Genome-wide regulatory gene-derived SSRs reveal genetic differentiation and population structure in fiber flax genotypes. J. Appl. Genet. 2019, 60, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kimaro, D.; Melis, R.; Sibiya, J.; Shimelis, H.; Shayanowako, A. Analysis of Genetic Diversity and Population Structure of Pigeon pea [Cajanuscajan (L.) Millsp.] Accessions Using SSR Markers. Plants 2020, 9, 1643. [Google Scholar] [CrossRef]
- Singh, J.; Dhall, R.K.; Vikal, Y. Genetic diversity studies in Indian germplasm of pea (Pisum sativum L.) using morphological and microsatellite markers. Genetika 2021, 53, 473–491. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rai, R.; Singh, B.D.; Chand, R.; Srivastava, C.P. Validation of SSR markers associated with rust (Uromycesfabae) resistance in pea (Pisumsativum L.). Physiol. Mol. Biol. Plants 2015, 21, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, V.; Kushwaha, K.P.S.; Pandey, P. Molecular screening of pea germplasm for rust disease resistance using SSR Marker. J. Pure Appl. Microbiol. 2017, 11, 343–348. [Google Scholar] [CrossRef]
- DARwin Software. Available online: http://darwin.cirad.fr/darwin (accessed on 1 March 2022).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Muse, S.V. Power Marker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research: An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Ram, H.; Hedau, N.; Chaudhari, G.; Mukesh Choudhary, M.; Lakshmi Kant, L. Genetic Diversity Assessment in Pea (Pisum sativum L.) using Microsatellite Markers. Int. J. Bio-Resour. Stress 2021, 12, 402–408. [Google Scholar] [CrossRef]
- Bouhadida, M.; Srarfi, F.; Saadi, I.; Kharrat, M. Molecular characterization of pea (Pisumsativum L.) using microsatellite markers. J. Appl. Chem. 2013, 5, 57–61. [Google Scholar]
- Duque-Zapata, J.D.; Muñoz, J.E.; Checa-Coral, O. Molecular characterization with SSR markers for 50 genotypes of shrub peas (Pisumsativum L.) of the GRICAND Collection, Colombia. Rev. Colomb. Cienc. Hortic. 2019, 13, 208–218. [Google Scholar] [CrossRef]
- Handerson, C.; Noren, S.K.; Wricha, T.; Meetei, N.T.; Khanna, V.K.; Pattanayak, A.; Datt, S.A.; Choudhury, P.; Kumar, M. Assessment of genetic diversity in pea (Pisumsativum L.) using morphological and molecular markers. Indian J. Genet. Plant Breed. 2014, 74, 205. [Google Scholar]
- Mohamed, A.; García-Martínez, S.; Loumerem, M.; Carbonell, P.; Ruiz, J.J.; Boubaker, M. Assessment of genetic diversity among local pea (Pisumsativum L.) accessions cultivated in the arid regions of Southern Tunisia using agro-morphological and SSR molecular markers. Genet. Resour. Crop Evol. 2019, 66, 1189–1203. [Google Scholar] [CrossRef]
- Haliloglu, K.; Turkoglu, A.; Tan, M.; Poczai, P. SSR-Based Molecular Identification and Population Structure Analysis for Forage Pea (Pisum sativum var. arvense L.) Landraces. Genes 2022, 13, 1086. [Google Scholar] [CrossRef]
- Sharma, R.; Dar, A.A.; Mahajan, R.; Sharma, S. Molecular and Biochemical Characterisation of Indian Germplasm of Pisum sativum L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 103–111. [Google Scholar] [CrossRef]
- Teshome, A.; Bryngelsson, T.; Dagne, K.; Geleta, M. Assessment of genetic diversity in Ethiopian field pea (Pisum sativum L.) accessions with newly developed EST-SSR markers. BMC Genet. 2015, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Singh, A.K.; Singh, B.D.; Joshi, A.K.; Chand, R.; Srivastava, C.P. Molecular mapping for resistance to pea rust caused by Uromyces fabae (Pers.) de-Bary. Theor. Appl. Genet. 2011, 123, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Scarano, D.; Rubio, F.; Ruiz, J.J.; Rao, R.; Corrado, G. Morphological and genetic diversity among and within common bean (Phaseolus vulgaris L.) landraces from the Campania region (Southern Italy). Sci. Hortic. 2014, 180, 72–78. [Google Scholar] [CrossRef]
- Ahmad, S.; Kaur, S.; Lamb-Palmer, N.D.; Lefsrud, M.; Singh, J. Genetic diversity and population structure of Pisum sativum accessions for marker-trait association of lipid content. Crop J. 2015, 3, 238–245. [Google Scholar] [CrossRef]
- Ferradini, N.; Torricelli, R.; Terzaroli, N.; Albertini, E.; Russi, L. The Genetic Structure of the Field Pea Landrace “Roveja di Civita di Cascia”. Sustainability 2019, 11, 6493. [Google Scholar] [CrossRef]
- Hanci, F. Genetic variability in peas (Pisumsativum L.) from Turkey assessed with molecular and morphological markers. Folia. Hortic. 2019, 31, 101–116. [Google Scholar] [CrossRef]
- Kumari, P.; Basal, N.; Singh, A.K.; Rai, V.P.; Srivastava, C.P.; Singh, P.K. Genetic diversity studies in pea (Pisum sativum L.) using simple sequence repeat markers. Genet. Mol. Res. 2013, 12, 3540–3550. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.A.; Lateef, D.D.; Omer, D.A.; Kareem, S.H.; Ahmad, D.A.; Khal, L.H. Genetic diversity and structure analysis of pea grown in Iraq using microsatellite markers. Jordan J. Biol. Sci. 2018, 11, 201–207. [Google Scholar]

| Origin | Accession Number |
|---|---|
| Australia | EC865919, EC865920, EC865921, EC865922, EC865923, EC865924, EC865925, EC865926, EC865927, EC865928, EC865929, EC865930, EC865931, EC865932, EC865933, EC865934, EC865935, EC865936, EC865937, EC865938, EC865939, EC865940, EC865941, EC865942, EC865943, EC865944, EC865945, EC865946, EC865947, EC865948, EC865949, EC865950, EC865951, EC865952, EC865953, EC865954, EC865955, EC865956, EC865957, EC865958, EC865959, EC865960, EC865961, EC865962, EC865963, EC865964, EC865965, EC865966, EC865967, EC865968, EC865969, EC865970, EC865971, EC865972, EC865973, EC865974, EC865975, EC865976, EC865977, EC865978, EC865979, EC865980, EC865981, EC865982, EC865983, EC865984, EC865985, EC865986, EC865987, EC865988, EC865989, EC865990, EC865991, EC865992, EC865993, EC865994, EC865995, EC865996, EC865997, EC865998, EC865999, EC866000, EC866001, EC866002, EC866003, EC866004, EC866005, EC866006, EC866007, EC866008, EC866009, EC866010, EC866011, EC866012, EC866013, EC866014, EC866015, EC866016, EC866017, EC866018, EC866019, EC866020, EC866021, EC866022, EC866023, EC866024, EC866025, EC866026, EC866027, EC866028, EC866029, EC866030, EC866031, EC866032, EC866033 |
| India | HUDP-15, HFP-8909, HFP-4, HFP-9907 |
| No | SSR Marker | No of Alleles (Na) | Major Allele Frequency (A) | Gene Diversity (He) | Heterozygosity (Ho) | PIC * |
|---|---|---|---|---|---|---|
| 1 | AA135 | 2 | 0.588 | 0.484 | 0.000 | 0.367 |
| 2 | AA176 | 2 | 0.907 | 0.167 | 0.000 | 0.153 |
| 3 | AA206 | 2 | 0.991 | 0.016 | 0.000 | 0.016 |
| 4 | AA345-145 | 2 | 0.932 | 0.125 | 0.000 | 0.117 |
| 5 | AA372.1 | 2 | 0.705 | 0.415 | 0.000 | 0.329 |
| 6 | AA416 | 2 | 0.563 | 0.492 | 0.000 | 0.371 |
| 7 | AA446 | 2 | 0.806 | 0.311 | 0.000 | 0.263 |
| 8 | AA505 | 2 | 0.529 | 0.498 | 0.000 | 0.374 |
| 9 | AA57 | 4 | 0.617 | 0.505 | 0.697 | 0.419 |
| 10 | AA61 | 2 | 0.983 | 0.033 | 0.000 | 0.032 |
| 11 | AA98 | 4 | 0.714 | 0.412 | 0.563 | 0.334 |
| 12 | AB24 | 2 | 0.949 | 0.095 | 0.000 | 0.091 |
| 13 | AB60 | 2 | 0.655 | 0.451 | 0.000 | 0.349 |
| 14 | AD146 | 2 | 0.789 | 0.331 | 0.000 | 0.276 |
| 15 | AD147 | 2 | 0.991 | 0.016 | 0.000 | 0.016 |
| 16 | AD237 | 4 | 0.403 | 0.657 | 0.000 | 0.584 |
| 17 | AD70290 | 2 | 0.991 | 0.016 | 0.000 | 0.016 |
| 18 | B14 | 2 | 0.537 | 0.497 | 0.000 | 0.373 |
| 19 | B16 | 9 | 0.420 | 0.630 | 0.352 | 0.555 |
| 20 | P46 | 2 | 0.806 | 0.311 | 0.000 | 0.263 |
| 21 | PSAB60 | 2 | 0.529 | 0.498 | 0.000 | 0.374 |
| 22 | S244 | 2 | 0.857 | 0.244 | 0.000 | 0.214 |
| 23 | CAASESP527 | 2 | 0.882 | 0.207 | 0.000 | 0.186 |
| 24 | S144 | 2 | 0.974 | 0.049 | 0.000 | 0.047 |
| 24 | S85 | 3 | 0.680 | 0.439 | 0.000 | 0.350 |
| 26 | B179 | 2 | 0.899 | 0.181 | 0.000 | 0.164 |
| 27 | CAASESP1173 | 2 | 0.907 | 0.167 | 0.000 | 0.153 |
| 28 | P282 | 3 | 0.672 | 0.494 | 0.000 | 0.443 |
| 29 | CAASESP524 | 2 | 0.781 | 0.341 | 0.000 | 0.283 |
| 30 | CAASESP1193 | 2 | 0.672 | 0.440 | 0.000 | 0.343 |
| 31 | P255 | 3 | 0.420 | 0.653 | 0.000 | 0.579 |
| Mean | 3 | 0.747 | 0.328 | 0.052 | 0.272 | |
| Max. | 9 | 0.991 | 0.657 | 0.697 | 0.584 | |
| Min. | 2 | 0.403 | 0.016 | 0.000 | 0.016 |
| Entry No. | Accession No. | AUDPC | Entry No. | Accession No. | AUDPC | Entry No. | Accession No. | AUDPC |
|---|---|---|---|---|---|---|---|---|
| 1 | EC865919 | 592 | 41 | EC865959 | 872 | 81 | EC865999 | 745 |
| 2 | EC865920 | 700 | 42 | EC865960 | 1091 | 82 | EC866000 | 547 |
| 3 | EC865921 | 334 | 43 | EC865961 | 560 | 83 | EC866001 | 481 |
| 4 | EC865922 | 834 | 44 | EC865962 | 476 | 84 | EC866002 | 543 |
| 5 | EC865923 | 723 | 45 | EC865963 | 575 | 85 | EC866003 | 876 |
| 6 | EC865924 | 698 | 46 | EC865964 | 843 | 86 | EC866004 | 679 |
| 7 | EC865925 | 463 | 47 | EC865965 | 546 | 87 | EC866005 | 881 |
| 8 | EC865926 | 537 | 48 | EC865966 | 628 | 88 | EC866006 | 845 |
| 9 | EC865927 | 794 | 49 | EC865967 | 527 | 89 | EC866007 | 507 |
| 10 | EC865928 | 682 | 50 | EC865968 | 491 | 90 | EC866008 | 530 |
| 11 | EC865929 | 344 | 51 | EC865969 | 544 | 91 | EC866009 | 744 |
| 12 | EC865930 | 469 | 52 | EC865970 | 871 | 92 | EC866010 | 562 |
| 13 | EC865931 | 824 | 53 | EC865971 | 850 | 93 | EC866011 | 872 |
| 14 | EC865932 | 833 | 54 | EC865972 | 794 | 94 | EC866012 | 436 |
| 15 | EC865933 | 799 | 55 | EC865973 | 756 | 95 | EC866013 | 796 |
| 16 | EC865934 | 538 | 56 | EC865974 | 849 | 96 | EC866014 | 539 |
| 17 | EC865935 | 540 | 57 | EC865975 | 292 | 97 | EC866015 | 845 |
| 18 | EC865936 | 536 | 58 | EC865976 | 782 | 98 | EC866016 | 861 |
| 19 | EC865937 | 868 | 59 | EC865977 | 839 | 99 | EC866017 | 878 |
| 20 | EC865938 | 826 | 60 | EC865978 | 459 | 100 | EC866018 | 853 |
| 21 | EC865939 | 572 | 61 | EC865979 | 896 | 101 | EC866019 | 746 |
| 22 | EC865940 | 762 | 62 | EC865980 | 873 | 102 | EC866020 | 650 |
| 23 | EC865941 | 723 | 63 | EC865981 | 860 | 103 | EC866021 | 548 |
| 24 | EC865942 | 676 | 64 | EC865982 | 842 | 104 | EC866022 | 843 |
| 25 | EC865943 | 452 | 65 | EC865983 | 869 | 105 | EC866023 | 527 |
| 26 | EC865944 | 389 | 66 | EC865984 | 550 | 106 | EC866024 | 576 |
| 27 | EC865945 | 871 | 67 | EC865985 | 861 | 107 | EC866025 | 588 |
| 28 | EC865946 | 706 | 68 | EC865986 | 861 | 108 | EC866026 | 383 |
| 29 | EC865947 | 852 | 69 | EC865987 | 546 | 109 | EC866027 | 916 |
| 30 | EC865948 | 881 | 70 | EC865988 | 524 | 110 | EC866028 | 382 |
| 31 | EC865949 | 876 | 71 | EC865989 | 656 | 111 | EC866029 | 857 |
| 32 | EC865950 | 886 | 72 | EC865990 | 910 | 112 | EC866030 | 853 |
| 33 | EC865951 | 344 | 73 | EC865991 | 549 | 113 | EC866031 | 345 |
| 34 | EC865952 | 883 | 74 | EC865992 | 565 | 114 | EC866032 | 383 |
| 35 | EC865953 | 825 | 75 | EC865993 | 550 | 115 | EC866033 | 351 |
| 36 | EC865954 | 915 | 76 | EC865994 | 492 | 116 * | HUDP-15 | 371 |
| 37 | EC865955 | 757 | 77 | EC865995 | 542 | 117 * | HFP-8909 | 851 |
| 38 | EC865956 | 569 | 78 | EC865996 | 866 | 118 * | HFP-4 | 1136 |
| 39 | EC865957 | 849 | 79 | EC865997 | 566 | 119 * | HFP-9907 | 514 |
| 40 | EC865958 | 860 | 80 | EC865998 | 864 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.S.; Singh, A.K.; Chand, R.; Vaish, S.S. Genetic Characterization and Population Structure of Pea (Pisum sativum L.) by Molecular Markers against Rust (Uromyces viciae-fabae) in Newly Developed Genotypes. Sustainability 2022, 14, 15082. https://doi.org/10.3390/su142215082
Yadav AS, Singh AK, Chand R, Vaish SS. Genetic Characterization and Population Structure of Pea (Pisum sativum L.) by Molecular Markers against Rust (Uromyces viciae-fabae) in Newly Developed Genotypes. Sustainability. 2022; 14(22):15082. https://doi.org/10.3390/su142215082
Chicago/Turabian StyleYadav, Anmol Singh, Anil Kumar Singh, Ramesh Chand, and Shyam Saran Vaish. 2022. "Genetic Characterization and Population Structure of Pea (Pisum sativum L.) by Molecular Markers against Rust (Uromyces viciae-fabae) in Newly Developed Genotypes" Sustainability 14, no. 22: 15082. https://doi.org/10.3390/su142215082
APA StyleYadav, A. S., Singh, A. K., Chand, R., & Vaish, S. S. (2022). Genetic Characterization and Population Structure of Pea (Pisum sativum L.) by Molecular Markers against Rust (Uromyces viciae-fabae) in Newly Developed Genotypes. Sustainability, 14(22), 15082. https://doi.org/10.3390/su142215082






